CONSPECTUS
There are a growing range of innovations in the field of nanobiotechnology and nanomedicine. However, the increased number of engineered nanomaterials (ENMs) and their novel physicochemical properties pose a new challenge of understanding the full spectrum of their interactions at the nano/bio interface, including the potential to engage in hazardous interactions. A comprehensive understanding of these interactions is required, including the physicochemical properties that control bioavailability and how this knowledge could be used for safer nanomaterial design. To this end, considerable knowledge generation and exploration is required to understand how material properties influence ENM uptake, transport and fate, as well as the biological consequences of these interactions at cellular level. The toxicity mechanisms of different ENMs differ with nanosize/nanosurface which directly correlates to the physicochemical activities of ENMs in vivo. So, to explore their underlying physicochemical processes of ENMs in cells will be essentially helpful for definitely understanding the toxicity of ENMs. In addition, the in vitro results are indispensable for modeling the biokinetics of ENMs. Nevertheless, we need to proceed such extrapolation with due caution, because the dosage relevance between the in vitro and in vivo exposure largely influences outcomes of the toxic response.
In this Account, we delineate our view of the impact of ENM physicochemical properties on cellular bioprocessing based on the research performed in our laboratories. Because organic, inorganic, and hybrid ENMs can be produced in various sizes, shapes, surface modifications and compositions, and their widely tunable compositions and structures that can be dynamically modified under different biological and environmental use conditions. Therefore, a description of how ENM chemical properties such as (1) hydrophobicity and hydropholicity, (2) material composition, (3) surface functionalization and charge, (4) dispersal state, and (5) adsorption of proteins on the surface determine ENM cellular uptake, intracellular biotransformation, and bioelimination or bioaccumulation, were included. We will also review how physical properties such as size, aspect ratio and surface area influence these interactions and their potential risks. We discuss this conceptual framework from the perspective of actual experimental findings and show how tuning of these properties can be used to control the uptake, biotransformation, fate and hazard of ENMs. The current review on ENM biological behavior and safety issues will provide specific and concentrated information with the principles of both nano-bio interactions and dominating natural biological rules. This knowledge gathering also assists us in developing safer nanotherapeutics and guiding the design of new materials that can execute novel functions at the nano-bio interface.
Introduction
With the ability to manipulate structures at nanoscale, significant breakthroughs have been achieved in material design to impact industrial use of ENMs as well as their application for nanomedicine.1 However, the dramatic increase in the number of new ENMs and their novel physicochemical properties introduce the potential to generate adverse biological outcomes in humans and the environment.2–4 In order to understand material hazard and develop safer ENMs, we need a platform that allows rational exploration of the cellular nano-bio interface, including predictions for how ENM physicochemical properties relate to cellular bioavailability, uptake and bioprocessing.
Numerous studies have attempted to address the role of physicochemical properties on ENM uptake, transport and fate. These ENM physicochemical properties include: (1) surface chemistry;5–8 (2) physical properties (size, shape and surface area);7,9 (3) surface modifications under biological conditions (e.g. acquisition of a protein corona);7,10,11 (4) dispersion, aggregation and agglomeration of the ENMs12,13 and (5) stability in physiological conditions.14–16 However, most published research on the bioprocessing and biological fate of ENMs lack information to allow interpretation of quantitative property-activity relationships.17 This lack of knowledge hampers a solid understanding of the biological behavior, beneficial use and safety assessment of nanomaterials. For this field to further evolve, we need to develop a scientific approach to understand how ENM physicochemical properties relate to biological behavior and how designs of those properties could be used to optimize the utility of the ENMs for therapeutic use and safety.
In order to address the uptake, transport and fate of ENMs, our understanding should transcend the knowledge of the biological behavior of traditional small molecules or micron scale particles. Generally, most organic and inorganic ENMs cannot be described only in terms of chemical composition but also have to take into consideration of size, shape, and surface modification. Moreover, their tunable compositions and structural features lead ENMs to undergo dynamic and subtle changes under biological conditions. This leads to the emergence of a series of distinct ENM behaviors under biological conditions, including the impact on cells during the uptake, transport and fate of ENMs. Most small drug molecules enter the cell through passive diffusion,17 whereas most ENMs are taken up by active processes such as phagocytosis or pinocytosis depending on a dynamic series of physicochemical properties.4,6,8,9,18 This introduces a range of biological response differences that could be used to therapeutic advantage or to understand and study hazard potential. Moreover, the intracellular fate and biotransformation of ENMs could differ from small molecules or larger particles due to the complex interaction of ENM compositions and physicochemical properties with cellular molecules and structures.19 This could introduce additional biological variation. The intracellular fate and toxicity of biopersistant ENMs could be very complicated.12,18
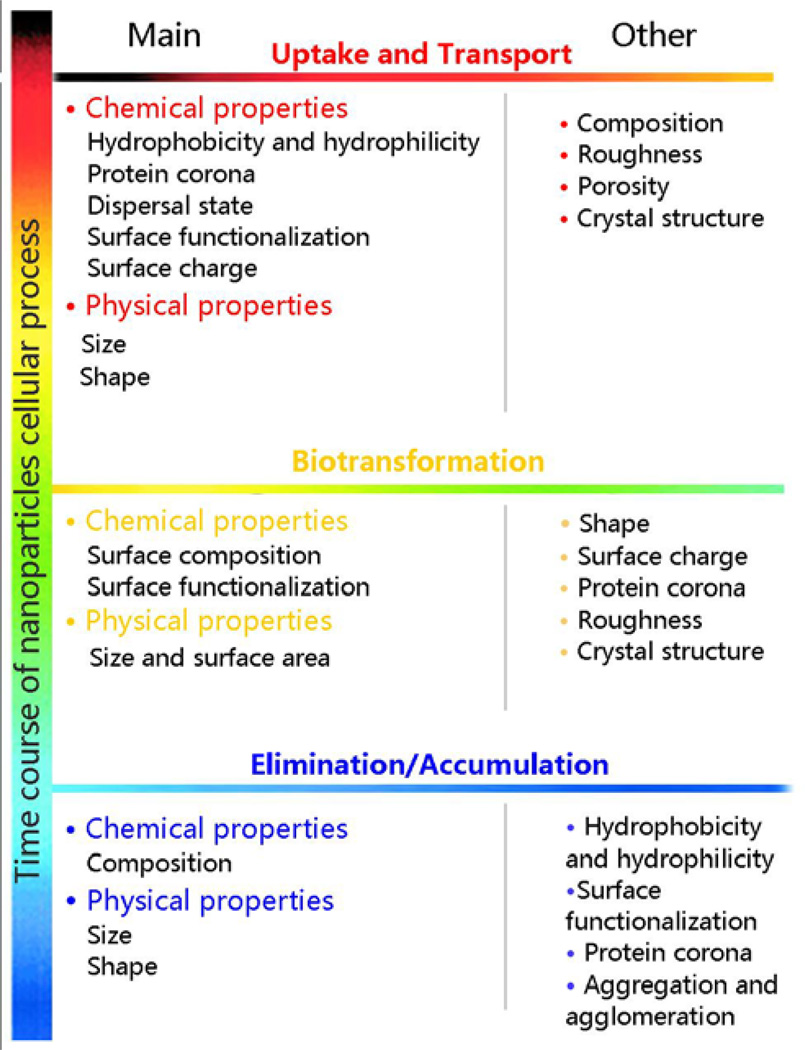
In this Account, the major physicochemical properties (Figure 1) of ENMs that impact biological interactions at cellular level, including uptake, fate, accumulation and biotransformation, are discussed. We will endeavor to explain the principal chemical and physical properties of ENMs that impact bioprocessing by providing examples of the biological events at the nano-bio interface and nanotoxicology emerging from our laboratories.
Figure 1.
Scheme of the main physicochemical properties govern the cellular process of ENMs which would be introduced in this Accounts. Other properties which were not elucidated in this Accounts but also involved in ENM cellular process were listed as other.
Impact of Chemical Properties on Nanomaterial Cellular Uptake, Transport, and Accumulation
When nanomaterials encounter cells, what do the cells see? And how do the cells respond? The chemical properties at the nanomaterial surface play an important role in determining interactions at the nano-bio interface.4,19 The composition, coating, charge, placement of ligands and wettability of the material surface play roles in the adsorption of biomolecules in cellular fate and uptake.11–13 These surface properties also determine interactions with membranes, ions, organelles, nucleic acids etc, and thus are capable of influencing the structure and function of biomolecules and cells to affect homeostasis or induction of toxicity. Surface composition also determines the stability and fate of ENMs in biology.12,14,16,20,21 Here, we will focus on the impact of the above properties in cellular uptake, biotransformation, fate and safety, and illustrate some successful approaches that improve the ENM biocompatibility and safety by adjusting the surface properties.
Impact of surface hydrophobicity and hydrophilicity
Hydrophobic nanoparticles are generally not stable and poorly dispersed in biological fluids and culture medium.11–13 Hydrophobic interactions promote hydrophobic nanoparticles forming aggregates, or interact with hydrophobic residues of blood proteins or peptides to enhance their dispersion.10,22 ENMs taken up as aggregates or agglomerates also tend to be less avidly cleared by the host. The residual nanoparticles in macrophages or stromal cells could last for one up to several months, thus leading to cumulative toxicity.12,15 It also seems that increased hydrophobicity is favored for blood protein binding.10,11,22 According to our recent findings, when nanoparticles enter a biological milieu, their original surface will have contact with proteins and other biomolecules that form a dynamic protein corona whose composition varies over time due to continuous protein association and dissociation as well as changes in the environment.10 The composition of the protein-corona depends chiefly on particle surface chemistry (primarily hydrophobicity or charge) and compositions.10,22 Our recent research indicates that serum proteins could competitively bind on the SWCNTs hydrophobic surface. The π-π stacking interactions between SWCNT and hydrophobic residues Tyr, Phe and Trp play key roles in determining their absorption capacity on the SWCNT surface.11 The formation of the protein corona is one of the most significant alterations of ENMs’ surface chemical properties, and may, in turn, strongly influence the uptake, biotransformation and biocompatibility of these particles.10,11 For instance, we found that by pre-incubatiing CNTs with serum protein, CNTs can be individually dispersed and be taken up at higher concentrations into human mesenchymal stem cells, HeLa cells, monocytes/macrophages as well as bronchial epithelial and endothelial cells.11,12 It is worthy to note that the high dosage of intracellular SWCNTs did not cause any apparent acute cytotoxicity.23 This also implicates that looking at the chronic toxicity in vivo is of most importance. In contrast, non-coated and agglomerated CNTs were less bioavailable and did not induce pro-fibrogenic cellular responses and pulmonary fibrosis to the same extent as dispersed tubes.12
An additional effect of protein adsorption to the surface of CNTs is opsonization and the removal by phagocytic cells such as monocytes and macrophages in the liver and spleen within minutes.24 Opsonization of therapeutic nanoparticles could lead to significant removal by the cells of the reticuloendothelial (RES), leading to a decrease of circulating ENMs and reduced bioavailability at the intended delivery site.24,25 Thus, with the view to improve the bioavailability and decrease toxicity, modification of polyethylene glycol (PEG) onto the nanoparticle surface is frequently used to improve ENM dispersibility and decrease subsequent opsonization.25 If combined with polyethyleneimine (PEI) in a PEI-PEG co-polymer in mesoporous silica nanoparticles (MSNP), the particle dispersal became better by electrostatic repulsion, which reduced opsonization and increased both the circulatory time as well passive drug delivery to a tumor site.26
Impact of surface functionalization and surface charge
Use of ENMs for therapeutic or diagnostic purposes often involve functionalization of nanomaterials with specific biomolecules (e.g., peptides, ligands) or chemical groups to achieve drug, nucleic acid, or dual drug nucleic acid delivery to cells and targeted disease sites. The interaction strength between nanoparticle surface groups and membrane receptors can be controlled by the type of biomolecules/chemicals (e.g. affinity) or by changing the density of surface biomolecules/chemicals (e.g. avidity).5,8,19
The cell membrane consists of an anionic hydrophilic outer surface. In contrast to neutral or anionic nanoparticles, cationic particles attach more readily to the cell surface, from where they may also be taken up more avidly if size permits.7 Therefore, cationic surface is frequently used to promote cellular entry for drug and gene delivery applications.6,8 We showed that cellular uptake of cationic PEI-coated MSNP is considerably enhanced compared to unmodified MSNP (silanol surface) or particles coated with phosphonate or PEG groups.6 Both the rate and abundance of cellular uptake are enhanced by a positive surface charge.7 In the case of PEI, this effect is tunable by the attachment of longer length polymers that display a higher density of cationic surface groups that are asymmetrically displayed and more amenable to attach to negatively charged membrane phospholipids than shorter length polymers.6 However, this comes at the expense of increased toxicity, because high cationic density could lead to physical membrane damage that is associated with increased intracellular calcium flux and cytotoxicity.5,6 Besides the generation of surface membrane damage, cationic particles coated with unsaturated amines can also initiate intracellular damage when taken up into the lysosomal compartment. According to the proton sponge hypothesis,19 polyamine groups with high proton binding affinity could lead to buffering and exaggerated proton pump activity. This toxicity results from chloride influx to maintain charge neutrality, thereby leading to osmotic swelling and lysosomal rupture.19 For instance, we have shown that cationic polystyrene (PS) nanoparticles with amine-functionalized surfaces are associated with a high rate of macrophage cell death following lysosomal rupture, intracellular calcium flux and mitochondrial injury.5,27
In order to achieve a therapeutically beneficial cationic nanoparticle it is necessary to control cationic density. We evaluated PEI polymer sizes ranging from 0.6 to 25 kD MW to balance the efficiency of intracellular delivery and cytotoxicity.6 We demonstrated that the reduction of the polymer size was capable of scaling back the cytotoxic effect of higher MW PEI. Particles coated with PEI polymers of 10 kD or less maintained the feature of facilitated cellular uptake due to high membrane binding avidity and ability to be efficiently wrapped by the surface membrane. Additionally, MSNP particles coated with PEI polymers ≤10 kD in length can efficiently bind and deliver siRNA, with significant gene knock down and without provoking cytotoxicity.5,8 Therefore, careful selection and control of surface cationic groups can achieve the goal of constructing cationic ENMs capable of enhanced intracellular siRNA delivery with minimal or no cytotoxicity.
Most of the delivered nanoparticles may get entrapped in endomembrane compartments, such as late endosome or lysosome.28 To escape from endosomal pathways into cytoplasm, cationic groups such as reducible polyethylenimine (PEI) or cell penetrating peptides (CPPs) are frequently applied. For those out-of-endosomal target delivery, the transportation must minimally satisfy the following requirements: it must (i) avoid or escape from endosomal/lysosomal pathways, (ii) possess a organelle localization signal (sorting signals), such as nuclear localization signal (NLS) or mitochondrial leader peptides to interact with the nuclear pore complex or mitochondria;29 and if the target is in nucleus: (iii) be small enough (<30 nm) to cross the nuclear membrane.30
Impact of material and surface composition
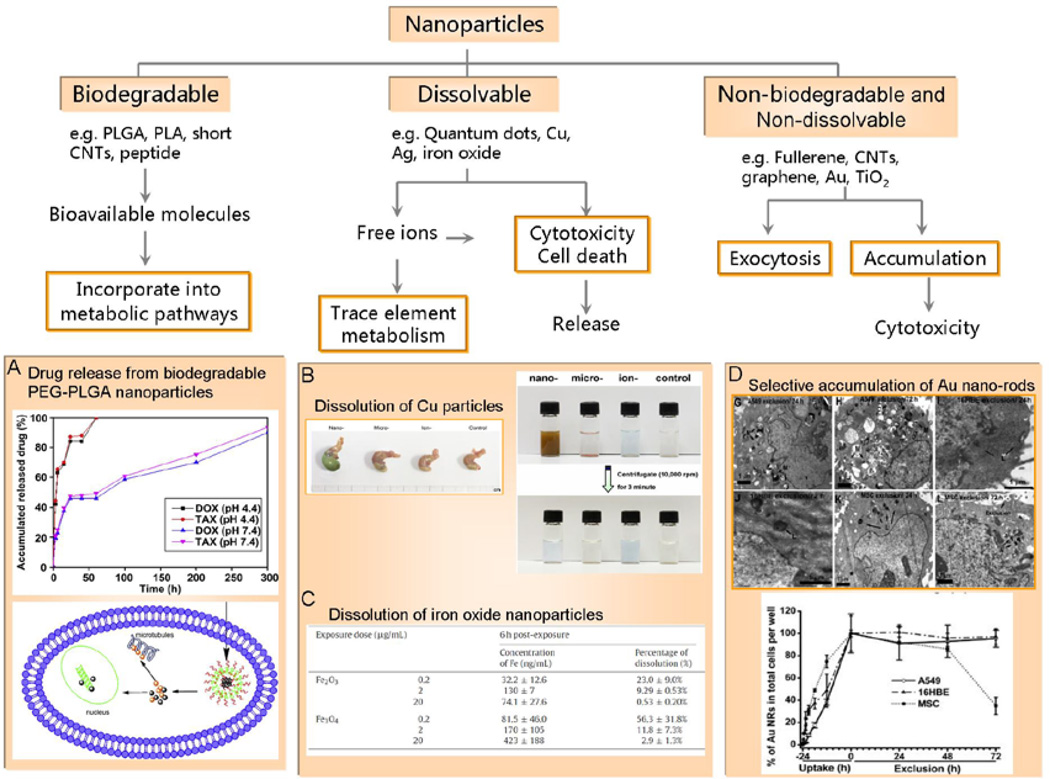
ENMs entering the cell by endocytosis are directed to a series of early and late endosomes.24 Some of these ensomes undergo acidification that could vary from a slightly acidic environment (pH 6.2–6.5) in early endosomes to more pronounced acidity (pH~ 4.5 and 5.5) in late endosomes and lysosomes. This process is also accompanied by enzyme recruitment to these compartments to digest vesicular content. In the case of nanoparticles, the material composition and surface coatings are important in determining the intracellular fate and biopersistance in this destructive environment. From the perspective of the material and surface stability, nanoparticles may be regarded as: (i) biodegradable (e.g. biodegradable polymer, peptide);31,32 (ii) dissolvable (e.g. quantum dots, zinc oxide, copper, silver, iron oxide)14–16,21,33 and (iii) non-biodegradable and non-dissolvable nanomaterials (e.g. CNTs, graphene, gold) (Figure 2). For biodegradable polymers such as poly (D,L-lactide-co-glycolide) (PLGA) and polylactide (PLA), their hydrolytic degradation is accelerated in low pH endosomal or lysosomal environments.31,32 The metabolic products that form such as lactic acid and glycolic acid, could become incorporated into biocompatible metabolic pathways.31 The biodegradation rate and release kinetics of the encapsulated guest molecules are controlled by particle size, composition and molecular weight of the shell polymer (Figure 2A).31,32
Figure 2.
Biotransformation and fate of biodegradable, dissolvable and non-dissolved and non-biodegradable nanomaterials. (A) Modulating drugs release by PLGA-nanoparticles;31 (B) Dissolution difference between small size (23.5 nm) and big size (17 micron) copper nanoparticles in stomach of murine and in artificial acidic stomach fuild;16 (C) Dissolution of iron oxide nanoparticles by human monocytes;14 (D) Selective accumulation of Au nano-rods in cancer and normal cells result in distinct cytotoxicity.18 PLGA: poly (D,L-lactide-co-glycolide); PLA: polylactide; Cu: copper; CNTs: carbon nanotubes; TiO2: titanium dioxide.
The dissolution of metallic nanoparticles, such as quantum dots, copper nanoparticles and magnetic iron oxide nanoparticles, is a dynamic process under biological conditions (Figure 2B&C).14,16,34 Material solubility depends on solvent properties (e.g. pH, ionic strength and concentration) and may therefore vary from one to another cellular compartment (e.g., early endosome, lysosome and cytosol). Dissolution of metallic nanomaterials might persist over periods of weeks to months to get rid of nanoscale materials.34 In case of exhaustion of enzymes or proton pump activity, nanomaterial overload may perturb cellular homeostasis or induce cell death, leading to the release of undigested material that could start a vicious cycle.14 Dissolution of hybrid nanomaterials with a core-shell structure may proceed layer by layer. Thus, contents shielded by the shell can be shielded from being degraded or biotransformed until at a more advanced stage of biotransformation. Cadmium (Cd)-containing quantum dots (QDs) are somewhat cytotoxic due to the presence of free Cd (QDs core degradation) or interaction of QDs with intracellular components. By manipulating the outer coating (capping material, functional groups), reduction of the interfacial exposure QDs could minimize cytotoxicity.20,21 Our recent study showed that different chirality of biomolecules (e.g., D- and L-glutathione, GSH) on the QD surface determines the ligands exchange between QD-surface group and the intrinsic homochiral glutathione. This ultimately determined the shell degradation of QDs, and their toxicity.20
Impact of Physical Properties on Nanomaterials Cellular Uptake, Transport, and Accumulation
Impact of nanoscale size
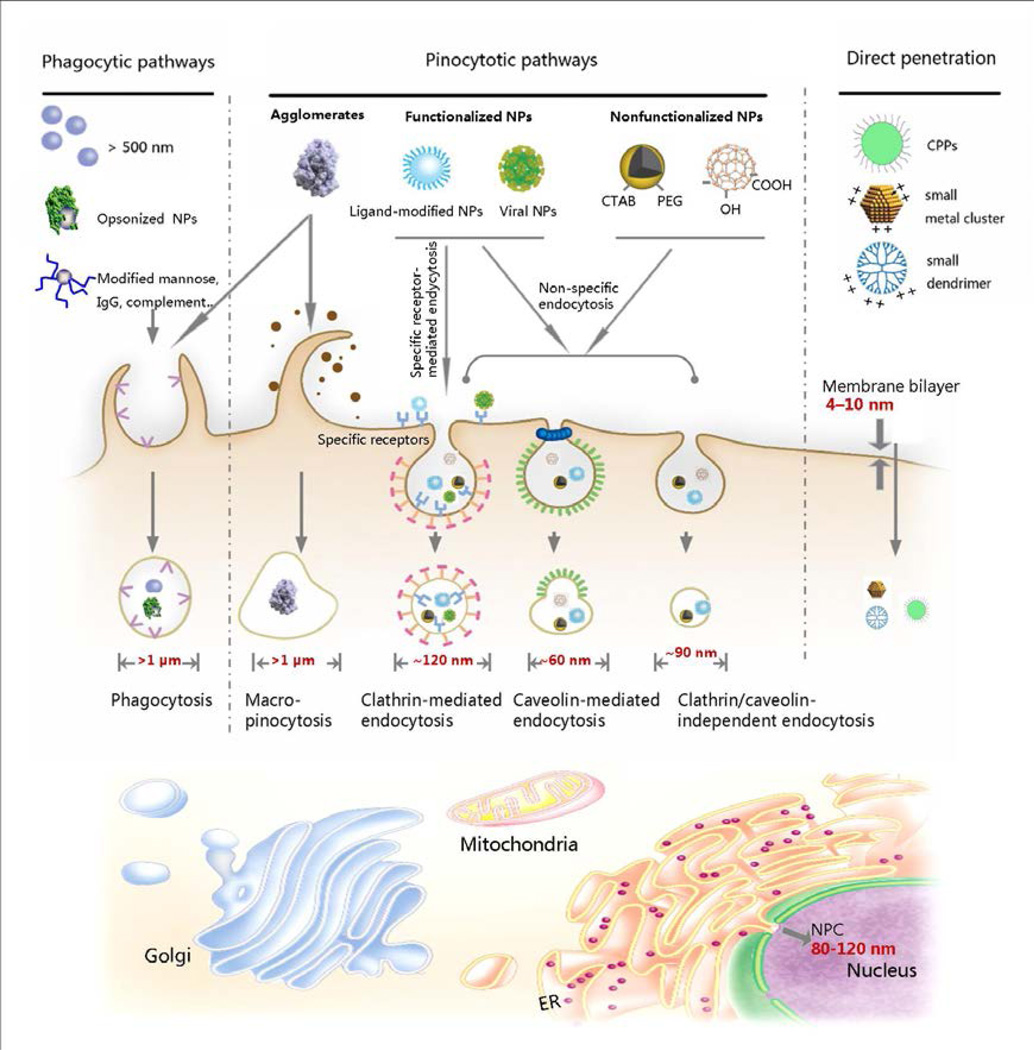
The most important physical property of a nanomaterial in determining cellular uptake, transport and accumulation is its nanoscale size. Organisms have highly tuned and precise function of regulating the uptake and transportation of nanosize biological components. There also exist some scale-rules within the cell. For example, most membrane bilayers exhibit a thickness of 4–10 nm. The vertebrate nuclear pore complex is approximately 80–120 nm in diameter.17 These natural size-restricted structures execute their barrier functions when nanoparticles enter and exit. Figure 3 illustrates the most crucial sizes involved in different ways of cellular uptake, transport, and accumulation. Therefore, the convergence of spatial sizes indicates that behaviors such as uptake, transport and ENM accumulation are restricted by the innate rules of biology that includes regulation at the nanoscale level. In the following discussions, the role of ENMs physical properties such as size (for 0-dimentional ENMs), aspect ratio (for 1-dimentional ENMs) and surface area will be discussed in terms of impact on cellular uptake, transport and bioaccumulation.
Figure 3.
Natural size-rules and gatekeepers within a mammalian cell. The thickness of membrane bilayer is typically 4–10 nm. The nuclear pore complex (NPC) is approximately 80–120 nm in diameter.17 The sizes of endocytic vesicles in both phagocytosis and pinocytosis pathways for nanoparticles internalization were also introduced.24 Phagocytes could uptake large particles (or nanoparticle aggragates), opsonized nanoparticles, or nanoparticles with certain liagnds modification via phagocytosis. Nanoparticles internalization in non-phagocytic mammalian cell is mainly through pinocytosis or direct penetration. With different surface modifications, nanoparticles may be taken up via specific (receptor-mediated) endocytosis or non-specific endocytosis. The heterogeneity of nanoparticles suface and dispersion always take multiple uptake pathways involved. These natural size-restricted structures execute their barrier functions when nanoparticle comes in and out. Therefore, the convergence of spatial sizes indicates that the behaviors (uptake, transport and accumulation) of ENMs are restricted by the innate rules of biology. MR: mannose receptor; PRRs: pattern-recognition receptors, FcγR: immunoglobulin Fcγ receptor, CR: complement receptor, CPPs: cell penetrating peptide; IgG: immunoglobulin G, ER: endoplasmic reticulum; Golgi: Golgi apparatus
To obtain direct bilayer penetration independent of endocytosis, the ENM size must be small (only a few nanometers) and its surface properties well designed to facilitate cellular entry.35,36 Larger particles or particles with high density cationic surfaces may lead to generating holes in the membrane, thereby generating cytotoxicity.35 ENMs taken up via endocytosis-mediated internalization are restricted by the size of each endocytotic portal (Figure 3). Mammalian cells exhibit five endocytic pathways for nanoparticle endocytosis: phagocytosis, macropinocytosis, clathrin-mediated, caveolin-mediated and clathrin/caveolin-independent endocytosis (Figure 3).24 Each of these portals has its own dynamics and size rules. For example, ligand-modified nanoparticles are typically taken up by clathrin-coated vesicles, which are ~120 nm in diameter (Figure 3). Ligands-modified nanoparticles larger than 120 nm are less facilely endocytosed via clathrin-mediated pathway.37
Impact of the aspect ratio
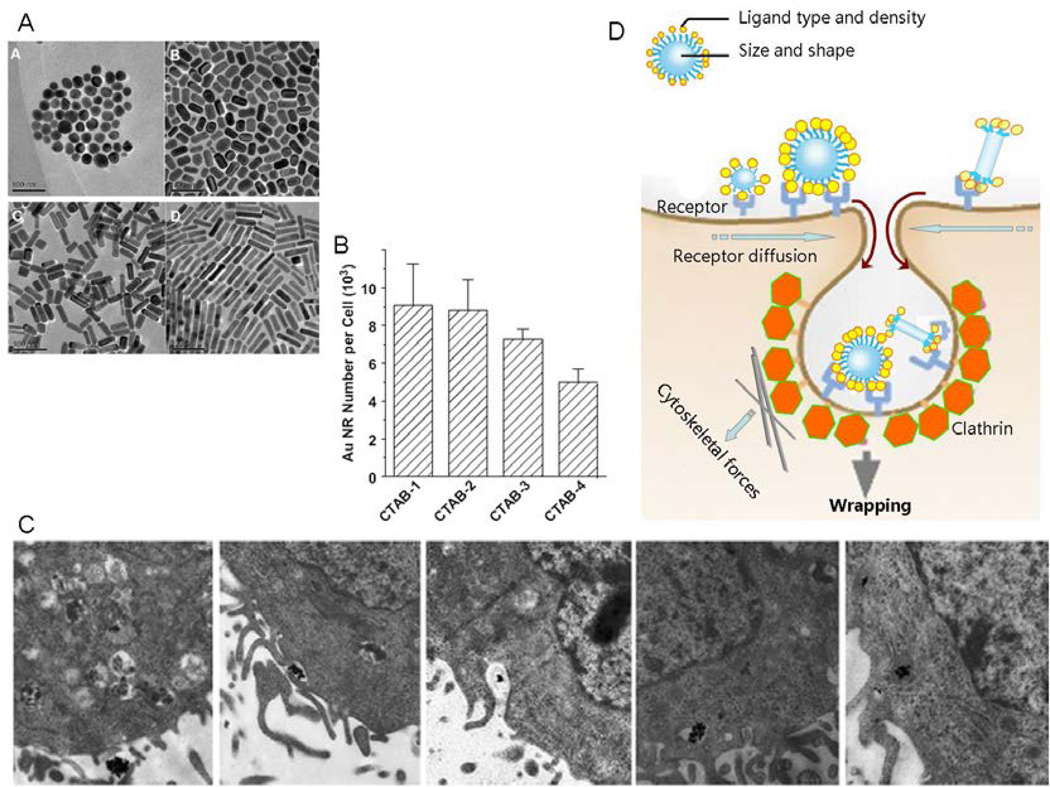
When nanoparticle size falls within the restricted size range of 120 nm, the aspect ratio of the material could make an additional independent impact on uptake and transport. ENM uptake typically proceeds through a 4-step process, namely nano/bio recognition, membrane binding, membrane wrapping, and pinching off.37 Both ENM size and aspect ratio impact membrane wrapping (Figure 4).9,38–40 To investigate how aspect ratio impacts cellular uptake, we constructed an ENM library in which a series of MSNPs with different aspect ratios were synthesized. This library included spheres and different nanorods with aspect ratios of 1 to 4.5. MSNP spheres are of 110 nm diameter. Rod-shaped MSNP cylinders are with dimensions of 110–130/60–80 nm (AR from 1.5–1.7), 160–190/60–90 nm (AR from 2.1–2.5), and 260–300/50–70 nm (AR from 4–4.5). We demonstrated that rod-shaped particles are preferentially taken up in HeLa and A549 cells. Particles exhibiting an aspect ratio of 2.1–2.5 were taken up faster and in larger quantities compared to spheres as well as shorter and longer length rods. We further showed that the intermediary length rods can be taken up via a macropinocytosis process. The rods with intermediary aspect ratio induced the maximal number of filopodia, actin polymerization, and activation of small GTP-binding proteins involved in the assembly of the actin cytoskeleton and filopodia formation.9 In another study, we demonstrated that Au nano-rods of longer aspect ratio [aspect ratio ranges from 1 to 4, with the sizes about 33 × 30, 40× 21, 50× 17, and 55 ×14, (length × diameter/nm), respectively] are internalized slower than shorter Au nano-rods. We believe that this is mainly attributed to the longer membrane wrapping time required for the longer rod-shaped particles (Figure 4).7,39 Comparing spherical nanoparticles with rod-shaped nanoparticles, the cellular uptake of spherical Au was 5–7 times faster than rod-shaped Au particles.37
Figure 4.
Impact of size and aspect ratio on ENMs cellular uptake. (A) Au nano-rods of different aspect ratio of 1.0 (CTAB-1), 2.0 (CTAB-2), 2.9 (CTAB-3) and 4.2 (CTAB-4), respectively. CTAB: cetyltrimethylammonium bromide;7 (B) Numbers of Au nano-rods within human breast adenocarcinoma (MCF-7) cells;7 (C) TEM image showing the process of cellular uptake of Au nano-rods. The Au nano-rods wrapping into vesicle and further get into lysosome;7 (D) Sketch map for how size and shape affect membrane wrapping kenetics in cell endocytosis. Changes in nanoparticle size may affect the surface ligand density, ligand conformation, surface curvature and relative orientation during nanoparticles membrane docking. Changes in nanoparticle aspect ratio may affect the position of surface ligand and wrapping time.
The physicochemical properties which regulate the exocytosis of nanoparticles are still not clear, but it appears to be largely impacted by size and aspect ratio. For instance, transferrin coated spherical-shaped Au nanoparticles (Tf-Au) are exocytosed in a linear relationship to size.39 Smaller Tf-Au appeared to exocytose at a faster rate and at a higher percentage than large Tf-Au. The fraction of spherical-shaped Tf-Au exocytosed (Fexo) could be written as: Fexo = α·N0/S, here, α: a constant that depends on the cell type and its value is determined experimentally; N0: the number of Tf-Au internalized at the beginning of the exocytosis process; S: surface area of each Tf-Au. Rod-shaped Tf-Au exocytosed was higher than spherical-shaped nanoparticles.39 However, in case of much longer and more rigid multi-walled CNTs (MWCNTs), the clearance of these high aspect ratio carbon nanomaterials proceed by an extremely slow rate in vivo.12 Inability to efficiently clear the aggregated MWCNTs that form rigid and fiber-like stacks could lead to toxicity by initiating frustrated phagocytosis.12
Impact of the surface area, dissolvability and degradability
Beyond cellular uptake, a key question becomes which ENM properties determine the materials' elimination kinetics or cellular retention, biotransformation, biodegradability, and metabolic pathways. Under certain conditions, the dissolution rate constant (k) of dissolvable ENMs depends on the surface area of particles (A) as shown: k=A×D/V×h, (D: diffusion coefficient of solute molecule, V: volume of solution, h: thickness of diffusion layer).34 Therefore, nanosized materials are often expected to dissolve more quickly and to a greater extent than large particles of the same material. The free ions released by dissolvable nanomaterials may be utilized as trace element or induce heavy metal toxicity (Figure 2). We experimentally determined that there is a major difference in the dissolution of small (23.5 nm) or big (17 micron) copper particles. The released copper ions lead to the accumulation of excessive alkaline substances in vivo and overload of heavy metal ions (copper ions) (Figure 2B).16 Our most recent research also indicates that inhaled magnetic iron oxide nanoparticles (MIONs) might be excreted from the cells in the form of breakdown products or ions via extracellular secreted membrane vesicles (named exosomes).41
It is also very important to understand the fate of ENMs that are not readily dissolved or biodegraded such as CNTs, graphene, Au, titanium dioxide nanomaterials, etc. These materials may either be cleared from or accumulate inside the cell. The limited literature on clearance of non-degradable nanomaterials suggested that it mainly occurs by exocytosis that depends on the endomembrane system.9,18,39 Furthermore, besides the physicochemical properties, the cellular trafficking and intracellular fate of nanoparticles are also cell-type and cell-phase dependent.18,39 For instance, the Au-nanoparticles show different exocytosis processes in Hela cells, SNB19, and STO cells, which could influence the cellular accumulation and clearance rates of the particles.39 Also, the Au nano-rods in cancer and normal cells show selective accumulation (Figure 2D).18 Au nano-rods within tumor cells could translocate to mitochondria, inducing decreased mitochondrial membrane potentials, increased oxidation stress and finally reduced cell viability. This is a god-given character in development of tumor cell targeted nanomedicines with low-toxicity to normal cells. Recent studies also indicated that internalization of nanoparticles by cells could be ranked according to the different phases: G2/M> S > G0/G1.42 Partitioning of nanoparticles in cell division is random and asymmetric uptake of nanoparticles by cells is also influenced by their cell cycle phase.43
Conclusion and Perspectives
Although ENMs have had numerous brilliant applications over the last decade, understanding of their bioprocesses is still on the way. Among these correlations between cellular trafficking and intracellular fate of ENMs and their physicochemical properties are the underlying fundaments. A thorough understanding of biological behavior and safety issues of ENMs needs to further know how nanoparticles interact with biological membranes, organelles, biomolecules, and what their biological consequences are,11 these generally lack of systematical investigation so far. Up to the present, experimental findings can provide us with very useful information but are still limited to help prediction of certain physicochemical properties on the cellular behavior of ENMs, especially on the processes of biotransformation and elimination of ENMs. A major challenge of identifying the causative relationships between physicochemical properties of ENMs and their toxicity responses from the viewpoint of cellular trafficking is the lack of better probing technique or methodology, in particular, the real time, in situ, rapid, and quantitative analyses methodology for characterizing cellular behavior of ENMs, which urgently needs to make a breakthrough in the future. Because of the large number of variables in nanomaterials, experimental exploration needs a long time and much costs to clarify the cellular uptake, transport and fate of each ENMs. Thus, modeling from in vitro data to in vivo metabolism using computer simulation becomes a grant challenge but is urgently needed to be developed to assist designs of biologically safer nanomaterials or nanoplatforms. The knowledge we gained from the dynamic processes of ENMs in biological systems like living cells would feed back to the rational design of safer ENMs. In general, the in vitro results at cellular level are more useful for understanding the mechanism of biokinetics (ADME) of ENMs in vivo, and for predicting the possibly potential toxic responses at a whole body level when a living body is exposed to a given ENM. For example, the results obtained in vitro can be gathered to predict in vivo ADME/Tox (absorption, distribution, metabolism, excretion and toxicity) of the ENMs through systematical information on (a) the most effective cellular uptake and bioavailability at target sites; (b) cellular metabolism and organ toxicity; (c) cellular excretion and tissue accumulation and long-term risks. All these are essential knowledge for us towards the development of a sustainable nanotechnology.
Acknowledgments
This work was supported by the National Basic Research Program of China (2011CB933403, 2012CB934000 and 2012CB932504), National Natural Science Foundation of China (31100721), National Science Foundation and the Environmental Protection Agency (Cooperative Agreement Number DBI 0830117) as well as the US Public Health Service Grants U19 ES019528 and RO1 ES016746.
Biographies
Motao Zhu is an Assistant Professor in National Center for Nanoscience and Technology of China. She obtained her Ph.D in Bioinorganic Chemistry from Institute of High Energy Physics, Chinese Academy of Sciences (CAS) in 2009. Her research interests focus on the biological trafficking of nanomaterials, nanotoxicology and drug delivery using natural membrane vesicles.
Guangjun Nie obtained his Ph.D in Biochemistry and Biophysics at Institute of Biophysics, CAS in 2002. He is currently CAS 100 Talents Scientist and Chief Scientist of National Basic Research Programs. His research interests mainly foucus on cancer biology, blood physiology, design of bio-inspired biomaterials to overcome the current barriers in tumor therapy.
Huan Meng, Researcher in the Division of NanoMedicine at UCLA, received his PhD in Bioinorganic Chemistry from Institute of High Energy Physics, CAS in 2008. His research interests include nanotoxicology and nanomedicine.
Tian Xia is an Adjunct Assistant Professor in the Division of NanoMedicine at UCLA and Project Leader of UCLA CNPT. He received his Ph.D in Biophysics from Peking University in 2001. His research interests are nanobiology, nanotoxicology, and nanomedicine.
André Nel, Professor of Medicine and Chief of the Division of NanoMedicine at UCLA, directs the UC CEIN and UCLA Center for Nanobiology and Predictive Toxicology (UCLA CNPT). He obtained his MD and Doctorate degrees in Cape Town, South Africa. His chief research interests are nano EHS, nanobiology and nanotherapeutics.
Yuliang Zhao received his Ph.D at Tokyo Metropolitan University and worked at RIKEN (Japan). He moved to Chinese Academy of Sciences and became the full professor as a Hundred Elite Professorship in 2001. His research interests include nanotoxicology, nanobioanalytical chemistry, cancer nanotechnology and radiochemistry. He has published more than 200 papers and authored/edited 10 books. The “Nanotoxicology” published in USA (2007) is the first textbook worldwide in the field. He has delivered 125 invited lectures and is serving as Associated Editor or Editorial Borad Member for 8 international SCI journals in USA and Europe.
References
- 1.Yan L, Zheng YB, Zhao F, Li S, Gao X, Xu B, Weiss PS, Zhao Y. Chemistry and physics of a single atomic layer: strategies and challenges for functionalization of graphene and graphene-based materials. Chem. Soc. Rev. 2011;41:97–114. doi: 10.1039/c1cs15193b. [DOI] [PubMed] [Google Scholar]
- 2.Gilbert N. Nanoparticle safety in doubt. Nature. 2009;460:937. doi: 10.1038/460937a. [DOI] [PubMed] [Google Scholar]
- 3.Zhao YL, Xing GM, Chai ZF. Nanotoxicology: Are carbon nanotubes safe? Nat. Nanotechnol. 2008;3:191–192. doi: 10.1038/nnano.2008.77. [DOI] [PubMed] [Google Scholar]
- 4.Zhao F, Zhao Y, Liu Y, Chang X, Chen C. Cellular uptake, intracellular trafficking, and cytotoxicity of nanomaterials. Small. 2011;7:1322–1337. doi: 10.1002/smll.201100001. [DOI] [PubMed] [Google Scholar]
- 5.Xia T, Kovochich M, Liong M, Zink JI, Nel AE. Cationic polystyrene nanosphere toxicity depends on cell-specific endocytic and mitochondrial injury pathways. ACS Nano. 2008;2:85–96. doi: 10.1021/nn700256c. [DOI] [PubMed] [Google Scholar]
- 6.Xia T, Kovochich M, Liong M, Meng H, Kabehie S, George S, Zink JI, Nel AE. Polyethyleneimine coating enhances the cellular uptake of mesoporous silica nanoparticles and allows safe delivery of siRNA and DNA constructs. ACS Nano. 2009;3:3273–3286. doi: 10.1021/nn900918w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Qiu Y, Liu Y, Wang L, Xu L, Bai R, Ji Y, Wu X, Zhao Y, Li Y, Chen C. Surface chemistry and aspect ratio mediated cellular uptake of Au nanorods. Biomaterials. 2010;31:7606–7619. doi: 10.1016/j.biomaterials.2010.06.051. [DOI] [PubMed] [Google Scholar]
- 8.Meng H, Liong M, Xia T, Li Z, Ji Z, Zink JI, Nel AE. Engineered design of mesoporous silica nanoparticles to deliver doxorubicin and P-glycoprotein siRNA to overcome drug resistance in a cancer cell line. ACS Nano. 2010;4:4539–4550. doi: 10.1021/nn100690m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meng H, Yang S, Li Z, Xia T, Chen J, Ji Z, Zhang H, Wang X, Lin S, Huang C, Zhou ZH, Zink JI, Nel AE. Aspect ratio determines the quantity of mesoporous silica nanoparticle uptake by a small GTPase-dependent macropinocytosis mechanism. ACS Nano. 2011;5:4434–4447. doi: 10.1021/nn103344k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cedervall T, Lynch I, Lindman S, Berggard T, Thulin E, Nilsson H, Dawson KA, Linse S. Understanding the nanoparticle-protein corona using methods to quantify exchange rates and affinities of proteins for nanoparticles. Proc. Natl. Acad.Sc.i U. S. A. 2007;104:2050–2055. doi: 10.1073/pnas.0608582104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ge C, Du J, Zhao L, Wang L, Liu Y, Li D, Yang Y, Zhou R, Zhao Y, Chai Z, Chen C. Binding of blood proteins to carbon nanotubes reduces cytotoxicity. Proc. Natl. Acad. Sci. U. S. A. 2011;108:16968–16973. doi: 10.1073/pnas.1105270108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang X, Xia T, Addo Ntim S, Ji Z, Lin S, Meng H, Chung CH, George S, Zhang H, Wang M, Li N, Yang Y, Castranova V, Mitra S, Bonner JC, Nel AE. The Dispersal State of Multi-walled Carbon Nanotubes Elicits Pro-Fibrogenic Cellular Responses that Correlate with Fibrogenesis Biomarkers and Fibrosis in the Murine Lung. ACS Nano. 2011;5:9772–9787. doi: 10.1021/nn2033055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang X, Xia T, Ntim SA, Ji Z, George S, Meng H, Zhang H, Castranova V, Mitra S, Nel AE. Quantitative techniques for assessing and controlling the dispersion and biological effects of multiwalled carbon nanotubes in mammalian tissue culture cells. ACS Nano. 2010;4:7241–7252. doi: 10.1021/nn102112b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhu MT, Wang B, Wang Y, Yuan L, Wang HJ, Wang M, Ouyang H, Chai ZF, Feng WY, Zhao YL. Endothelial dysfunction and inflammation induced by iron oxide nanoparticle exposure: Risk factors for early atherosclerosis. Toxicol. Lett. 2011;203:162–171. doi: 10.1016/j.toxlet.2011.03.021. [DOI] [PubMed] [Google Scholar]
- 15.Zhu MT, Feng WY, Wang Y, Wang B, Wang M, Ouyang H, Zhao YL, Chai ZF. Particokinetics and extrapulmonary translocation of intratracheally instilled ferric oxide nanoparticles in rats and the potential health risk assessment. Toxicol. Sci. 2009;107:342–351. doi: 10.1093/toxsci/kfn245. [DOI] [PubMed] [Google Scholar]
- 16.Meng H, Chen Z, Xing G, Yuan H, Chen C, Zhao F, Zhang C, Zhao Y. Ultrahigh reactivity provokes nanotoxicity: explanation of oral toxicity of nano-copper particles. Toxicol. Lett. 2007;175:102–110. doi: 10.1016/j.toxlet.2007.09.015. [DOI] [PubMed] [Google Scholar]
- 17.Alber F, Dokudovskaya S, Veenhoff LM, Zhang WH, Kipper J, Devos D, Suprapto A, Karni-Schmidt O, Williams R, Chait BT, Sali A, Rout MP. The molecular architecture of the nuclear pore complex. Nature. 2007;450:695–701. doi: 10.1038/nature06405. [DOI] [PubMed] [Google Scholar]
- 18.Wang L, Liu Y, Li W, Jiang X, Ji Y, Wu X, Xu L, Qiu Y, Zhao K, Wei T, Li Y, Zhao Y, Chen C. Selective Targeting of Gold Nanorods at the Mitochondria of Cancer Cells: Implications for Cancer Therapy. Nano Lett. 2011;11:772–780. doi: 10.1021/nl103992v. [DOI] [PubMed] [Google Scholar]
- 19.Nel AE, Madler L, Velegol D, Xia T, Hoek EM, Somasundaran P, Klaessig F, Castranova V, Thompson M. Understanding biophysicochemical interactions at the nano-bio interface. Nat. Mater. 2009;8:543–557. doi: 10.1038/nmat2442. [DOI] [PubMed] [Google Scholar]
- 20.Li Y, Zhou Y, Wang HY, Perrett S, Zhao Y, Tang Z, Nie G. Chirality of glutathione surface coating affects the cytotoxicity of quantum dots. Angew. Chem. Int. Ed. Engl. 2011;50:5860–5864. doi: 10.1002/anie.201008206. [DOI] [PubMed] [Google Scholar]
- 21.Chen Z, Chen H, Meng H, Xing G, Gao X, Sun B, Shi X, Yuan H, Zhang C, Liu R, Zhao F, Zhao Y, Fang X. Bio-distribution and metabolic paths of silica coated CdSeS quantum dots. Toxicol. Appl. Pharmacol. 2008;230:364–371. doi: 10.1016/j.taap.2008.03.022. [DOI] [PubMed] [Google Scholar]
- 22.Cedervall T, Lynch I, Foy M, Berggard T, Donnelly SC, Cagney G, Linse S, Dawson KA. Detailed identification of plasma proteins adsorbed on copolymer nanoparticles. Angew. Chem. Int. Ed. Engl. 2007;46:5754–5756. doi: 10.1002/anie.200700465. [DOI] [PubMed] [Google Scholar]
- 23.Holt BD, Dahl KN, Islam MF. Quantification of Uptake and Localization of Bovine Serum Albumin-Stabilized Single-Wall Carbon Nanotubes in Different Human Cell Types. Small. 2011;7:2348–2355. doi: 10.1002/smll.201100437. [DOI] [PubMed] [Google Scholar]
- 24.Dobrovolskaia MA, McNeil SE. Immunological properties of engineered nanomaterials. Nat. Nanotechnol. 2007;2:469–478. doi: 10.1038/nnano.2007.223. [DOI] [PubMed] [Google Scholar]
- 25.Gref R, Minamitake Y, Peracchia MT, Trubetskoy V, Torchilin V, Langer R. Biodegradable long-circulating polymeric nanospheres. Science. 1994;263:1600–1603. doi: 10.1126/science.8128245. [DOI] [PubMed] [Google Scholar]
- 26.Meng H, Xue M, Xia T, Ji Z, Tarn DY, Zink JI, Nel AE. Use of size and a copolymer design feature to improve the biodistribution and the enhanced permeability and retention effect of doxorubicin-loaded mesoporous silica nanoparticles in a murine xenograft tumor model. ACS Nano. 2011;5:4131–4144. doi: 10.1021/nn200809t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xia T, Kovochich M, Brant J, Hotze M, Sempf J, Oberley T, Sioutas C, Yeh JI, Wiesner MR, Nel AE. Comparison of the abilities of ambient and manufactured nanoparticles to induce cellular toxicity according to an oxidative stress paradigm. Nano Lett. 2006;6:1794–1807. doi: 10.1021/nl061025k. [DOI] [PubMed] [Google Scholar]
- 28.Chou LY, Ming K, Chan WC. Strategies for the intracellular delivery of nanoparticles. Chem. Soc. Rev. 2011;40:233–245. doi: 10.1039/c0cs00003e. [DOI] [PubMed] [Google Scholar]
- 29.Entelis NS, Kolesnikova OA, Martin RP, Tarassov IA. RNA delivery into mitochondria. Adv. Drug. Deliv. Rev. 2001;49:199–215. doi: 10.1016/s0169-409x(01)00135-1. [DOI] [PubMed] [Google Scholar]
- 30.Tkachenko AG, Xie H, Coleman D, Glomm W, Ryan J, Anderson MF, Franzen S, Feldheim DL. Multifunctional gold nanoparticle-peptide complexes for nuclear targeting. J. Am. Chem. Soc. 2003;125:4700–4701. doi: 10.1021/ja0296935. [DOI] [PubMed] [Google Scholar]
- 31.Wang H, Zhao Y, Wu Y, Hu YL, Nan K, Nie G, Chen H. Enhanced anti-tumor efficacy by co-delivery of doxorubicin and paclitaxel with amphiphilic methoxy PEG-PLGA copolymer nanoparticles. Biomaterials. 2011;32:8281–8290. doi: 10.1016/j.biomaterials.2011.07.032. [DOI] [PubMed] [Google Scholar]
- 32.Miao QH, Xu DX, Wang Z, Xu L, Wang TW, Wu Y, Lovejoy DB, Kalinowski DS, Richardson DR, Nie GJ, Zhao YL. Amphiphilic hyper-branched co-polymer nanoparticles for the controlled delivery of anti-tumor agents. Biomaterials. 2010;31:7364–7375. doi: 10.1016/j.biomaterials.2010.06.012. [DOI] [PubMed] [Google Scholar]
- 33.Zhu MT, Feng WY, Wang B, Wang TC, Gu YQ, Wang M, Wang Y, Ouyang H, Zhao YL, Chai ZF. Comparative study of pulmonary responses to nano- and submicron-sized ferric oxide in rats. Toxicology. 2008;247:102–111. doi: 10.1016/j.tox.2008.02.011. [DOI] [PubMed] [Google Scholar]
- 34.Borm P, Klaessig FC, Landry TD, Moudgil B, Pauluhn J, Thomas K, Trottier R, Wood S. Research strategies for safety evaluation of nanomaterials, part V: role of dissolution in biological fate and effects of nanoscale particles. Toxicol. Sci. 2006;90:23–32. doi: 10.1093/toxsci/kfj084. [DOI] [PubMed] [Google Scholar]
- 35.Verma A, Uzun O, Hu Y, Han HS, Watson N, Chen S, Irvine DJ, Stellacci F. Surface-structure-regulated cell-membrane penetration by monolayer-protected nanoparticles. Nat. Mater. 2008;7:588–595. doi: 10.1038/nmat2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Won YW, Lim KS, Kim YH. Intracellular organelle-targeted non-viral gene delivery systems. J. Control Release. 2011;152:99–109. doi: 10.1016/j.jconrel.2011.01.013. [DOI] [PubMed] [Google Scholar]
- 37.Gao H, Shi W, Freund LB. Mechanics of receptor-mediated endocytosis. Proc. Natl. Acad. Sci. U. S. A. 2005;102:9469–9474. doi: 10.1073/pnas.0503879102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jiang W, Kim BY, Rutka JT, Chan WC. Nanoparticle-mediated cellular response is size-dependent. Nat. Nanotechnol. 2008;3:145–150. doi: 10.1038/nnano.2008.30. [DOI] [PubMed] [Google Scholar]
- 39.Chithrani BD, Chan WC. Elucidating the mechanism of cellular uptake and removal of protein-coated gold nanoparticles of different sizes and shapes. Nano Lett. 2007;7:1542–1550. doi: 10.1021/nl070363y. [DOI] [PubMed] [Google Scholar]
- 40.Chithrani BD, Ghazani AA, Chan WC. Determining the size and shape dependence of gold nanoparticle uptake into mammalian cells. Nano Lett. 2006;6:662–668. doi: 10.1021/nl052396o. [DOI] [PubMed] [Google Scholar]
- 41.Zhu M, Li Y, Shi J, Feng W, Nie G, Zhao Y. Exosomes as extrapulmonary signaling conveyors for nanoparticle-induced systemic immune activation. Small. 2011;8:404–412. doi: 10.1002/smll.201101708. [DOI] [PubMed] [Google Scholar]
- 42.Kim JA, Aberg C, Salvati A, Dawson KA. Role of cell cycle on the cellular uptake and dilution of nanoparticles in a cell population. Nat. Nanotechnol. 2012;7:62–68. doi: 10.1038/nnano.2011.191. [DOI] [PubMed] [Google Scholar]
- 43.Summers HD, Rees P, Holton MD, Brown MR, Chappell SC, Smith PJ, Errington RJ. Statistical analysis of nanoparticle dosing in a dynamic cellular system. Nat. Nanotechnol. 2011;6:170–174. doi: 10.1038/nnano.2010.277. [DOI] [PubMed] [Google Scholar]