Abstract
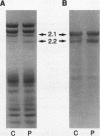
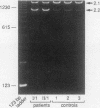
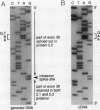
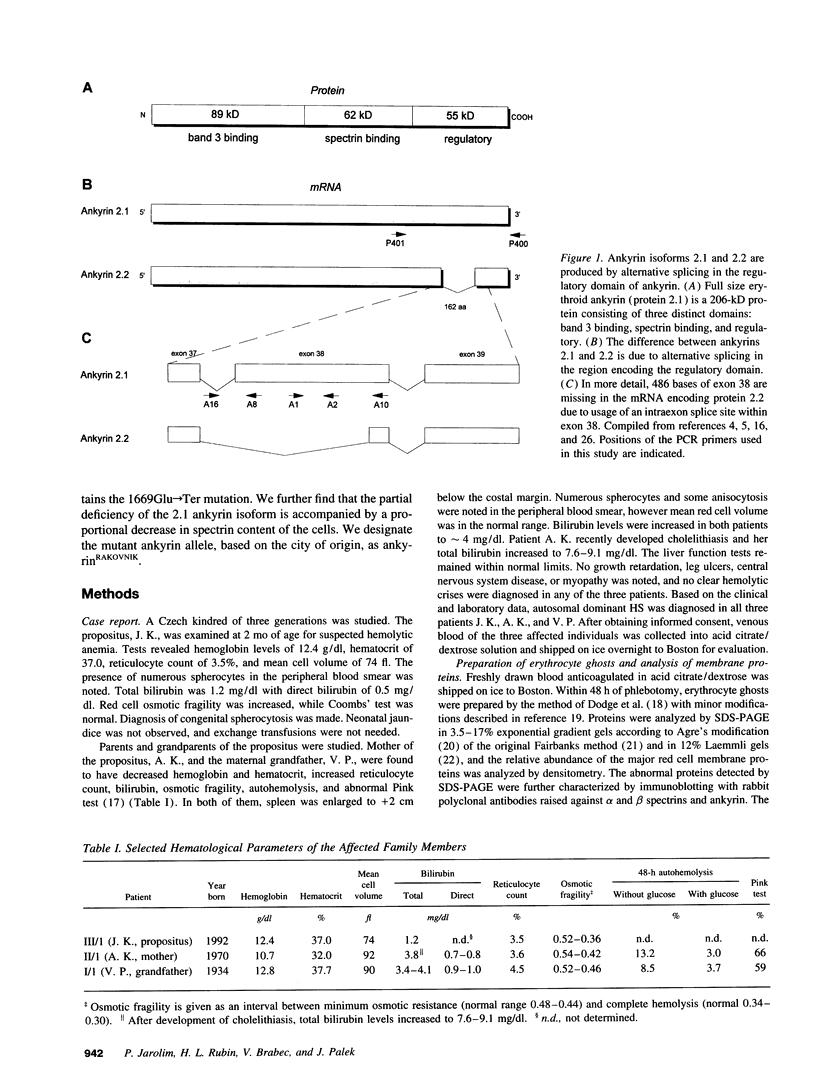
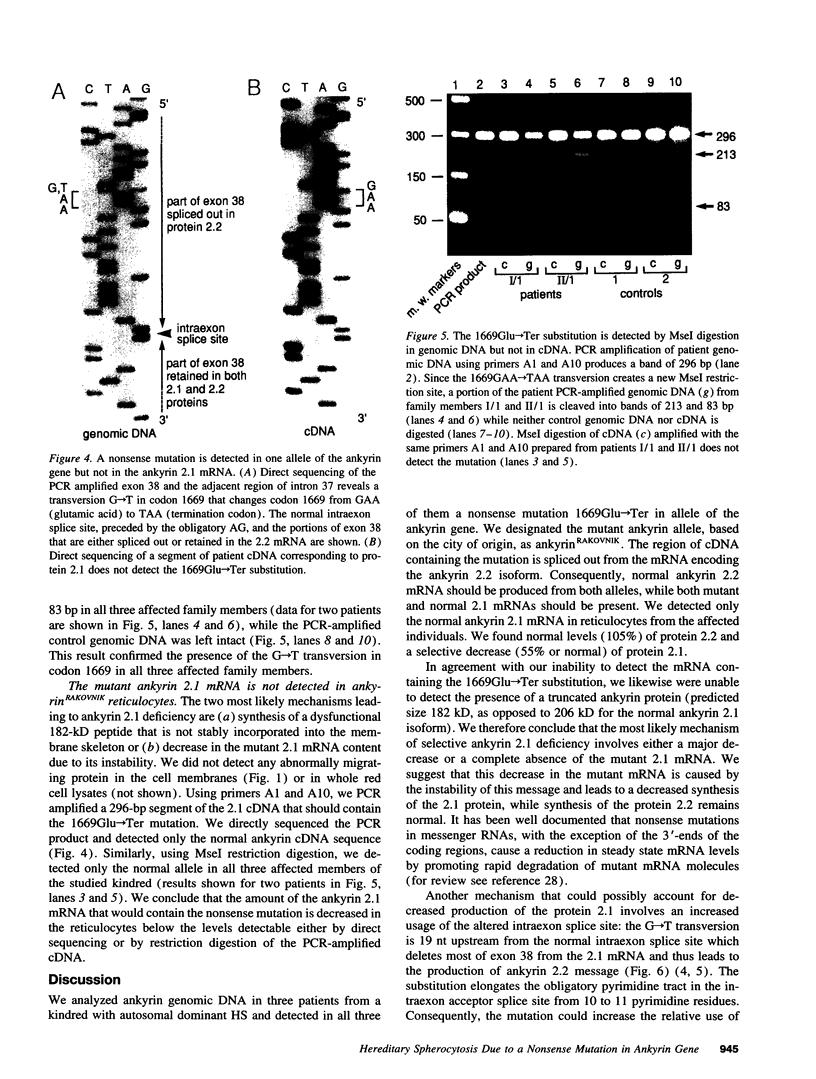
We describe a nonsense mutation in the regulatory domain of erythroid ankyrin associated with autosomal dominant hereditary spherocytosis with a selective deficiency of the ankyrin isoform 2.1 (55% of normal), a deficiency of spectrin (58% of normal) proportional to the decrease in ankyrin 2.1, and a normal content of the other main ankyrin isoform, protein 2.2. PCR amplification of cDNA encoding the regulatory domain of ankyrin revealed a marked decreased in the ratio of ankyrin 2.1 mRNA to the ankyrin 2.2 mRNA. Sequencing of ankyrin gene in the region where the 2.1 and 2.2 mRNA differ detected a nonsense mutation 1669Glu-->Ter (GAA-->TAA) in one ankyrin allele. Only normal ankyrin 2.1 mRNA was detected in the reticulocyte RNA. Since the alternative splicing within the regulatory domain of ankyrin retains codon 1669 in ankyrin 2.1 mRNA and removes it from ankyrin 2.2 mRNA, we propose that the 1669Glu-->Ter mutation decreases the stability of the abnormal ankyrin 2.1 mRNA allele leading to a decreased synthesis of ankyrin 2.1 and a secondary deficiency of spectrin.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agre P., Casella J. F., Zinkham W. H., McMillan C., Bennett V. Partial deficiency of erythrocyte spectrin in hereditary spherocytosis. 1985 Mar 28-Apr 3Nature. 314(6009):380–383. doi: 10.1038/314380a0. [DOI] [PubMed] [Google Scholar]
- Bennett V. Ankyrins. Adaptors between diverse plasma membrane proteins and the cytoplasm. J Biol Chem. 1992 May 5;267(13):8703–8706. [PubMed] [Google Scholar]
- Birkenmeier C. S., White R. A., Peters L. L., Hall E. J., Lux S. E., Barker J. E. Complex patterns of sequence variation and multiple 5' and 3' ends are found among transcripts of the erythroid ankyrin gene. J Biol Chem. 1993 May 5;268(13):9533–9540. [PubMed] [Google Scholar]
- Cobb C. E., Beth A. H. Identification of the eosinyl-5-maleimide reaction site on the human erythrocyte anion-exchange protein: overlap with the reaction sites of other chemical probes. Biochemistry. 1990 Sep 11;29(36):8283–8290. doi: 10.1021/bi00488a012. [DOI] [PubMed] [Google Scholar]
- Costa F. F., Agre P., Watkins P. C., Winkelmann J. C., Tang T. K., John K. M., Lux S. E., Forget B. G. Linkage of dominant hereditary spherocytosis to the gene for the erythrocyte membrane-skeleton protein ankyrin. N Engl J Med. 1990 Oct 11;323(15):1046–1050. doi: 10.1056/NEJM199010113231507. [DOI] [PubMed] [Google Scholar]
- DODGE J. T., MITCHELL C., HANAHAN D. J. The preparation and chemical characteristics of hemoglobin-free ghosts of human erythrocytes. Arch Biochem Biophys. 1963 Jan;100:119–130. doi: 10.1016/0003-9861(63)90042-0. [DOI] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Gallagher P. G., Tse W. T., Scarpa A. L., Lux S. E., Forget B. G. Large numbers of alternatively spliced isoforms of the regulatory region of human erythrocyte ankyrin. Trans Assoc Am Physicians. 1992;105:268–277. [PubMed] [Google Scholar]
- Goossens M., Kan Y. Y. DNA analysis in the diagnosis of hemoglobin disorders. Methods Enzymol. 1981;76:805–817. doi: 10.1016/0076-6879(81)76159-7. [DOI] [PubMed] [Google Scholar]
- Hall T. G., Bennett V. Regulatory domains of erythrocyte ankyrin. J Biol Chem. 1987 Aug 5;262(22):10537–10545. [PubMed] [Google Scholar]
- Hanspal M., Yoon S. H., Yu H., Hanspal J. S., Lambert S., Palek J., Prchal J. T. Molecular basis of spectrin and ankyrin deficiencies in severe hereditary spherocytosis: evidence implicating a primary defect of ankyrin. Blood. 1991 Jan 1;77(1):165–173. [PubMed] [Google Scholar]
- Jackson R. J. Cytoplasmic regulation of mRNA function: the importance of the 3' untranslated region. Cell. 1993 Jul 16;74(1):9–14. doi: 10.1016/0092-8674(93)90290-7. [DOI] [PubMed] [Google Scholar]
- Jarolim P., Palek J., Rubin H. L., Prchal J. T., Korsgren C., Cohen C. M. Band 3 Tuscaloosa: Pro327----Arg327 substitution in the cytoplasmic domain of erythrocyte band 3 protein associated with spherocytic hemolytic anemia and partial deficiency of protein 4.2. Blood. 1992 Jul 15;80(2):523–529. [PubMed] [Google Scholar]
- Jarolim P., Rubin H. L., Liu S. C., Cho M. R., Brabec V., Derick L. H., Yi S. J., Saad S. T., Alper S., Brugnara C. Duplication of 10 nucleotides in the erythroid band 3 (AE1) gene in a kindred with hereditary spherocytosis and band 3 protein deficiency (band 3PRAGUE). J Clin Invest. 1994 Jan;93(1):121–130. doi: 10.1172/JCI116935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings L. K., Brown L. K., Dockter M. E. Quantitation of protein 3 content of circulating erythrocytes at the single-cell level. Blood. 1985 May;65(5):1256–1262. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lambert S., Bennett V. From anemia to cerebellar dysfunction. A review of the ankyrin gene family. Eur J Biochem. 1993 Jan 15;211(1-2):1–6. doi: 10.1111/j.1432-1033.1993.tb19863.x. [DOI] [PubMed] [Google Scholar]
- Lambert S., Yu H., Prchal J. T., Lawler J., Ruff P., Speicher D., Cheung M. C., Kan Y. W., Palek J. cDNA sequence for human erythrocyte ankyrin. Proc Natl Acad Sci U S A. 1990 Mar;87(5):1730–1734. doi: 10.1073/pnas.87.5.1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lux S. E., John K. M., Bennett V. Analysis of cDNA for human erythrocyte ankyrin indicates a repeated structure with homology to tissue-differentiation and cell-cycle control proteins. Nature. 1990 Mar 1;344(6261):36–42. doi: 10.1038/344036a0. [DOI] [PubMed] [Google Scholar]
- Lux S. E., Tse W. T., Menninger J. C., John K. M., Harris P., Shalev O., Chilcote R. R., Marchesi S. L., Watkins P. C., Bennett V. Hereditary spherocytosis associated with deletion of human erythrocyte ankyrin gene on chromosome 8. Nature. 1990 Jun 21;345(6277):736–739. doi: 10.1038/345736a0. [DOI] [PubMed] [Google Scholar]
- Moon R. T., Ngai J., Wold B. J., Lazarides E. Tissue-specific expression of distinct spectrin and ankyrin transcripts in erythroid and nonerythroid cells. J Cell Biol. 1985 Jan;100(1):152–160. doi: 10.1083/jcb.100.1.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palek J., Jarolim P. Clinical expression and laboratory detection of red blood cell membrane protein mutations. Semin Hematol. 1993 Oct;30(4):249–283. [PubMed] [Google Scholar]
- Pekrun A., Eber S. W., Kuhlmey A., Schröter W. Combined ankyrin and spectrin deficiency in hereditary spherocytosis. Ann Hematol. 1993 Aug;67(2):89–93. doi: 10.1007/BF01788132. [DOI] [PubMed] [Google Scholar]
- Peters L. L., Lux S. E. Ankyrins: structure and function in normal cells and hereditary spherocytes. Semin Hematol. 1993 Apr;30(2):85–118. [PubMed] [Google Scholar]
- Peters L. L., White R. A., Birkenmeier C. S., Bloom M. L., Lux S. E., Barker J. E. Changing patterns in cytoskeletal mRNA expression and protein synthesis during murine erythropoiesis in vivo. Proc Natl Acad Sci U S A. 1992 Jul 1;89(13):5749–5753. doi: 10.1073/pnas.89.13.5749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savvides P., Shalev O., John K. M., Lux S. E. Combined spectrin and ankyrin deficiency is common in autosomal dominant hereditary spherocytosis. Blood. 1993 Nov 15;82(10):2953–2960. [PubMed] [Google Scholar]
- Vettore L., Zanella A., Molaro G. L., De Matteis M. C., Pavesi M., Mariani M. A new test for the laboratory diagnosis of spherocytosis. Acta Haematol. 1984;72(4):258–263. doi: 10.1159/000206398. [DOI] [PubMed] [Google Scholar]
- Yu J., Goodman S. R. Syndeins: the spectrin-binding protein(s) of the human erythrocyte membrane. Proc Natl Acad Sci U S A. 1979 May;76(5):2340–2344. doi: 10.1073/pnas.76.5.2340. [DOI] [PMC free article] [PubMed] [Google Scholar]