Abstract
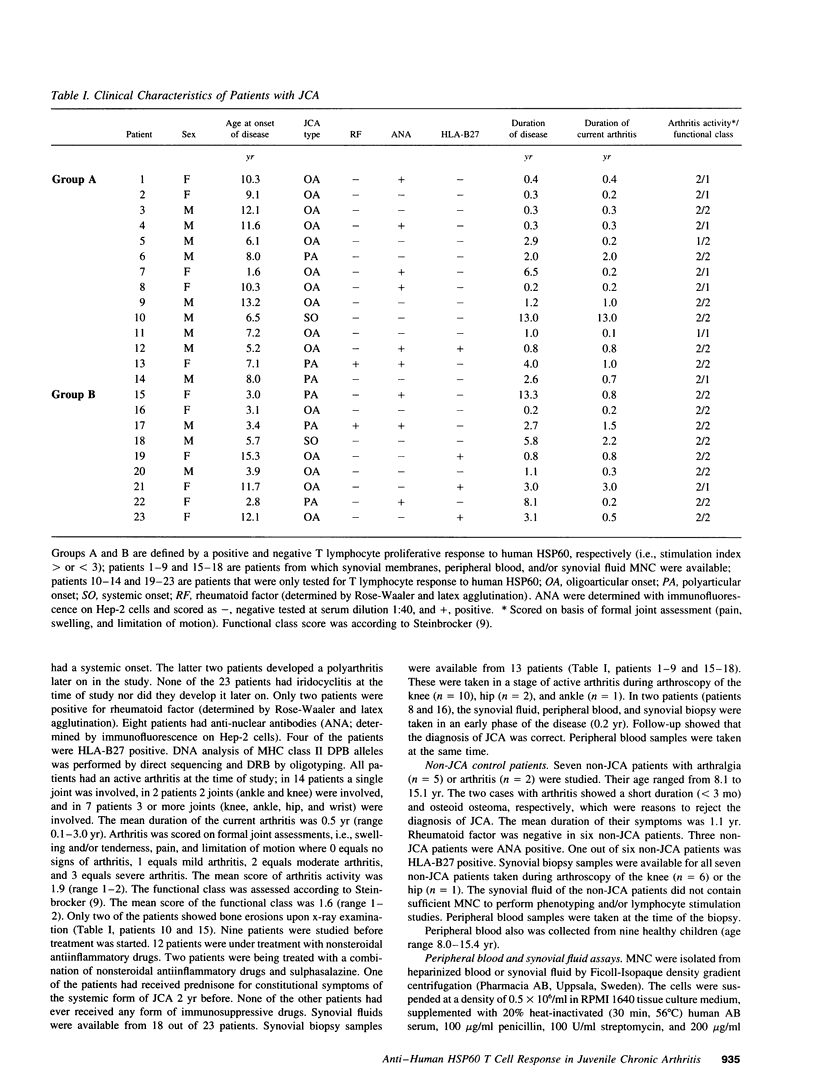
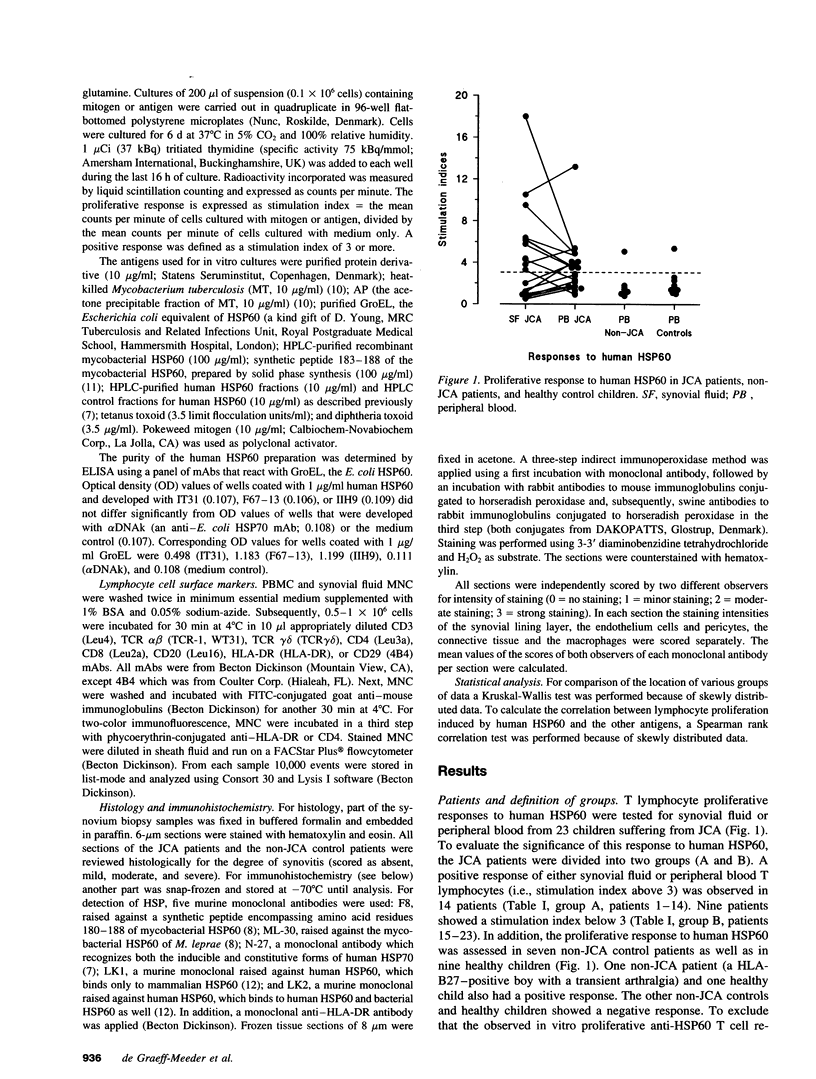
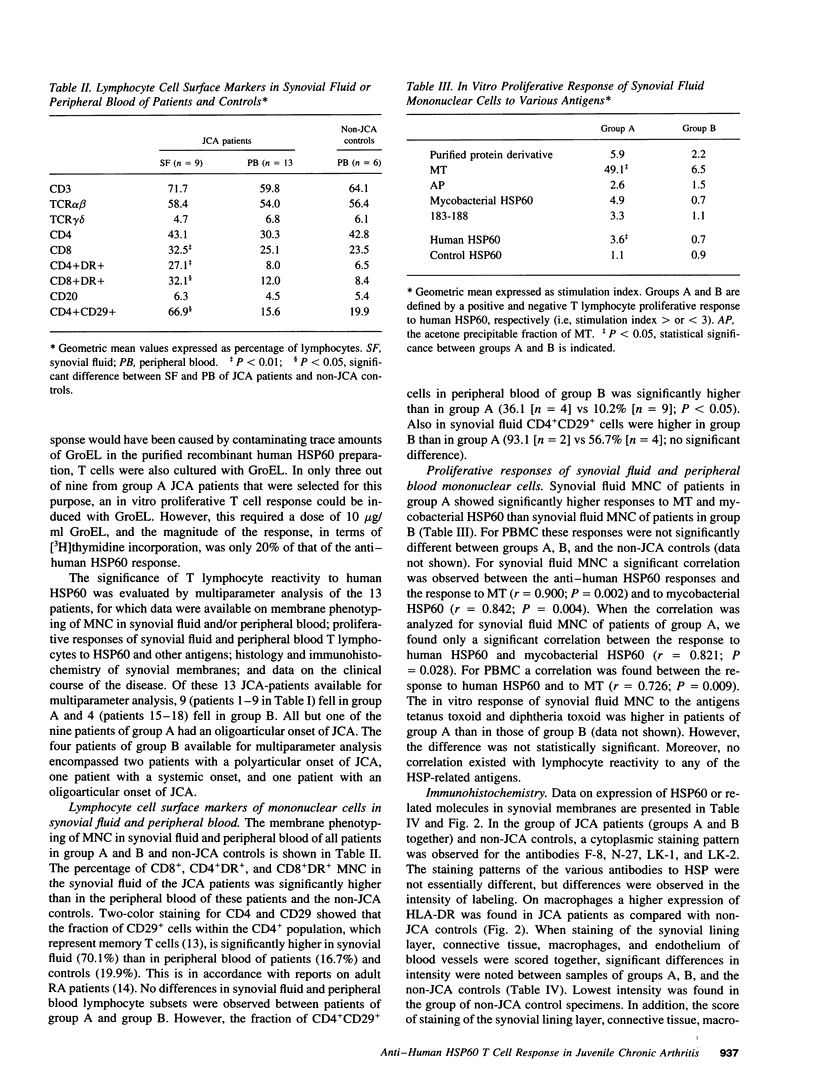
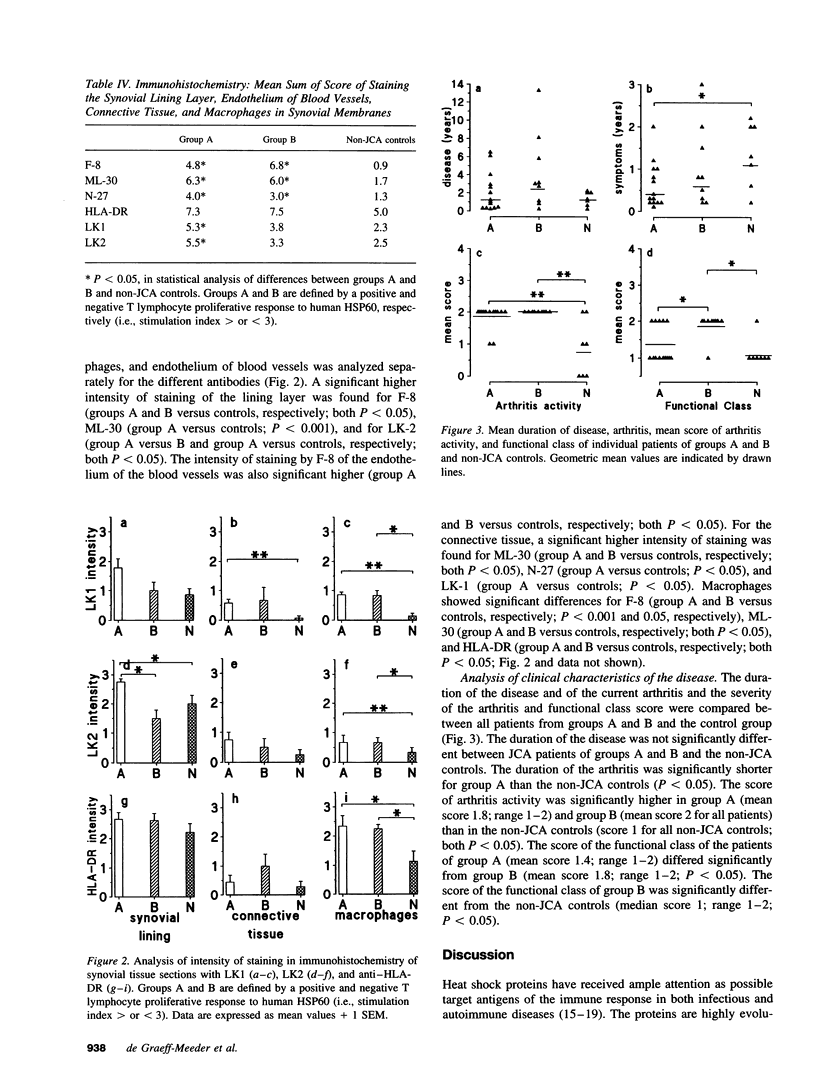
Synovial fluid and peripheral blood mononuclear cell proliferative responses to the 60-kD human heat shock protein (HSP60) were studied in 23 patients with juvenile chronic arthritis (JCA) and 7 non-JCA control patients. All patients showed active arthritis at the time of study. The patients were divided into two groups according to the presence (group A) or absence (group B) of T lymphocyte reactivity to human HSP60. We show that reactivity to human HSP60 is primarily, though not exclusively, occurring in patients with a remitting course of disease, i.e., the subgroup of HLA-B27 negative JCA patients with an oligoarticular onset. Immunohistochemical analysis of HSP expression in synovial membranes showed a significantly higher intensity of staining in JCA patients than in non-JCA controls. The results suggest that, in accordance with the earlier observation made in experimental models, T lymphocyte reactivity to human HSP60 in this subgroup of JCA patients may be part of T cell regulatory mechanisms that control the development of arthritis.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boog C. J., de Graeff-Meeder E. R., Lucassen M. A., van der Zee R., Voorhorst-Ogink M. M., van Kooten P. J., Geuze H. J., van Eden W. Two monoclonal antibodies generated against human hsp60 show reactivity with synovial membranes of patients with juvenile chronic arthritis. J Exp Med. 1992 Jun 1;175(6):1805–1810. doi: 10.1084/jem.175.6.1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cush J. J., Lipsky P. E. Cellular basis for rheumatoid inflammation. Clin Orthop Relat Res. 1991 Apr;(265):9–22. [PubMed] [Google Scholar]
- De Graeff-Meeder E. R., van der Zee R., Rijkers G. T., Schuurman H. J., Kuis W., Bijlsma J. W., Zegers B. J., van Eden W. Recognition of human 60 kD heat shock protein by mononuclear cells from patients with juvenile chronic arthritis. Lancet. 1991 Jun 8;337(8754):1368–1372. doi: 10.1016/0140-6736(91)93057-g. [DOI] [PubMed] [Google Scholar]
- Gaston J. S. Heat shock proteins and autoimmunity. Semin Immunol. 1991 Jan;3(1):35–42. [PubMed] [Google Scholar]
- Hermann E., Lohse A. W., Van der Zee R., Van Eden W., Mayet W. J., Probst P., Poralla T., Meyer zum Büschenfelde K. H., Fleischer B. Synovial fluid-derived Yersinia-reactive T cells responding to human 65-kDa heat-shock protein and heat-stressed antigen-presenting cells. Eur J Immunol. 1991 Sep;21(9):2139–2143. doi: 10.1002/eji.1830210923. [DOI] [PubMed] [Google Scholar]
- Holoshitz J., Klajman A., Drucker I., Lapidot Z., Yaretzky A., Frenkel A., van Eden W., Cohen I. R. T lymphocytes of rheumatoid arthritis patients show augmented reactivity to a fraction of mycobacteria cross-reactive with cartilage. Lancet. 1986 Aug 9;2(8502):305–309. doi: 10.1016/s0140-6736(86)90003-6. [DOI] [PubMed] [Google Scholar]
- Jindal S., Dudani A. K., Singh B., Harley C. B., Gupta R. S. Primary structure of a human mitochondrial protein homologous to the bacterial and plant chaperonins and to the 65-kilodalton mycobacterial antigen. Mol Cell Biol. 1989 May;9(5):2279–2283. doi: 10.1128/mcb.9.5.2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann S. H. Heat shock proteins and the immune response. Immunol Today. 1990 Apr;11(4):129–136. doi: 10.1016/0167-5699(90)90050-j. [DOI] [PubMed] [Google Scholar]
- Kluin-Nelemans H. C., van der Linden J. A., Gmelig Meyling F. H., Schuurman H. J. HLA-DR positive T lymphocytes in blood and synovial fluid in rheumatoid arthritis. J Rheumatol. 1984 Jun;11(3):272–276. [PubMed] [Google Scholar]
- Lamb J. R., Bal V., Mendez-Samperio P., Mehlert A., So A., Rothbard J., Jindal S., Young R. A., Young D. B. Stress proteins may provide a link between the immune response to infection and autoimmunity. Int Immunol. 1989;1(2):191–196. doi: 10.1093/intimm/1.2.191. [DOI] [PubMed] [Google Scholar]
- Lang B. A., Shore A. A review of current concepts on the pathogenesis of juvenile rheumatoid arthritis. J Rheumatol Suppl. 1990 Mar;21:1–15. [PubMed] [Google Scholar]
- Life P., Hassell A., Williams K., Young S., Bacon P., Southwood T., Gaston J. S. Responses to gram negative enteric bacterial antigens by synovial T cells from patients with juvenile chronic arthritis: recognition of heat shock protein HSP60. J Rheumatol. 1993 Aug;20(8):1388–1396. [PubMed] [Google Scholar]
- Maksymowych W. P., Glass D. N. Population genetics and molecular biology of the childhood chronic arthropathies. Baillieres Clin Rheumatol. 1988 Dec;2(3):649–671. doi: 10.1016/s0950-3579(88)80033-5. [DOI] [PubMed] [Google Scholar]
- Nepom B. S., Glass D. N. Juvenile rheumatoid arthritis and HLA: report of the Park City III workshop. J Rheumatol Suppl. 1992 Apr;33:70–74. [PubMed] [Google Scholar]
- Sanders M. E., Makgoba M. W., Shaw S. Human naive and memory T cells: reinterpretation of helper-inducer and suppressor-inducer subsets. Immunol Today. 1988 Jul-Aug;9(7-8):195–199. doi: 10.1016/0167-5699(88)91212-1. [DOI] [PubMed] [Google Scholar]
- Thompson S. J., Rook G. A., Brealey R. J., Van der Zee R., Elson C. J. Autoimmune reactions to heat-shock proteins in pristane-induced arthritis. Eur J Immunol. 1990 Nov;20(11):2479–2484. doi: 10.1002/eji.1830201118. [DOI] [PubMed] [Google Scholar]
- Van Vollenhoven R. F., Soriano A., McCarthy P. E., Schwartz R. L., Garbrecht F. C., Thorbecke G. J., Siskind G. W. The role of immunity to cartilage proteoglycan in adjuvant arthritis. Intravenous injection of bovine proteoglycan enhances adjuvant arthritis. J Immunol. 1988 Aug 15;141(4):1168–1173. [PubMed] [Google Scholar]
- Winfield J. B. Stress proteins, arthritis, and autoimmunity. Arthritis Rheum. 1989 Dec;32(12):1497–1504. doi: 10.1002/anr.1780321202. [DOI] [PubMed] [Google Scholar]
- Young R. A., Elliott T. J. Stress proteins, infection, and immune surveillance. Cell. 1989 Oct 6;59(1):5–8. doi: 10.1016/0092-8674(89)90861-1. [DOI] [PubMed] [Google Scholar]
- Young R. A. Stress proteins and immunology. Annu Rev Immunol. 1990;8:401–420. doi: 10.1146/annurev.iy.08.040190.002153. [DOI] [PubMed] [Google Scholar]
- de Graeff-Meeder E. R., Voorhorst M., van Eden W., Schuurman H. J., Huber J., Barkley D., Maini R. N., Kuis W., Rijkers G. T., Zegers B. J. Antibodies to the mycobacterial 65-kd heat-shock protein are reactive with synovial tissue of adjuvant arthritic rats and patients with rheumatoid arthritis and osteoarthritis. Am J Pathol. 1990 Nov;137(5):1013–1017. [PMC free article] [PubMed] [Google Scholar]
- van Eden W. Heat-shock proteins as immunogenic bacterial antigens with the potential to induce and regulate autoimmune arthritis. Immunol Rev. 1991 Jun;121:5–28. doi: 10.1111/j.1600-065x.1991.tb00821.x. [DOI] [PubMed] [Google Scholar]
- van Eden W., Thole J. E., van der Zee R., Noordzij A., van Embden J. D., Hensen E. J., Cohen I. R. Cloning of the mycobacterial epitope recognized by T lymphocytes in adjuvant arthritis. Nature. 1988 Jan 14;331(6152):171–173. doi: 10.1038/331171a0. [DOI] [PubMed] [Google Scholar]
- van den Broek M. F., Hogervorst E. J., Van Bruggen M. C., Van Eden W., van der Zee R., van den Berg W. B. Protection against streptococcal cell wall-induced arthritis by pretreatment with the 65-kD mycobacterial heat shock protein. J Exp Med. 1989 Aug 1;170(2):449–466. doi: 10.1084/jem.170.2.449. [DOI] [PMC free article] [PubMed] [Google Scholar]


