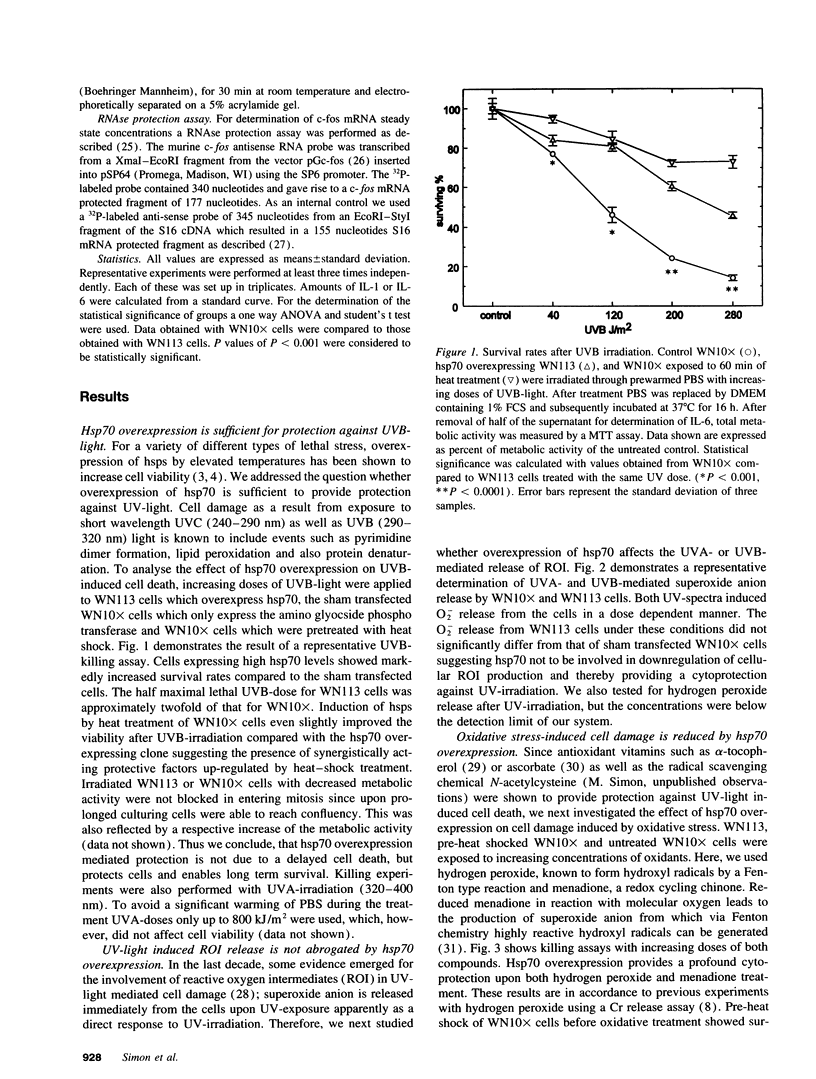
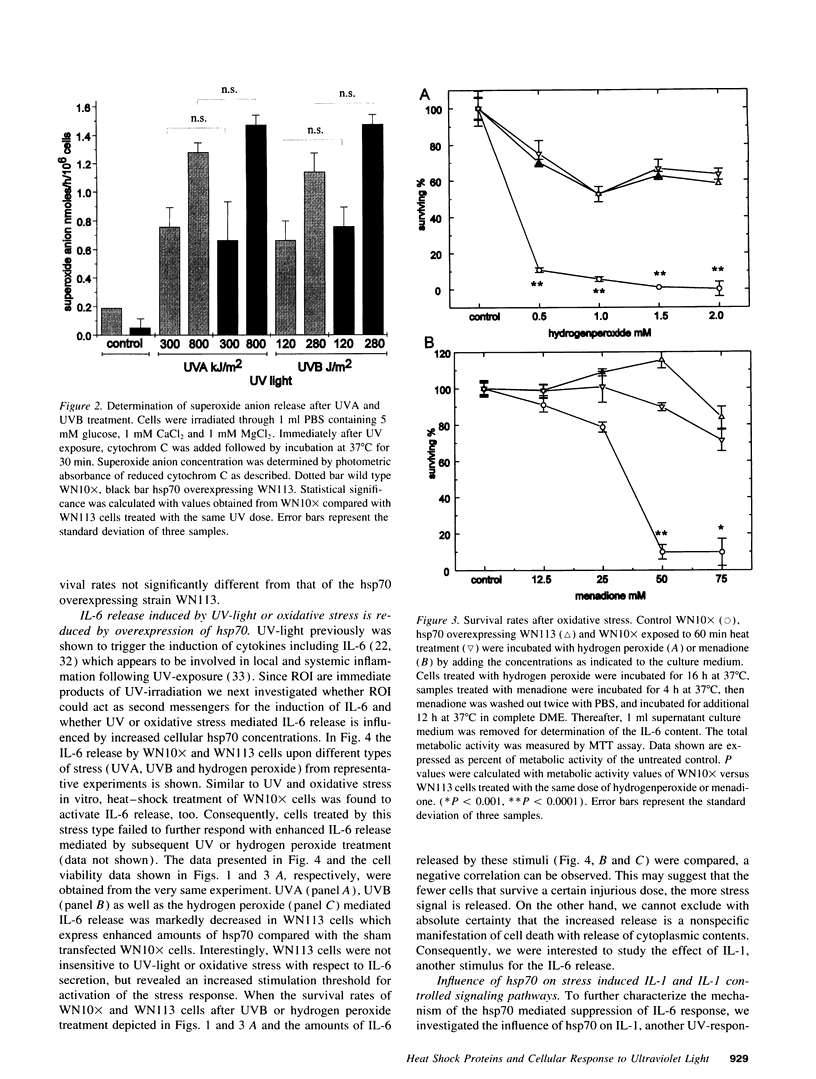
Abstract
To elucidate cellular concepts for protection against ultraviolet (UV) light we investigated the effect of heat shock protein 70 (hsp70) overexpression on cell viability and on the secretion of UV-inducible immunological cytokines. Transfected murine fibrosarcoma cells (WEHI-S), overexpressing hsp70 or a sham transfected control were used. Overexpression of hsp70 was sufficient to markedly increase cell viability upon treatment with UVB (290-320 nm). Since long wave UV (UVA, 320-400 nm) as well as UVB turned out to stimulate the release of O2- radicals we studied the cell viability upon oxidative stress. Hsp70 overexpression increased viability upon treatment with hydrogen peroxide or menadione, but had no influence on UV-induced O2- release. UV-light is known to upregulate immunologic and proinflammatory cytokines such as IL-1 and IL-6. Oxidative stress appeared to exert a similar effect. Hsp70 overexpression markedly decreased the release of IL-6 induced by UVA, UVB and oxidative stress. To test whether the hsp70 mediated suppression is confined to events caused by UV-light we determined IL-1-mediated effects. IL-1-induced IL-6 release was reduced by hsp70 overexpression, whereas the IL-1 mediated activation of nuclear factor kappa B was not affected. Our data suggests that hsp70 plays a central role not only in cell protection against UV-light, but also in the regulation of proinflammatory cytokine release induced by UV-exposure.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aida Y., Pabst M. J. Neutrophil responses to lipopolysaccharide. Effect of adherence on triggering and priming of the respiratory burst. J Immunol. 1991 Feb 15;146(4):1271–1276. [PubMed] [Google Scholar]
- Baeuerle P. A. The inducible transcription activator NF-kappa B: regulation by distinct protein subunits. Biochim Biophys Acta. 1991 Apr 16;1072(1):63–80. doi: 10.1016/0304-419x(91)90007-8. [DOI] [PubMed] [Google Scholar]
- Bergers G., Reikerstorfer A., Braselmann S., Graninger P., Busslinger M. Alternative promoter usage of the Fos-responsive gene Fit-1 generates mRNA isoforms coding for either secreted or membrane-bound proteins related to the IL-1 receptor. EMBO J. 1994 Mar 1;13(5):1176–1188. doi: 10.1002/j.1460-2075.1994.tb06367.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black H. S. Potential involvement of free radical reactions in ultraviolet light-mediated cutaneous damage. Photochem Photobiol. 1987 Aug;46(2):213–221. doi: 10.1111/j.1751-1097.1987.tb04759.x. [DOI] [PubMed] [Google Scholar]
- Braselmann S., Graninger P., Busslinger M. A selective transcriptional induction system for mammalian cells based on Gal4-estrogen receptor fusion proteins. Proc Natl Acad Sci U S A. 1993 Mar 1;90(5):1657–1661. doi: 10.1073/pnas.90.5.1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darr D., Combs S., Dunston S., Manning T., Pinnell S. Topical vitamin C protects porcine skin from ultraviolet radiation-induced damage. Br J Dermatol. 1992 Sep;127(3):247–253. doi: 10.1111/j.1365-2133.1992.tb00122.x. [DOI] [PubMed] [Google Scholar]
- De la Harpe J., Nathan C. F. A semi-automated micro-assay for H2O2 release by human blood monocytes and mouse peritoneal macrophages. J Immunol Methods. 1985 Apr 22;78(2):323–336. doi: 10.1016/0022-1759(85)90089-4. [DOI] [PubMed] [Google Scholar]
- DeNagel D. C., Pierce S. K. A case for chaperones in antigen processing. Immunol Today. 1992 Mar;13(3):86–89. doi: 10.1016/0167-5699(92)90147-Y. [DOI] [PubMed] [Google Scholar]
- Ferris D. K., Harel-Bellan A., Morimoto R. I., Welch W. J., Farrar W. L. Mitogen and lymphokine stimulation of heat shock proteins in T lymphocytes. Proc Natl Acad Sci U S A. 1988 Jun;85(11):3850–3854. doi: 10.1073/pnas.85.11.3850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gething M. J., Sambrook J. Protein folding in the cell. Nature. 1992 Jan 2;355(6355):33–45. doi: 10.1038/355033a0. [DOI] [PubMed] [Google Scholar]
- Girotti A. W. Mechanisms of lipid peroxidation. J Free Radic Biol Med. 1985;1(2):87–95. doi: 10.1016/0748-5514(85)90011-x. [DOI] [PubMed] [Google Scholar]
- Griffin G. E., Leung K., Folks T. M., Kunkel S., Nabel G. J. Activation of HIV gene expression during monocyte differentiation by induction of NF-kappa B. Nature. 1989 May 4;339(6219):70–73. doi: 10.1038/339070a0. [DOI] [PubMed] [Google Scholar]
- Gromkowski S. H., Yagi J., Janeway C. A., Jr Elevated temperature regulates tumor necrosis factor-mediated immune killing. Eur J Immunol. 1989 Sep;19(9):1709–1714. doi: 10.1002/eji.1830190927. [DOI] [PubMed] [Google Scholar]
- Hopkins S. J., Humphreys M. Simple, sensitive and specific bioassay of interleukin-1. J Immunol Methods. 1989 Jun 21;120(2):271–276. doi: 10.1016/0022-1759(89)90252-4. [DOI] [PubMed] [Google Scholar]
- Hunt C., Morimoto R. I. Conserved features of eukaryotic hsp70 genes revealed by comparison with the nucleotide sequence of human hsp70. Proc Natl Acad Sci U S A. 1985 Oct;82(19):6455–6459. doi: 10.1073/pnas.82.19.6455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Israël A., Le Bail O., Hatat D., Piette J., Kieran M., Logeat F., Wallach D., Fellous M., Kourilsky P. TNF stimulates expression of mouse MHC class I genes by inducing an NF kappa B-like enhancer binding activity which displaces constitutive factors. EMBO J. 1989 Dec 1;8(12):3793–3800. doi: 10.1002/j.1460-2075.1989.tb08556.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jättelä M. Overexpression of major heat shock protein hsp70 inhibits tumor necrosis factor-induced activation of phospholipase A2. J Immunol. 1993 Oct 15;151(8):4286–4294. [PubMed] [Google Scholar]
- Jättelä M., Pinola M., Saksela E. Heat shock inhibits the cytotoxic action of TNF-alpha in tumor cells but does not alter its noncytotoxic actions in endothelial and adrenal cells. Lymphokine Cytokine Res. 1991 Apr;10(1-2):119–125. [PubMed] [Google Scholar]
- Jättelä M., Saksela K., Saksela E. Heat shock protects WEHI-164 target cells from the cytolysis by tumor necrosis factors alpha and beta. Eur J Immunol. 1989 Aug;19(8):1413–1417. doi: 10.1002/eji.1830190810. [DOI] [PubMed] [Google Scholar]
- Jättelä M., Wissing D., Bauer P. A., Li G. C. Major heat shock protein hsp70 protects tumor cells from tumor necrosis factor cytotoxicity. EMBO J. 1992 Oct;11(10):3507–3512. doi: 10.1002/j.1460-2075.1992.tb05433.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jättelä M., Wissing D. Heat-shock proteins protect cells from monocyte cytotoxicity: possible mechanism of self-protection. J Exp Med. 1993 Jan 1;177(1):231–236. doi: 10.1084/jem.177.1.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr J. B., McElroy C. T. Evidence for large upward trends of ultraviolet-B radiation linked to ozone depletion. Science. 1993 Nov 12;262(5136):1032–1034. doi: 10.1126/science.262.5136.1032. [DOI] [PubMed] [Google Scholar]
- Kirnbauer R., Köck A., Neuner P., Förster E., Krutmann J., Urbanski A., Schauer E., Ansel J. C., Schwarz T., Luger T. A. Regulation of epidermal cell interleukin-6 production by UV light and corticosteroids. J Invest Dermatol. 1991 Apr;96(4):484–489. doi: 10.1111/1523-1747.ep12470181. [DOI] [PubMed] [Google Scholar]
- Kripke M. L. Immunology and photocarcinogenesis. New light on an old problem. J Am Acad Dermatol. 1986 Jan;14(1):149–155. doi: 10.1016/s0190-9622(86)70017-0. [DOI] [PubMed] [Google Scholar]
- Kuperberg G., Ellis J., Marcinkiewicz J., Chain B. M. Temperature-induced stress abrogates co-stimulatory function in antigen-presenting cells. Eur J Immunol. 1991 Nov;21(11):2791–2795. doi: 10.1002/eji.1830211121. [DOI] [PubMed] [Google Scholar]
- Kupper T. S., Chua A. O., Flood P., McGuire J., Gubler U. Interleukin 1 gene expression in cultured human keratinocytes is augmented by ultraviolet irradiation. J Clin Invest. 1987 Aug;80(2):430–436. doi: 10.1172/JCI113090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köck A., Schwarz T., Kirnbauer R., Urbanski A., Perry P., Ansel J. C., Luger T. A. Human keratinocytes are a source for tumor necrosis factor alpha: evidence for synthesis and release upon stimulation with endotoxin or ultraviolet light. J Exp Med. 1990 Dec 1;172(6):1609–1614. doi: 10.1084/jem.172.6.1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis M. J., Pelham H. R. Involvement of ATP in the nuclear and nucleolar functions of the 70 kd heat shock protein. EMBO J. 1985 Dec 1;4(12):3137–3143. doi: 10.1002/j.1460-2075.1985.tb04056.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G. C., Li L., Liu R. Y., Rehman M., Lee W. M. Heat shock protein hsp70 protects cells from thermal stress even after deletion of its ATP-binding domain. Proc Natl Acad Sci U S A. 1992 Mar 15;89(6):2036–2040. doi: 10.1073/pnas.89.6.2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libermann T. A., Baltimore D. Activation of interleukin-6 gene expression through the NF-kappa B transcription factor. Mol Cell Biol. 1990 May;10(5):2327–2334. doi: 10.1128/mcb.10.5.2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindquist S., Craig E. A. The heat-shock proteins. Annu Rev Genet. 1988;22:631–677. doi: 10.1146/annurev.ge.22.120188.003215. [DOI] [PubMed] [Google Scholar]
- Lindquist S. The heat-shock response. Annu Rev Biochem. 1986;55:1151–1191. doi: 10.1146/annurev.bi.55.070186.005443. [DOI] [PubMed] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983 Dec 16;65(1-2):55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Nelson R. J., Ziegelhoffer T., Nicolet C., Werner-Washburne M., Craig E. A. The translation machinery and 70 kd heat shock protein cooperate in protein synthesis. Cell. 1992 Oct 2;71(1):97–105. doi: 10.1016/0092-8674(92)90269-i. [DOI] [PubMed] [Google Scholar]
- Nutter L. M., Ngo E. O., Fisher G. R., Gutierrez P. L. DNA strand scission and free radical production in menadione-treated cells. Correlation with cytotoxicity and role of NADPH quinone acceptor oxidoreductase. J Biol Chem. 1992 Feb 5;267(4):2474–2479. [PubMed] [Google Scholar]
- Res P. C., Thole J. E., de Vries R. R. Heat shock proteins in immunopathology. Curr Opin Immunol. 1991 Dec;3(6):924–929. doi: 10.1016/s0952-7915(05)80015-9. [DOI] [PubMed] [Google Scholar]
- Riabowol K. T., Mizzen L. A., Welch W. J. Heat shock is lethal to fibroblasts microinjected with antibodies against hsp70. Science. 1988 Oct 21;242(4877):433–436. doi: 10.1126/science.3175665. [DOI] [PubMed] [Google Scholar]
- Schreiber E., Matthias P., Müller M. M., Schaffner W. Rapid detection of octamer binding proteins with 'mini-extracts', prepared from a small number of cells. Nucleic Acids Res. 1989 Aug 11;17(15):6419–6419. doi: 10.1093/nar/17.15.6419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen R., Baltimore D. Multiple nuclear factors interact with the immunoglobulin enhancer sequences. Cell. 1986 Aug 29;46(5):705–716. doi: 10.1016/0092-8674(86)90346-6. [DOI] [PubMed] [Google Scholar]
- Shimizu H., Mitomo K., Watanabe T., Okamoto S., Yamamoto K. Involvement of a NF-kappa B-like transcription factor in the activation of the interleukin-6 gene by inflammatory lymphokines. Mol Cell Biol. 1990 Feb;10(2):561–568. doi: 10.1128/mcb.10.2.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirakawa F., Chedid M., Suttles J., Pollok B. A., Mizel S. B. Interleukin 1 and cyclic AMP induce kappa immunoglobulin light-chain expression via activation of an NF-kappa B-like DNA-binding protein. Mol Cell Biol. 1989 Mar;9(3):959–964. doi: 10.1128/mcb.9.3.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon M. M., Aragane Y., Schwarz A., Luger T. A., Schwarz T. UVB light induces nuclear factor kappa B (NF kappa B) activity independently from chromosomal DNA damage in cell-free cytosolic extracts. J Invest Dermatol. 1994 Apr;102(4):422–427. doi: 10.1111/1523-1747.ep12372194. [DOI] [PubMed] [Google Scholar]
- Stein B., Rahmsdorf H. J., Steffen A., Litfin M., Herrlich P. UV-induced DNA damage is an intermediate step in UV-induced expression of human immunodeficiency virus type 1, collagenase, c-fos, and metallothionein. Mol Cell Biol. 1989 Nov;9(11):5169–5181. doi: 10.1128/mcb.9.11.5169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbanski A., Schwarz T., Neuner P., Krutmann J., Kirnbauer R., Köck A., Luger T. A. Ultraviolet light induces increased circulating interleukin-6 in humans. J Invest Dermatol. 1990 Jun;94(6):808–811. doi: 10.1111/1523-1747.ep12874666. [DOI] [PubMed] [Google Scholar]
- Vitelli L., Kemler I., Lauber B., Birnstiel M. L., Busslinger M. Developmental regulation of micro-injected histone genes in sea urchin embryos. Dev Biol. 1988 May;127(1):54–63. doi: 10.1016/0012-1606(88)90188-1. [DOI] [PubMed] [Google Scholar]
- Werninghaus K., Handjani R. M., Gilchrest B. A. Protective effect of alpha-tocopherol in carrier liposomes on ultraviolet-mediated human epidermal cell damage in vitro. Photodermatol Photoimmunol Photomed. 1991 Dec;8(6):236–242. [PubMed] [Google Scholar]
- Wlaschek M., Bolsen K., Herrmann G., Schwarz A., Wilmroth F., Heinrich P. C., Goerz G., Scharffetter-Kochanek K. UVA-induced autocrine stimulation of fibroblast-derived-collagenase by IL-6: a possible mechanism in dermal photodamage? J Invest Dermatol. 1993 Aug;101(2):164–168. doi: 10.1111/1523-1747.ep12363644. [DOI] [PubMed] [Google Scholar]
- Wlaschek M., Heinen G., Poswig A., Schwarz A., Krieg T., Scharffetter-Kochanek K. UVA-induced autocrine stimulation of fibroblast-derived collagenase/MMP-1 by interrelated loops of interleukin-1 and interleukin-6. Photochem Photobiol. 1994 May;59(5):550–556. doi: 10.1111/j.1751-1097.1994.tb02982.x. [DOI] [PubMed] [Google Scholar]