SUMMARY
Introduction
Drug hepatotoxicity is a major clinical issue. Acetaminophen (APAP) overdose is especially common. Serum biomarkers used to follow patient progress reflect either liver injury or function, but focus on biomarkers that can provide insight into the basic mechanisms of hepatotoxicity is increasing and enabling us to translate mechanisms of toxicity from animal models to humans.
Areas covered
We review recent advances in mechanistic serum biomarker research in drug hepatotoxicity. Specifically, biomarkers for reactive drug intermdiates, mitochondrial dysfunction, nuclear DNA damage, mode of cell death and inflammation are discussed, as well as microRNAs. Emphasis is placed on APAP-induced liver injury.
Expert Opinion
Several serum biomarkers of reactive drug intermediates, mitochondrial damage, nuclear DNA damage, apoptosis and necrosis, and inflammation have been described. These studies have provided evidence that mitochondrial damage is critical in APAP hepatotoxicity in humans, while apoptosis has only a minor role, and inflammation is important for recovery and regeneration after APAP overdose. Additionally, mechanistic serum biomarkers have been shown to predict outcome as well as, or better than, some clinical scores. In the future, such biomarkers will help determine the need for liver transplantation and, with improved understanding of the human pathophysiology, identify novel therapeutic targets.
Keywords: Acetaminophen, Apoptosis, Biomarkers, Hepatotoxicity, Inflammation, Mechanisms, Mitochondria, Necrosis
1. INTRODUCTION
Drug hepatotoxicity is currently the single greatest cause of acute liver failure (ALF) in the U.S. and several other Western countries [1]. Acetaminophen (APAP) is foremost among the drugs responsible. By itself, APAP overdose is the most common etiology of ALF, as well as the primary cause of ALF-related deaths [1]. Liver injury is also a major reason for post-marketing withdrawal of drugs [2]. The standard clinical assays used to assess drug hepatotoxicity reflect only liver damage or liver function. Serum aminotransferases (ALT and AST) and other enzymes indicate liver cell death, while other parameters like prothrombin time and bilirubin can show the loss and later recovery of basic liver functions. These markers, though useful for tracking the overall progression and resolution of organ damage, provide little or no insight into mechanisms of hepatotoxicity. They are also limited in prognostic utility. Since by definition they follow, rather than precede, the tissue damage, they cannot be used to predict patient outcome at early time points. ALT, in particular, has repeatedly been shown to be a poor prognostic indicator [3,4], regardless of the time of sample collection. For these reasons, recent research has focused on the development and application of circulating biomarkers capable of providing insight into the molecular mechanisms of drug-induced liver injury.
Mechanistic serum biomarkers have several advantages over current standard clinical assays. The first and most obvious is their potential for use in translational research, where they may help to confirm or reject potential therapeutic targets discovered through preclinical work. Another advantage is that mechanistic serum biomarkers are somewhat more specific. For example, only xenobiotics that cause mitochondrial damage would be expected to increase serum biomarkers of mitochondrial damage, whereas ALT could be elevated in any form of liver injury regardless of etiology. Thus, in the future it may be possible that certain causes of hepatotoxicity could be diagnosed faster or with greater confidence based on a profile or signature of serum mechanistic biomarkers. Also, because the mechanisms leading to liver injury must precede the injury itself, it is possible that the serum levels of some mechanistic biomarkers could be elevated before there are any clinical signs of hepatotoxicity. This is especially true for small compounds that do not require cell necrosis for release. Finally, one might expect that the serum levels of at least some mechanistic biomarkers would have an association with severity of clinical outcome [3,4].
Here, we review recent findings in mechanistic serum biomarker research. Compared to other drugs that can cause liver injury, overdose of APAP is both common and well-characterized. As a result, most studies of mechanistic serum biomarkers in drug hepatotoxicity have involved APAP overdose patients. For this reason, emphasis is placed on APAP hepatotoxicity. We will begin with an overview of the basic mechanisms of APAP hepatotoxicity, then move on to discussion of individual serum biomarkers.
2. MECHANISMS OF APAP HEPATOTOXICITY IN RODENT MODELS
APAP is likely the most-studied hepatotoxicant because of its reproducibility, convenience and clinical relevance. The mechanism of APAP-induced liver injury has been studied in rodents, especially mice, for more than 40 years [5]. Key to APAP-induced liver injury is the fraction of the dose metabolized by P450 enzymes, predominantly Cyp2E1 and 1A2, leading to formation of a reactive metabolite (N-acetyl-p-benzoquinone imine, NAPQI) [6, 7]. NAPQI reacts with sulfhydryl groups of cysteine in glutathione (detoxification) and in proteins [8]. These protein adducts can be measured in the liver and also in blood and are direct evidence for reactive metabolite formation [9, 10]. Importantly, adducts can be detected in plasma long before cell necrosis and the adduct levels in blood reflect the dose the animals was exposed to [10]. It is generally assumed that the overall protein binding in the cell is less important than the specific binding to mitochondrial proteins [11]. The mitochondrial adducts may be responsible for the initial mitochondrial dysfunction and oxidant stress, which activates a number of MAP kinases including ASK1, MLK3, and RIP1/RIP3 [12,13,14]. The upstream kinase signaling eventually leads to activation (phosphorylation) of c-jun-N-terminal kinase (P-JNK), which translocates to the mitochondria and enhances the oxidant stress [15,16]. The oxidant stress and peroxynitrite trigger the formation of the mitochondrial membrane permeability transition pore (MPT) resulting in the collapse of the membrane potential [17,18] and necrotic cell death [19]. The central role of the mitochondrial oxidant stress for APAP-induced liver injury is supported by the protective effect of scavenging ROS in mitochondria [20] and the aggravation of APAP-induced liver injury in animals with impaired mitochondrial antioxidant defense including SOD2 [21,22] and peroxiredoxin-6 [23]. Furthermore, removal of damaged mitochondria by autophagy also limits APAP-mediated cell death [24]. Thus, it seems that mitochondrial dysfunction is central to the mechanisms of APAP hepatotoxicity [25]. Mitochondria are thought to play a role in liver injury caused by many other drugs as well [25,26].
In addition to mitochondrial dysfunction, nuclear DNA damage and karyorrhexis are hallmarks of APAP-induced liver damage in mice [27,28]. Nuclear DNA fragmentation is not caused by direct damage by reactive oxygen species (ROS) but has to involve endonucleases because of the characteristic DNA ladder observed after APAP treatment [27,29]. Since there is no evidence for activation of pro-apoptotic caspases after APAP overdose and caspase inhibitors do not protect [19,28], it is unlikely that the caspase-activated DNase is responsible for the nuclear DNA damage. In contrast, DNA damage correlates with mitochondrial dysfunction [29] and translocation of the mitochondrial intermembrane proteins endonuclease G and apoptosis-inducing factor to the nucleus [30]. The release of these intermembrane proteins is caused initially by formation of a bax pore in the outer membrane and later by the rupture of the outer membrane due to matrix swelling after the MPT [31]. The resulting nuclear DNA fragments are detectable in the cytosol and also in plasma [32,33].
The extensive cell necrosis after APAP overdose leads to the release of damage-associated molecular patterns (DAMPs) including high mobility group box 1 (HMGB1), glutamate dehydrogenase (GDH), mtDNA, nuclear DNA fragments, argininosuccinate synthetase (ASS), micro-RNA-122 (miR-122) and many other endogenous compounds. Some of these DAMPs can stimulate toll-like receptors (TLRs) on resident macrophages to induce transcriptional activation of cytokines [34]. These pro-inflammatory mediators recruit first neutrophils [35] and later monocytes to the liver [36]. Although the impact of neutrophils and the resident and newly recruited macrophages on the injury is controversial, the preponderance of experimental evidence suggests these leukocytes are needed for the repair process but do not aggravate APAP-induced liver injury [37]. In addition to soluble mediators, it is also possible to analyze circulating leukocytes in blood. Previous studies have demonstrated that the activation of circulating neutrophils correlates with the status of neutrophils recruited into the liver [38,39].
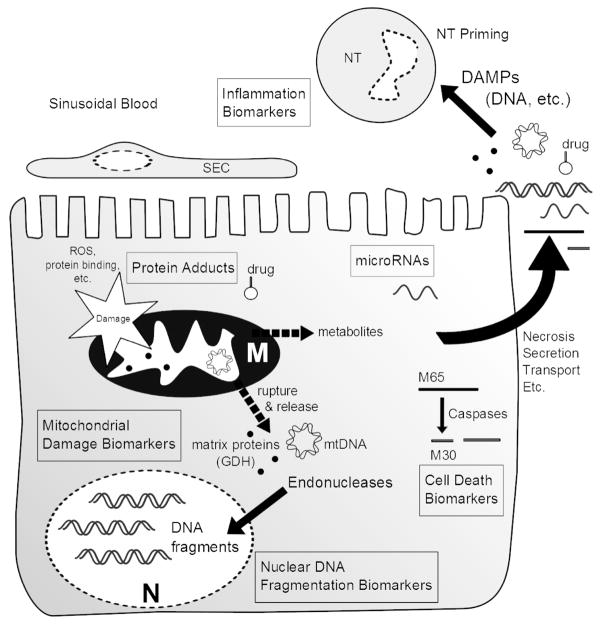
Because many aspects of the mechanisms of APAP-induced liver injury are well established, it is not surprising that much of the recent work on mechanistic serum biomarkers in drug hepatotoxicity has been done using samples from APAP overdose patients. The knowledge gained from preclinical models assists in the development of new mechanistic biomarkers and is critical for the interpretation of biomarker data in clinical samples. We can divide these serum biomarkers into several major groups: 1) markers of reactive drug intermediates, 2) markers of mitochondrial damage, 3) markers of nuclear DNA fragmentation, 4) markers of the mode of cell death, 5) inflammation markers, and 6) microRNAs with possible mechanistic significance (Figure 1). While these could also be divided into biomarkers of exposure (reactive drug intermediates and macromolecule adducts) and biomarkers of effect (mitochondrial damage, etc.), our intention is to present biomarkers of mechanisms. Also, because the serum availability of these biomarkers is of critical importance for their interpretation, data on the mechanisms of biomarker release, when available, will be discussed.
Figure 1. Mechanistic biomarkers of drug hepatotoxicity.
Mechanistic biomarkers are released into circulation and reflect events at the molecular level. Six major types of mechanistic biomarkers are indicated in boxes. Some of these biomarkers (mitochondrial and nuclear DNA and mitochondrial proteins) may also act as damage-associated molecular patterns (DAMPs). M, mitochondrion; N, nucleus; NT, neutrophil; SEC, sinusoidal endothelial cell.
3. MECHANISTIC BIOMARKERS
3.1 Serum biomarkers of reactive drug intermediates
Serum biomarkers of reactive drug intermediates are unique in that they are inherent to the drug itself. That is to say that these biomarkers are not endogenous proteins or metabolites, but are the direct result of drug metabolism intermdiates reacting with such endogenous compounds. Covalent protein and DNA adducts have been used as serum biomarkers of exposure to several toxicants. However, the mechanistic significance of this is rarely discussed. In the case of APAP, it has long been known from rodent studies that the drug is converted to an electrophile that can bind to proteins and that this is critical in the mechanism of toxicity [5,10]. Interestingly, shortly after the introduction of immunological methods to measure APAP-protein adducts [40], it was found that these adducts can be detected not only in the livers of APAP-treated mice but also in serum [41]. Importantly, it was later shown that APAP-protein adducts can also be detected in the serum of APAP overdose patients [9,42], demonstrating that protein binding occurs in humans just as it does in mice. This showed that the first step in the mechanism of APAP hepatotoxicity in rodents, covalent protein modification, could be the same in humans. These data were complementary to earlier work suggesting GSH binding and GSH depletion after exposure to APAP [43,44]. It is also worth noting that products of APAP-GSH conjugates, also the result of the reactive metabolite of APAP, can also be detected after APAP exposure [45].
More recently the potential for diagnostic use of serum APAP-protein adducts in APAP overdose patients was demonstrated [42,46]. A major advantage of measuring these adducts compared with measuring serum APAP levels is that adducts have a much longer half-life in serum [47], so they can be used to accurately diagnose APAP-induced liver injury long after APAP ingestion. With the advent of more sensitive techniques [9,48], it has become possible to measure very low concentrations of protein-derived APAP-cysteine. It is now known that APAP-protein adducts are also detectable in serum after subtoxic or even just therapeutic doses in both mice and humans [10,49]. The mechanism by which these adducts appear in circulation in the absence of cell death has not been fully elucidated, but there is evidence that it could involve secretion of proteins adducted in the liver and/or in situ adduction of proteins in the serum after diffusion of the reactive metabolite of APAP out of hepatocytes [10]. Because these adducts are detectable in serum at low doses, it is important to select an appropriate threshold for clinical diagnosis. A combination of APAP-cysteine concentration ≥ 1.1 μM and ALT > 1,000 U/L has been suggested [49]. In any case, the fact that APAP-protein adducts form at doses that do not cause liver injury suggests that protein binding, although necessary, is not sufficient for APAP hepatotoxicity.
3.2 Serum biomarkers of mitochondrial damage
Because of the central role of mitochondria in APAP-induced cell death in mouse liver, it was hypothesized that mitochondrial damage is also involved in human liver. Interestingly, it has been shown that both the mitochondrial matrix enzyme GDH (measured by kinetic assay) and mtDNA (measured by absolute quantification real-time PCR) are increased in serum from mice and humans with APAP-induced liver injury [4,33,50,51]. However, neither is significantly increased in samples from mice treated with hepatotoxic doses of furosemide, a diuretic which causes liver injury but does not seem to affect mitochondria [33,52,53]. These data suggest that GDH and mtDNA can be measured in the circulation as biomarkers of mitochondrial damage. The large sizes of these macromolecules (GDH forms a homohexameric complex with molecular weight of approximately 300 kD [54]) likely makes it difficult to release either from mitochondria without extensive damage and membrane rupture. Importantly, the serum levels of both biomarkers correlate with outome, suggesting that mitochondrial damage is central in the mechanisms of APAP hepatotoxicity in humans [4]. This conclusion is supported by the fact that mitochondrial dysfunction precedes cell death in the metabolically-competent human liver cell line HepaRG [48].
Long chain fatty acids are conjugated to carnitine to be transported into mitochondria for degradation. Mitochondrial dysfunction leads to the impairment of this transport system and consequently accumulation of acylcarnitines. Thus, long chain acylcarnitines in serum are also potential biomarkers for mitochondrial damage or dysfunction in drug hepatotoxicity. Similar to GDH and mtDNA, these acylcarnitines are increased in the circulation of mice after treatment with toxic levels of APAP, but not after furosemide [53,55]. Although an increase in these metabolites could not be detected in human plasma after APAP overdose, this may have been due to the standard-of-care treatment N-acetylcysteine (NAC) [53]. The high doses of NAC used clinically do not only supply cysteine for GSH synthesis but are also degraded and support mitochondrial energy metabolism [20]. Thus, NAC treatment may interfere with the use of acylcarnitines as mitochondrial dysfunction biomarkers. However, circulating acylcarnitines are already measured in the clinic to diagnose genetic mitochondrial fatty acid oxidation disorders, and could be very useful in future studies of liver injury in the absence of NAC. It is not yet known if admission levels of serum acylcarnitines in late-presenting APAP overdose patients who have not yet received NAC correlate with later liver injury or with outcome, and this needs to be explored. It is possible that, if acylcarnitines do strongly correlate with the development of later liver injury, they could be measured at the time of patient admission to determine the need for NAC treatment, similar to what has been proposed for APAP-protein adducts.
Various other serum biomarkers of mitochondrial damage are currently being explored. It has recently been shown that another mitochondrial enzyme, argininosuccinate synthetase (ASS), increases early in serum of mice after hepatic ischemia-reperfusion and during endotoxin-induced liver injury [56,57]. ASS is also increased in mice early after APAP treatment, even after just subtoxic doses of APAP, as well as in humans after APAP overdose [58]. However, unlike the biomarkers of mitochondrial damage described here, serum ASS also increased in mice treated with furosemide, suggesting that its appearance in circulation is not specific for mitochondrial damage [58]. Nevertheless, due to its short half-life in plasma and other interesting features, ASS may be useful as a sensitive serum biomarker of acute liver injury in general.
3.3 Serum biomarkers of nuclear DNA damage
Nuclear DNA damage in the form of fragmentation has been shown to occur in both mice and humans during APAP hepatotoxicity [27,30,33]. The most common way to measure nuclear DNA fragments in serum is with an anti-histone ELISA with an anti-DNA secondary antibody. In principle, endonucleases can only cut nuclear DNA in the accessible regions between nucleosomes. When this occurs, there are more soluble nucleosomes free to bind to the primary antibody. Because mtDNA does not have nuclear histones, this assay does not detect mtDNA. However, nuclear DNA fragmentation during APAP hepatotoxicity in mice is mediated by endonucleases that are released from damaged mitochondria [30]. Thus, nuclear DNA fragments in serum may be considered additional possible serum biomarkers of mitochondrial damage during APAP-induced liver injury [4,33].
3.4 Serum biomarkers of cell death mode
Nucleosomes were initially measured as a serum marker of programmed cell death in cancer [59]. More recently, nuclear DNA fragments in the form of nucleosomes have also been measured in serum as a means to assess mode of cell death during liver injury [60]. In apoptosis, caspase-activated endonucleases can cleave nuclear DNA [61], and secondary necrosis may release the resulting DNA fragments. Although it has become common to associate this kind of DNA damage with apoptosis, it must be stressed that nuclear DNA fragmentation is not specific for this mode of cell death as it can occur through other mechanisms. An example is nuclear translocation of mitochondrial endonucleases during APAP-induced hepatocyte necrosis [30]. Although nuclear DNA fragments can be measured in the circulation of patients with APAP-induced liver injury, there is no evidence of caspase activation in these individuals [33].
A better serum biomarker of cell death mode is keratin 18 (K18). Caulín et al. [62] showed that K18 structural filaments can be cleaved by caspases and that they form condensed punctate structures during apoptosis in epithelial cells. This cleavage occurs at an aspartate-alanine-leucine-aspartate/serine amino acid (DALD/S) sequence near the C-terminal end of the protein and exposes an epitope that can be recognized by an antibody named M30 [63]. The antibody that recognizes the full-length form of K18 is called M65. Like nucleosomes, caspase-cleaved K18 was first measured as a serum biomarker of apoptosis and chemotherapeutic drug response in cancer patients [64,65,66,67]. It has since been shown to be elevated in a variety of liver diseases, including non-alcoholic steatohepatitis [68], viral hepatitis [69] and acute liver injury due to multiple etiologies [3,59]. However, when measuring caspase-cleaved K18, it is important to express it as a ratio of total serum K18. Although the caspase-cleaved form is elevated in serum of some patients during APAP hepatotoxicity [3,50], it makes up only about 15% or less of the total K18 released, suggesting that oncotic necrosis is the major form of cell death in humans [3]. The most common way to measure K18 is with the M30 and M65 ELISAs, which are commercially available as kits. However, it can also be done with mass spectrometry [3]. Interestingly, admission serum levels of the full-length necrotic form of K18 in APAP overdose patients may predict the severity of later injury [50]. Other caspase target proteins may also be useful as biomarkers of apoptosis. For example, spectrin can be a target of both caspases and non-apoptotic proteases and it has been suggested that different spectrin breakdown products assessed by immunoblotting can be used to differentiate between apoptosis and necrosis [70]. Measurement of serum spectrin has most recently been used to study cell death in rat models of stroke [71] and in neonates undergoing cardiac surgery [72] and may hold promise for future mechanistic studies of liver injury.
While the measurement of caspase-cleaved proteins by mass spectrometry or ELISA may be more sensitive, a more direct way to assess apoptotic signaling in drug hepatotoxicity using serum is to measure caspase activation. This can be done either by immunoblotting for active truncated caspase or using a kinetic assay for activity. Consistent with the results from K18, neither the active form of caspase 3 protein nor an increase in caspase 3 activity could be detected in samples from APAP overdose patients, even though full length caspase 3 was present [33]. In contrast, both cleaved caspase 3 protein and caspase 3 activities were measurable in the circulation of apoptosis models such as galactosamine/endotoxin-treated mice [33] and rats [73].
A final option is to measure the nuclear form of high mobility group box 1 protein (HMGB1). Because of its subcellular location, it is unlikely that this could be released from cells and into serum except in the case of severe damage and cell necrosis. It has been proposed then that nuclear HMGB1 can be measured in serum as a biomarker of necrosis, and this has been applied to APAP-induced liver injury in both mice and humans [3,74]. Importantly, it was found that HMGB1 is increased in serum during APAP hepatotoxicity in humans, and that serum levels correlate with poor outcome [3]. It is important to note that there are modified forms of this protein that can often be detected in circulation, and these can provide additional information about underlying mechanisms of liver injury, as discussed in the next section.
3.5 Serum biomarkers of inflammation
Although HMGB1 was first described as a nuclear protein which controls gene expression by binding to and altering the structure of DNA, it is now clear that it has a number of other roles outside of the nucleus. The function of HMGB1 and the balance of nuclear to cytoplasmic localization are determined by posttranslational modifications, particularly acetylation [75,76,77]. When hyperacetylated, HMGB1 is packaged in secretory vesicles and can be released into the extracellular space. This is the case in some immune cells including macrophages in the liver, which, when activated, release HMGB1 as an additional inflammatory signal. Thus, hyperacetylated HMGB1 has been measured in serum as a biomarker of inflammation, specifically macrophage activation, in liver injury [3,74]. Interestingly, increased serum acetylated HMGB1 concentration was found to correlate with worse injury after APAP overdose [3]. It was also found that serum acetylated HMGB1 increases relatively late in the time course of APAP hepatotoxicity in humans [3]. This is consistent with monocyte recruitment into the liver and activation of these cells during the regenerative phase of APAP-induced liver injury [78]. Likewise, neutrophil activation increases at late time points in APAP overdose patients, after the peak of injury and during regeneration [39]. Together, these data strongly suggest that sterile inflammation plays a critical role during recovery in APAP hepatotoxicity in experimental animals and in humans [37,39,78]. A delayed increase in acetylated HMGB1 in serum has also been reported in humans with mild heparin-induced liver injury [79]. The pathophysiological consequences of the latter are not yet clear.
A more obvious way to assess inflammation is to measure the levels of circulating cytokines. A number of cytokines have been shown to be increased in serum from patients with liver injury resulting from many different etiologies. In APAP hepatotoxicity in humans, circulating interleukin 6 (IL-6), IL-8 and monocyte chemoattractant protein 1 (MCP-1) are increased [78,80]. Interestingly, these cytokines have been shown to have immunomodulatory and hepatoprotective effects in APAP toxicity, supporting the idea that inflammation is important in the recovery phase rather than the injury [78,80]. More recently, pentraxin 3, which is released by immune cells in response to certain cytokines, has also been reported to increase in APAP hepatotoxicity in humans and to correlate with both the development of hepatic encephalopathy and death [81]. Also, mitochondrial DNA and proteins and nuclear DNA fragments can act as DAMPs and activate immune cells. In addition to these soluble biomarkers of inflammation, the activation status of immune cells in the periphery appears to correlate with the status of immune cells in the liver. Importantly, activation of circulating neutrophils occurs with activation of neutrophils in the liver during injury caused by galactosamine/endotoxin and by APAP [38,39]. Thus, CD11b immunolabeling and ROS or phagocytosis priming of blood neutrophils can provide some information about inflammation occurring in the liver. Using this approach, the time course of neutrophil status relative to the peak of injury during APAP hepatotoxicity was recently found to be similar in mice and humans [39]. In contrast to neutrophils, monocytes recruited into the liver differentiate into macrophages in the area of necrosis [78]. These macrophages are primed for phagocytosis of cell debris and generation of anti-inflammatory cytokines, which promotes the resolution of the sterile inflammatory response. However, in the periphery, these anti-inflammatory cytokines may contribute to the inactivation of monocytes, which can enhance the susceptibility to sepsis [78]. This may contribute to the development of infection in ALF patients, which is not uncommon [82]. It is also important to note that systemic inflammatory response syndrome (SIRS) alone, whether or not it is caused by infection, can predict worse outcome [82,83].
3.6 Serum microRNAs
Mature microRNAs are recently-discovered short (about 20 nucleotides long) non-coding RNAs. These small nucleic acids were first recognized as important antisense regulators of cell signaling processes during Caenorhabditis elegans development [84]. It is now clear that they are produced by nearly all living things and that they have a number of important biological functions. Although it has been known for nearly a century that cell-free nucleic acids can be detected in serum [85], the first successful attempts to measure microRNAs in circulation were undertaken within the last decade [86,87,88,89]. The potential for these molecules as non-invasive serum biomarkers of disease was immediately recognized, particularly within the cancer diagnostics field [86]. Blood-borne microRNAs are usually found within extracellular vesicles, or in association with proteins. While the functions of most of these small circulating RNAs are as yet undetermined, there is accumulating evidence that they are important mediators of cell-to-cell communication [85]. Combined, their importance as regulators of gene expression and their roles in intercellular communication mean that serum microRNAs have the potential to provide new mechanistic insights into diseases. In particular, the aim of a number of studies has been to characterize and quantify circulating microRNAs in drug hepatotoxicity. Wang et al. [90] reported significant increases in plasma concentrations of miR-122 and miR-192 during APAP-induced liver injury in mice. Interestingly, these changes were observed before the development of overt injury after toxic doses of APAP, and were seen even after sub-toxic doses. These findings were later extended to humans [91]. Serum concentrations of some microRNAs have also been found to be elevated in patients with viral hepatitis [92,93], non-alcoholic fatty liver disease and steatohepatitis [93], cirrhosis caused by hepatitis C or alcohol [94] and non-acetaminophen drug-induced liver injury [95], as well as rodent models of fatty liver [96], endotoxemia [92,97], cholestasis [97] and even herbal hepatotoxicity [98]. Although there is some evidence that serum microRNA panels could be used to develop biomarker signatures that are useful for diagnosis or prognosis [99], additional research in that area is needed to fully realize the potential of microRNAs. Because it is liver-specific and it is the most abundant single microRNA in the liver, miR-122 is currently the most popular individual microRNA serum biomarker of liver injury. Interestingly, it has been shown that miR-122 has functional roles in hepatocyte differentiation [100], tumor suppression [101], viral replication [102], lipid metabolism [103] and possibly alcoholic liver disease [104]. Although the exact role of miR-122 in drug hepatotoxicity is still unclear, it is interesting that admission levels of circulating miR-122 appear to be predictive of later liver injury in APAP overdose patients [50].
4. CONCLUSION
Recent developments in the identification and characterization of mechanistic serum biomarkers for use in drug hepatotoxicity research have allowed investigators to begin translating the molecular mechanisms of drug-induced liver injury from animal models to humans. In particular, serum markers of reactive drug intermediates, mitochondrial damage, nuclear DNA damage, mode of cell death, and inflammation have already provided new insights into the mechanisms of APAP toxicity in overdose patients. As mechanistic indicators, we expect that these serum biomarkers will shed light on the pathophysiology of other drug-induced liver injuries and on other liver diseases in the near future. Moreover, although the emerging serum biomarkers discussed in this manuscript have not yet made their way into the clinic, there is evidence that some of them may be useful for the prediction of patient outcome. Unfortunately, because different outcome endpoints (e.g. liver injury, death, transplantation, King’s College criteria, etc.) are used in different studies, it is difficult at this point in time to directly compare them in terms of predictive power, sensitivity and specificity. In the case of APAP hepatotoxicity, what we can say is that early circulating levels of HMGB1, full-length K18, and miR-122 seem to be able to predict later liver injury, while HMGB1, K18, GDH, mtDNA and nuclear DNA fragment levels may be associated with death. While the variation and overlap in each marker likely somewhat limits their individual prognostic utility, a combination of several of these parameters to create a panel or signature may prove to be clinically useful. A major disadvantage of most of the serum biomarkers reviewed here, however, is their large size, which prevents release without necrosis. Others, such as microRNAs and ASS, have the advantage of increasing in serum before aminotransferases, which may facilitate earlier prediction of injury and outcome. Currently, there is a tendency to combine all acute liver injury or acute liver failure patients into a single group in studies of liver injury patients. However, because mechanisms vary with the causes of liver injury, it will be important to separate such patients into different groups based on etiology when measuring mechanistic serum biomarkers in the future. As an example, although mitochondrial damage biomarkers are elevated in APAP overdose patients and may even predict patient outcome to some degree, this may not be the case in patients with immune-mediated idiosyncratic drug toxicity. The utility of mechanistic serum biomarkers, or combinations thereof, will obviously depend upon the mechanisms of injury.
5. EXPERT OPINION ON MECHANISTIC BIOMARKERS IN DRUG HEPATOTOXICITY
The examples discussed in this manuscript demonstrate that there are a few approaches to establishing mechanistic biomarkers. For example, post-translational modifications, such as HMGB1 acetylation, caspase cleavage, or covalent modification by a reactive metabolite, can provide mechanistic information [3,33,42]. Another approach is to look for molecules that can only appear in circulation when an organelle with critical function ruptures [33], or when a signaling pathway is activated. Alternatively, one can try to measure compounds that accumulate when a critical process is disrupted [53]. In any case, the strength of mechanistic blood biomarkers is that they permit the study of injury mechanisms in intact humans over any period of time desired. This is especially important, as rodent models are often insensitive to drugs known to cause liver injury in humans, and particularly to those that cause idiosyncratic hepatotoxicity. Idiosyncratic hepatotoxicity is especially difficult to study due to its rarity. Considerable effort has been made to predict and model drug-induced liver injury in humans using laboratory animals [105,106,107,108] with only little success and even with some controversy [109]. APAP hepatotoxicity in rodents is increasingly popular as an experimental model of drug-induced liver injury because it is relatively well-characterized, clinically relevant, and convenient. Moreover, the mechanisms of APAP hepatotoxicity in mice appear to be similar in humans [33,42,48]. However, even for APAP there are important species differences. For example, rats are much less susceptible to APAP-induced liver injury than mice [110]. Thus, translational serum biomarkers will likely be very important for the study of drug-induced liver injury in humans in the future.
Besides serum biomarkers, there are a number of other approaches to translational mechanistic research, including human cell cultures and patient tissue biopsies. Unfortunately, most human hepatocyte cell lines express only very low levels of drug-metabolizing enzymes (DMEs) when compared with primary human hepatocytes or intact human liver tissue [111,112,113], making most liver cell lines a poor choice for the study of any drug toxicity that requires biotransformation. One exception is the cell line HepaRG, which expresses several major DMEs, as well as the nuclear receptors that control their expression, at levels much closer to primary hepatocytes [48,111,112,114]. Occassionally, transfected hepatoma cells overexpressing one or more DMEs are used [111]. However, transfected cell lines are very artificial, and it is difficult to assess the physiological relevance of studies that use this approach. Even in the case of HepaRG cells, it is reasonable to expect that there will still be major differences in expression or activity of proteins important in cell signaling in hepatoma cells compared to intact liver. Additionally, each individual cell line available today originated from a single donor, who may or may not reflect population averages. Overall, rather than being considered tools for translational research, cell lines are better thought of as preclinical models that can provide critical leads for future investigations. Primary human hepatocytes are a much better choice for translational in vitro studies of drug hepatotoxicity in humans [115]. Nevertheless, even this approach has limits. Although DME expression in primary human hepatocytes is generally more stable than in primary rodent hepatocytes, it is still somewhat unpredictable. Significant changes in DME levels (both increases and decreases) have been shown to occur as a function of time in culture [116,117]. Moreover, the isolation procedure, which usually involves digestion of the extracellular matrix with high concentrations of collagenase and mechanical disruption, is likely traumatic and may itself alter gene expression. Also, because fresh human hepatocytes are not conveniently available for most investigators, much primary human hepatocyte research is done with cryopreserved cells, which introduces another variable and often has a negative impact of cell viability and on attachment to culture vessels. When one does work with cultured hepatocytes, it is best to use freshly isolated human hepatocytes if possible. When done properly, cell culture experiments can provide useful leads for serum biomarker research. In vitro approaches looking for endogenous molecules released into culture medium during injury have been used to identify novel biomarkers of necrosis in cardiac myocytes [118] and to examine release of extracellular vesicles (and their RNA content) during drug toxicity in hepatocytes [119]. However, one major disadvantage with this approach is the absence of nonparenchymal cells that are normally present in the intact liver.
It is sometimes possible to obtain tissue samples from biopsies, and biopsy samples are another alternative for translational research. Biopsy samples have been used to study JNK activation in APAP hepatotoxicity in humans [120]. One advantage of these intact tissues for translational research is that they are not subjected to the trauma of cell isolation and they do not have the changes in gene expression that occur during cell culture. However, biopsy samples have several major disadvantages; not least among them being the invasive nature of the sample procurement procedure. In general, biopsies cannot be obtained serially, making it difficult or impossible to perform time course studies. Thus, a biopsy can provide only a snapshot of what is occurring. Additionally, tissue samples are often not available in some cases of drug hepatotoxicity, namely APAP overdose, because biopsy is usually unnecessary for diagnosis, and may even be contraindicated. In these instances, samples used for study are usually obtained either post-mortem or at the time of liver transplantation, which may be days after the initiation or even peak of liver injury. The relevance of information obtained regarding detailed molecular mechanisms at these late time points is questionable at best.
A weakness shared by many of the mechanistic biomarkers discussed in this manuscript is limited validation in multiple models of liver injury. This is especially true for the mitochondrial biomarkers. The status of the mitochondrial biomarkers as indicators of mitochondrial damage depends in large part upon comparisons of APAP with furosemide. Unfortunately, few hepatotoxicants are characterized nearly as well as APAP, and among those that are furosemide stands out as the only one known to cause a pattern of liver injury that is very similar to APAP without altering mitochondrial function [52]. Future studies should validate these mitochondrial biomarkers in other models, and explore the utility of these biomarkers in other diseases.
It is our opinion that mechanistic blood biomarkers are useful tools for translational research. In the last few years, there have been many new developments in mechanistic biomarker research. This is especially true in the field of drug hepatotoxicity. With increasing interest in translational research in all areas of science and medicine, it is our opinion that this field will continue to expand well into the future and that mechanistic biomarkers will become an important part of the study of human drug hepatotoxicity, as well as other human diseases.
Table 1.
Mechanistic Serum Biomarkers to Study APAP Hepatotoxicity and Other Injury
| Mechanism | Biomarker | Ref. |
|---|---|---|
| Reactive drug intermediates/binding/adducts | ||
| APAP-protein adducts | 10,42,49 | |
| APAP-GSH or products | 45 | |
| Mitochondrial damage | ||
| GDH | 4,33 | |
| mtDNA | 4,33 | |
| nDNA fragments? (APAP) | 4,33 | |
| Long-chain acylcarnitines | 63 | |
| Nuclear DNA damage | ||
| nDNA fragments | 33,59 | |
| Mode of cell death | ||
| nDNA fragments? | 59 | |
| Caspase 3 processing/activity | 33 | |
| Full length and caspase-cleaved K18 | 3 | |
| Spectrin products* | 70 | |
| Inflammation | ||
| Peripheral leukocyte count/activation | 39,78 | |
| Acetylated HMGB1 | 3 | |
| Cytokines | 78,80 | |
| DAMPs (DNA, HMGB1, etc.) | 3,33,34 | |
| Other | ||
| microRNAs | 90,91,92 |
GDH, glutamate dehydrogenase; nDNA, nuclear DNA fragments; K18, keratin 18; DAMPs, damage-associated molecular patterns.
Not yet tested in liver injury.
HIGHLIGHTS.
Mechanistic serum biomarkers provide insight into the mechanisms of drug hepatotoxicity
Currently, serum biomarkers of mitochondrial damage, DNA damage, cell death mode, and inflammation exist
Mechanistic serum biomarkers have been extensively applied to acetaminophen hepatotoxicity
Serum MicroRNAs represent an important new class of potentially mechanistic biomarkers
Mechanistic serum biomarkers may be useful in diagnosis and prognosis in drug hepatotoxicity
References
- 1.Lee WM. Etiologies of acute liver failure. Semin Liver Dis. 2008;28:142–152. doi: 10.1055/s-2008-1073114. [DOI] [PubMed] [Google Scholar]
- 2.MacDonald JS, Robertson RT. Toxicity testing in the 21st century: a view from the pharmaceutical industry. Toxicol Sci. 2009;110:40–46. doi: 10.1093/toxsci/kfp088. [DOI] [PubMed] [Google Scholar]
- 3**.Antoine DJ, Jenkins RE, Dear JW, et al. Molecular forms of HMGB1 and keratin-18 as mechanistic biomarkers for mode of cell death and prognosis during clinical acetaminophen hepatotoxicity. J Hepatol. 2012;56:1070–1079. doi: 10.1016/j.jhep.2011.12.019. First measurement of HMGB1 and keratin 18 in APAP overdose patients. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 4*.McGill MR, Lee WM, Jaeschke H. Serum biomarkers of mitochondrial damage in survivors and non-survivors of acetaminophen-induced acute liver failure: implications for the mechanism of hepatotoxicity in humans (abstract) The Toxicologist (Toxicol Sci Suppl) 2014;138:224. First demonstration that mitochondrial damage biomarkers are higher in serum of non-survivors of APAP-induced acute liver failure patients than survivors. [Google Scholar]
- 5.Mitchell JR, Jollow DJ, Potter WZ, et al. Acetaminophen-induced hepatic necrosis. I. Role of drug metabolism. J Pharmacol Exp Ther. 1973;187:185–194. [PubMed] [Google Scholar]
- 6.Nelson SD. Molecular mechanisms of the hepatotoxicity caused by acetaminophen. Semin Liver Dis. 1990;10:267–78. doi: 10.1055/s-2008-1040482. [DOI] [PubMed] [Google Scholar]
- 7.McGill MR, Jaeschke H. Metabolism and disposition of acetaminophen: recent advances in relation to hepatotoxicity and diagnosis. Pharm Res. 2013;30:2174–2187. doi: 10.1007/s11095-013-1007-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cohen SD, Khairallah EA. Selective protein arylation and acetaminophen-induced hepatotoxicity. Drug Metab Rev. 1997;29:59–77. doi: 10.3109/03602539709037573. [DOI] [PubMed] [Google Scholar]
- 9**.Muldrew KL, James LP, Coop L, et al. Determination of acetaminophen-protein adducts in mouse liver and serum and human serum after hepatotoxic doses of acetaminophen using high-performance liquid chromatography with electrochemical detection. Drug Metab Dispos. 2002;30:446–451. doi: 10.1124/dmd.30.4.446. Description of first convenient analytical method to measure APAP-protein adducts and application in APAP overdose patient serum. [DOI] [PubMed] [Google Scholar]
- 10.McGill MR, Lebosfky M, Norris HR, et al. Plasma and liver acetaminophen-protein adduct levels in mice after acetaminophen treatment: dose-response, mechanisms, and clinical implications. Toxicol Appl Pharmacol. 2013;269:240–249. doi: 10.1016/j.taap.2013.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tirmenstein MA, Nelson SD. Subcellular binding and effects on calcium homeostasis produced by acetaminophen and a nonhepatotoxic regioisomer, 3′-hydroxyacetanilide, in mouse liver. J Biol Chem. 1989;264:9814–9819. [PubMed] [Google Scholar]
- 12.Nakagawa H, Maeda S, Hikiba Y, et al. Deletion of apoptosis signal-regulating kinase 1 attenuates acetaminophen-induced liver injury by inhibiting c-Jun N-terminal kinase activation. Gastroenterology. 2008;135:1311–1321. doi: 10.1053/j.gastro.2008.07.006. [DOI] [PubMed] [Google Scholar]
- 13.Sharma M, Gadang V, Jaeschke A. Critical role for mixed-lineage kinase 3 in acetaminophen-induced hepatotoxicity. Mol Pharmacol. 2012;82:1001–1007. doi: 10.1124/mol.112.079863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ramachandran A, McGill MR, Xie Y, et al. Receptor interacting protein kinase 3 is a critical early mediator of acetaminophen-induced hepatocyte necrosis in mice. Hepatology. 2013;58:2099–2108. doi: 10.1002/hep.26547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hanawa N, Shinohara M, Saberi B, et al. Role of JNK translocation to mitochondria leading to inhibition of mitochondria bioenergetics in acetaminophen-induced liver injury. J Biol Chem. 2008;283:13565–13577. doi: 10.1074/jbc.M708916200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Saito C, Lemasters JJ, Jaeschke H. c-Jun N-terminal kinase modulates oxidant stress and peroxynitrite formation independent of inducible nitric oxide synthase in acetaminophen hepatotoxicity. Toxicol Appl Pharmacol. 2010;246:8–17. doi: 10.1016/j.taap.2010.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kon K, Kim JS, Jaeschke H, Lemasters JJ. Mitochondrial permeability transition in acetaminophen-induced necrosis and apoptosis of cultured mouse hepatocytes. Hepatology. 2004;40:1170–1179. doi: 10.1002/hep.20437. [DOI] [PubMed] [Google Scholar]
- 18.Ramachandran A, Lebofsky M, Baines CP, et al. Cyclophilin D deficiency protects against acetaminophen-induced oxidant stress and liver injury. Free Radic Res. 2011;45:156–164. doi: 10.3109/10715762.2010.520319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gujral JS, Knight TR, Farhood A, et al. Mode of cell death after acetaminophen overdose in mice: apoptosis or oncotic necrosis? Toxicol Sci. 2002;67:322–328. doi: 10.1093/toxsci/67.2.322. [DOI] [PubMed] [Google Scholar]
- 20.Saito C, Zwingmann C, Jaeschke H. Novel mechanisms of protection against acetaminophen hepatotoxicity in mice by glutathione and N-acetylcysteine. Hepatology. 2010;51:246–254. doi: 10.1002/hep.23267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ramachandran A, Lebofsky M, Weinman SA, et al. The impact of partial manganese superoxide dismutase (SOD2)-deficiency on mitochondrial oxidant stress, DNA fragmentation and liver injury during acetaminophen hepatotoxicity. Toxicol Appl Pharmacol. 2011;251:226–233. doi: 10.1016/j.taap.2011.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Agarwal R, Hennings L, Rafferty TM, et al. Acetaminophen-induced hepatotoxicity and protein nitration in neuronal nitric-oxide synthase knockout mice. J Pharmacol Exp Ther. 2012;340:134–142. doi: 10.1124/jpet.111.184192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mohar I, Stampher BD, Rademacher PM, et al. Acetaminophen-induced liver damage in mice is associated with gender-specific adduction of peroxiredoxin-6. Redox Biol. 2014;2:377–387. doi: 10.1016/j.redox.2014.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ni HM, Bockus A, Boggess N, et al. Activation of autophagy protects against acetaminophen-induced hepatotoxicity. Hepatology. 2012;55:222–232. doi: 10.1002/hep.24690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jaeschke H, McGill MR, Ramachandran A. Oxidant stress, mitochondria, and cell death mechanisms in drug-induced liver injury: lessons learned from acetaminophen hepatotoxicity. Drug Metab Rev. 2012;44:88–106. doi: 10.3109/03602532.2011.602688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pessayre D, Fromenty B, Berson A, et al. Central role of mitochondria in drug-induced liver injury. Drug Metab Rev. 2012;44:34–87. doi: 10.3109/03602532.2011.604086. [DOI] [PubMed] [Google Scholar]
- 27.Ray SD, Sorge CL, Raucy JL, et al. Early loss of large genomic DNA in vivo with accumulation of Ca2+ in the nucleus during acetaminophen-induced liver injury. Toxicol Appl Pharmacol. 1990;106:346–351. doi: 10.1016/0041-008x(90)90254-r. [DOI] [PubMed] [Google Scholar]
- 28.Lawson JA, Fisher MA, Simmons CA, et al. Inhibition of Fas receptor (CD95)-induced hepatic caspase activation and apoptosis by acetaminophen in mice. Toxicol Appl Pharmacol. 1999;156:179–186. doi: 10.1006/taap.1999.8635. [DOI] [PubMed] [Google Scholar]
- 29.Cover C, Mansouri A, Knight TR, et al. Peroxynitrite-induced mitochondrial and endonuclease-mediated nuclear DNA damage in acetaminophen hepatotoxicity. J Pharmacol Exp Ther. 2005;315:879–887. doi: 10.1124/jpet.105.088898. [DOI] [PubMed] [Google Scholar]
- 30.Bajt ML, Cover C, Lemasters JJ, Jaeschke H. Nuclear translocation of endonuclease G and apoptosis-inducing factor during acetaminophen-induced liver cell injury. Toxicol Sci. 2006;94:217–225. doi: 10.1093/toxsci/kfl077. [DOI] [PubMed] [Google Scholar]
- 31.Bajt ML, Farhood A, Lemasters JJ, Jaeschke H. Mitochondrial Bax translocation accelerates DNA fragmentation and cell necrosis in a murine model of acetaminophen hepatotoxicity. J Pharmacol Exp Ther. 2008;324:8–14. doi: 10.1124/jpet.107.129445. [DOI] [PubMed] [Google Scholar]
- 32.Jahr S, Hentze H, Englisch S, et al. DNA fragments in the blood plasma of cancer patients: quantitations and evidence for their origin from apoptotic and necrotic cells. Cancer Res. 2001;61:1659–1665. [PubMed] [Google Scholar]
- 33**.McGill MR, Sharpe MR, Williams CD, et al. The mechanism underlying acetaminophen-induced hepatotoxicity in humans and mice involves mitochondrial damage and nuclear DNA fragmentation. J Clin Invest. 2012;122:1574–1583. doi: 10.1172/JCI59755. Characterization and measurement of mitochondrial damage biomarkers in serum of APAP overdose patients. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kubes P, Mehal WZ. Sterile inflammation in the liver. Gastroenterology. 2012;143:1158–1172. doi: 10.1053/j.gastro.2012.09.008. [DOI] [PubMed] [Google Scholar]
- 35.Lawson JA, Farhood A, Hopper RD, et al. The hepatic inflammatory response after acetaminophen overdose: role of neutrophils. Toxicol Sci. 2000;54:509–516. doi: 10.1093/toxsci/54.2.509. [DOI] [PubMed] [Google Scholar]
- 36.Holt MP, Cheng L, Ju C. Identification and characterization of infiltrating macrophages in acetaminophen-induced liver injury. J Leukoc Biol. 2008;84:1410–1421. doi: 10.1189/jlb.0308173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jaeschke H, Williams CD, Ramachandran A, Bajt ML. Acetaminophen hepatotoxicity and repair: the role of sterile inflammation and innate immunity. Liver Int. 2012;32:8–20. doi: 10.1111/j.1478-3231.2011.02501.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Williams CD, Bajt ML, Farhood A, Jaeschke H. Acetaminophen-induced hepatic neutrophil accumulation and inflammatory liver injury in CD18-deficient mice. Liver Int. 2010;30:1280–1292. doi: 10.1111/j.1478-3231.2010.02284.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39*.Williams CD, Bajt ML, Sharpe MR, et al. Neutrophil activation during acetaminophen hepatotoxicity and repair in mice and humans. Toxicol Appl Pharmacol. 2014;275:122–133. doi: 10.1016/j.taap.2014.01.004. Measurement of neutrophil activation in peripheral blood during APAP hepatotoxicity in humans. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Roberts DW, Pumford NR, Potter DW, et al. A sensitive immunochemical assay for acetaminophen-protein adducts. J Pharmacol Exp Ther. 1987;241:527–533. [PubMed] [Google Scholar]
- 41.Pumford NR, Hinson JA, Potter DW, et al. Immunochemical quantitation of the 3-(cystein-S-yl)acetaminophen adducts in serum and liver proteins of acetaminophen-treated mice. J Pharmacol Exp Ther. 1989;248:190–196. [PubMed] [Google Scholar]
- 42.Davern TJ, 2nd, James LP, Hinson JA, et al. Measurement of serum acetaminophen-protein adducts in patients with acute liver failure. Gastroenterology. 2006;130:687–694. doi: 10.1053/j.gastro.2006.01.033. [DOI] [PubMed] [Google Scholar]
- 43.Davis M, Ideo G, Harrison NG, Williams R. Hepatic glutathione depletion and impaired bromosulphthalein clearance early after paracetamol overdose in man and the rat. Clin Sci Mol Med. 1975;49:495–502. doi: 10.1042/cs0490495. [DOI] [PubMed] [Google Scholar]
- 44.Lauterburg BH, Mitchell JR. Therapeutic doses of acetaminophen stimulate the turnover of cysteine and glutathione in man. J Hepatol. 1987;4:206–211. doi: 10.1016/s0168-8278(87)80081-8. [DOI] [PubMed] [Google Scholar]
- 45.Gelotte CK, Auiler JF, Lynch JM, et al. Disposition of acetaminophen at 4, 6, and 8 g/day for 3 days in healthy young adults. Clin Pharmacol Ther. 2007;81:840–848. doi: 10.1038/sj.clpt.6100121. [DOI] [PubMed] [Google Scholar]
- 46.James LP, Alonso EM, Hynan LS, et al. Detection of acetaminophen protein adducts in children with acute liver failure of indeterminate cause. Pediatrics. 2006;118:e676–e6781. doi: 10.1542/peds.2006-0069. [DOI] [PubMed] [Google Scholar]
- 47.James LP, Letzig L, Simpson PM, et al. Pharmacokinetics of acetaminophen-protein adducts in adults with acetaminophen overdose and acute liver failure. Drug Metab Dispos. 2009;37:1779–1784. doi: 10.1124/dmd.108.026195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McGill MR, Yan HM, Ramachandran A, et al. HepaRG cells: a human model to study mechanisms of acetaminophen hepatotoxicity. Hepatology. 2011;53:974–982. doi: 10.1002/hep.24132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Heard KJ, Green JL, James LP, et al. Acetaminophen-cysteine adducts during therapeutic dosing and following overdose. BMC Gastroenterol. 2011;11:20. doi: 10.1186/1471-230X-11-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Antoine DJ, Dear JW, Lewis PS, et al. Mechanistic biomarkers provide early and sensitive detection of acetaminophen-induced acute liver injury at first presentation to hospital. Hepatology. 2013;58:777–787. doi: 10.1002/hep.26294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schomaker S, Warner R, Bock J, et al. Assessment of emerging biomarkers of liver injury in human subjects. Toxicol Sci. 2013;132:276–283. doi: 10.1093/toxsci/kft009. [DOI] [PubMed] [Google Scholar]
- 52.Wong SG, Card JW, Racz WJ. The role of mitochondrial injury in bromobenzene and furosemide induced hepatotoxicity. Toxicol Lett. 2000;116:171–181. doi: 10.1016/s0378-4274(00)00218-6. [DOI] [PubMed] [Google Scholar]
- 53.McGill MR, Li F, Sharpe MR, et al. Circulating acylcarnitines as biomarkers of mitochondrial dysfunction after acetaminophen overdose in mice and humans. Arch Toxicol. 2014;88:391–401. doi: 10.1007/s00204-013-1118-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li M, Li C, Stanley CA, Smith TJ. The structure and allosteric regulation of mammalian glutamate dehydrogenase. Arch Biochem Biophys. 2012;519:69–80. doi: 10.1016/j.abb.2011.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chen C, Krausz KW, Shah YM, et al. Serum metabolomics reveals irreversible inhibition of fatty acid beta-oxidation through the suppression of PPARalpha activation as a contributing mechanism of acetaminophen-induced hepatotoxicity. Chem Res Toxicol. 2009;22:699–707. doi: 10.1021/tx800464q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Svetlov SI, Xiang Y, Oli MW, et al. Identification and preliminary validation of novel biomarkers of acute hepatic ischemia/reperfusion injury using dual-platform proteomic/degradomic approaches. Biomarkers. 2006;11:355–369. doi: 10.1080/13547500600775110. [DOI] [PubMed] [Google Scholar]
- 57.Prima V, Cao M, Svetlov SI. ASS and SULT2A1 are novel and sensitive biomarkers of acute hepatic injury – a comparative study in animal models. J Liver. 2013;2 doi: 10.4172/2167-0889.1000115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.McGill MR, Cao M, Svetlov A, et al. Argininosuccinate synthetase as a plasma biomarker of liver injury after acetaminophen overdose in rodents and humans. Biomarkers. 2014 doi: 10.3109/1354750X.2014.897757. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Holdenrieder S, Stieber P, Förg T, et al. Apoptosis in serum of patients with solid tumors. Anticancer Res. 1999;19:2721–2724. [PubMed] [Google Scholar]
- 60.Craig DG, Lee P, Pryde EA, et al. Circulating apoptotic and necrotic cell death markers in patients with acute liver injury. Liver Int. 2011;31:1127–1136. doi: 10.1111/j.1478-3231.2011.02528.x. [DOI] [PubMed] [Google Scholar]
- 61.Nagata S, Nagase H, Kawane K, et al. Degradation of chromosomal DNA during apoptosis. Cell Death Differ. 2003;10:108–116. doi: 10.1038/sj.cdd.4401161. [DOI] [PubMed] [Google Scholar]
- 62.Caulin C, Salvesen GS, Oshima RG. Caspase cleavage of keratin 18 and reorganization of intermediate filaments during epithelial cell apoptosis. J Cell Biol. 1997;138:1379–1394. doi: 10.1083/jcb.138.6.1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Leers MP, Kölgen W, Björklund V, et al. Immunocytochemical detection and mapping of a cytokeratin 18 neo-epitope exposed during early apoptosis. J Pathol. 1999;187:567–572. doi: 10.1002/(SICI)1096-9896(199904)187:5<567::AID-PATH288>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 64.Ueno T, Toi M, Bivén K, et al. Measurement of an apoptotic product in the sera of breast cancer patients. Eur J Cancer. 2003;39:769–774. doi: 10.1016/s0959-8049(02)00865-1. [DOI] [PubMed] [Google Scholar]
- 65.Olofsson MH, Ueno T, Pan Y, et al. Cytokeratin-18 is a useful serum biomarker for early determination of response of breast carcinomas to chemotherapy. Clin cancer Res. 2007;13:3198–3206. doi: 10.1158/1078-0432.CCR-07-0009. [DOI] [PubMed] [Google Scholar]
- 66.Ulukaya E, Yilmaztepe A, Akgoz S, et al. The levels of caspase-cleaved cytokeratin 18 are elevated in serum from patients with lung cancer and helpful to predict the survival. Lung Cancer. 2007;56:399–404. doi: 10.1016/j.lungcan.2007.01.015. [DOI] [PubMed] [Google Scholar]
- 67.Otzurk B, Coskun U, Sancak B, et al. Elevated serum levels of M30 and M65 in patients with locally advanced head and neck tumors. Int Immunopharmacol. 2009;9:645–648. doi: 10.1016/j.intimp.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 68.Wieckowska A, Zein NN, Yerian LM, et al. In vivo assessment of liver cell apoptosis as a novel biomarker of disease severity in nonalcoholic fatty liver disease. Hepatology. 2006;44:27–33. doi: 10.1002/hep.21223. [DOI] [PubMed] [Google Scholar]
- 69.Bantel H, Lügering A, Heidemann J, et al. Detection of apoptotic caspase activation in sera from patients with chronic HCV infection is associated with fibrotic liver injury. Hepatology. 2004;40:1078–1087. doi: 10.1002/hep.20411. [DOI] [PubMed] [Google Scholar]
- 70.Zhang Z, Larner SF, Liu MC, et al. Multiple alphaII-spectrin breakdown products distinguish calpain and caspase dominated necrotic and apoptotic cell death pathways. Apoptosis. 2009;14:1289–1298. doi: 10.1007/s10495-009-0405-z. [DOI] [PubMed] [Google Scholar]
- 71.Ren C, Zoltewicz S, Guingab-Cagmat J, et al. Different expression of ubiquitin C-terminal hydrolase-L1 and αII-spectrin in ischemic and hemorrhagic stroke: potential biomarkers in diagnosis. Brain Res. 2013;1540:84–91. doi: 10.1016/j.brainres.2013.09.051. [DOI] [PubMed] [Google Scholar]
- 72.Jain P, Spaeder MC, Donofrio MT, et al. Detection of aplhaII-spectrin breakdown products in the serum of neonates with congenital heart disease. Pediatr Crit Care Med. 2014;15:229–235. doi: 10.1097/PCC.0000000000000059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gujral JS, Farhood A, Jaeschke H. Oncotic necrosis and caspase-dependent apoptosis during galactosamine-induced liver injury in rats. Toxicol Appl Pharmacol. 2003;190:37–46. doi: 10.1016/s0041-008x(03)00154-6. [DOI] [PubMed] [Google Scholar]
- 74.Antoine DJ, Williams DP, Kipar A, et al. High-mobility group box-1 protein and keratin-18, circulating serum proteins informative of acetaminophen-induced necrosis and apoptosis in vivo. Toxicol Sci. 2009;112:521–531. doi: 10.1093/toxsci/kfp235. [DOI] [PubMed] [Google Scholar]
- 75.Bonaldi T, Talamo F, Scaffidi P, et al. Monocytic cells hyperacetylate chromatin protein HMGB1 to redirect it towards secretion. EMBO J. 2003;22:5551–5560. doi: 10.1093/emboj/cdg516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Klune JR, Dhupar R, Cardinal J, et al. HMGB1: endogenous danger signaling. 2008;14:476–484. doi: 10.2119/2008-00034.Klune. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yang H, Antoine DJ, Andersson U, Tracey KJ. The many faces of HMGB1: molecular structure-functional activity in inflammation, apoptosis, and chemotaxis. J Leukoc Biol. 2013;93:865–873. doi: 10.1189/jlb.1212662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78**.Antoniades CG, Quaglia A, Taams LS, et al. Source and characterization of hepatic macrophages in acetaminophen-induced acute liver failure in humans. Hepatology. 2012;56:735–746. doi: 10.1002/hep.25657. First characterization of hepatic macrophages in APAP overdose patients. [DOI] [PubMed] [Google Scholar]
- 79.Harrill AH, Roach J, Fier I, et al. The effects of heparins on the liver: application of mechanistic serum biomarkers in a randomized study in healthy volunteers. Clin Pharmacol Ther. 2012;92:214–220. doi: 10.1038/clpt.2012.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.James LP, Simpson PM, Farrar HC, et al. Cytokines and toxicity in acetaminophen overdose. J Clin Pharmacol. 2005;45:1165–1171. doi: 10.1177/0091270005280296. [DOI] [PubMed] [Google Scholar]
- 81.Craig DG, Lee P, Pryde EA, et al. Elevated leves of the long pentraxin 3 in paracetamol-induced human acute liver injury. Eur J Gastroenterol Hepatol. 2013;25:359–367. doi: 10.1097/MEG.0b013e32835ac77a. [DOI] [PubMed] [Google Scholar]
- 82.Leber B, Spindelboeck W, Stadlbauer V. Infectious complications of acute and chronic liver disease. Semin Respir Crit Care Med. 2012;33:80–95. doi: 10.1055/s-0032-1301737. [DOI] [PubMed] [Google Scholar]
- 83.Antoniades CG, Berry PA, Wendon JA, et al. The importance of immune dysfunction in determining outcome in acute liver failure. J Hepatol. 2008;49:845–861. doi: 10.1016/j.jhep.2008.08.009. [DOI] [PubMed] [Google Scholar]
- 84.Ambros V. The evolution of our thinking about microRNAs. Nat Med. 2008;14:1036–1040. doi: 10.1038/nm1008-1036. [DOI] [PubMed] [Google Scholar]
- 85.Kosaka N, Yoshioka Y, Hagiwara K, et al. Trash or treasure: extracellular microRNAs and cell-to-cell communication. Front Genet. 2013;4:173. doi: 10.3389/fgene.2013.00173. eCollection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mitchell PS, Parkin RK, Kroh EM, et al. Circulating microRNAs as stable blood-based markers for cancer detection. Proc Natl Acad Sci US A. 2008;105:10513–10518. doi: 10.1073/pnas.0804549105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hunter MP, Ismail N, Zhang X, et al. Detection of microRNA expression in human peripheral blood microvesicles. PLoS One. 2008;3:e3694. doi: 10.1371/journal.pone.0003694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chim SS, Shing TK, Hung EC, et al. Detection and characterization of placental microRNAs in maternal plasma. Clin Chem. 2008;54:482–490. doi: 10.1373/clinchem.2007.097972. [DOI] [PubMed] [Google Scholar]
- 89.Gilad S, Meiri E, Yogev Y, et al. Serum microRNAs are promising novel biomarkers. PLoS One. 2008;3:e3148. doi: 10.1371/journal.pone.0003148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90*.Wang K, Zhang S, Marzolf B, et al. Circulating microRNAs, potential biomarkers for drug-induced liver injury. Proc Natl Acad Sci US A. 2009;106:4402–4407. doi: 10.1073/pnas.0813371106. First measurement of serum microRNAs in APAP hepatotoxicity in rodents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91**.Starkey Lewis PJ, Dear J, Platt V, et al. Circulating microRNAs as potential markers of human drug-induced liver injury. Hepatology. 2011;54:1767–1776. doi: 10.1002/hep.24538. First measurement of serum microRNAs in APAP hepatotoxicity in humans. [DOI] [PubMed] [Google Scholar]
- 92.Zhang Y, Jia Y, Zheng R, et al. Plasma microRNA-122 as a biomarker for viral-, alcohol-, and chemical-related hepatic diseases. Clin Chem. 2010;56:1830–1838. doi: 10.1373/clinchem.2010.147850. [DOI] [PubMed] [Google Scholar]
- 93.Cermelli S, Ruggieri A, Marrero JA, et al. Circulating microRNAs in patients with chronic hepatitis C and non-alcoholic fatty liver disease. PLoS One. 2011;6:e23937. doi: 10.1371/journal.pone.0023937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Roderburg C, Mollnow T, Bongaerts B, et al. Micro-RNA profiling in human serum reveals compartment-specific roles of miR-571 and miR-652 in liver cirrhosis. PLoS One. 2012;7:e32999. doi: 10.1371/journal.pone.0032999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Thulin P, Nordahl G, Gry M, et al. Keratin-18 and microRNA-122 complement alanine aminotransferase as novel safety biomarkers for drug-induced liver injury in two human cohorts. Liver Int. 2013 doi: 10.1111/liv.12322. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 96.Clarke JD, Sharapova T, Lake AD, et al. Circulating microRNA 122 in the methionine- and choline-deficient mouse model of non-alcoholic steatohepatitis. J Appl Toxicol. 2013 doi: 10.1002/jat.2960. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Woolbright BL, Antoine DJ, Jenkins RE, et al. Plasma biomarkers of liver injury and inflammation demonstrate a lack of apoptosis during obstructive cholestasis in mice. Toxicol Appl Pharmacol. 2013;273:524–531. doi: 10.1016/j.taap.2013.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Su YW, Chen X, Jiang ZZ, et al. A panel of serum microRNAs as specific biomarkers for diagnosis of compound- and herb-induced liver injury in rats. PLoS One. 2012;7:e37395. doi: 10.1371/journal.pone.0037395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ward J, Bala S, Petrasek J, Szabo G. Plasma microRNA profiles distinguish lethal injury in acetaminophen toxicity: a research study. World J Gastroenterol. 2012;18:2798–2804. doi: 10.3748/wjg.v18.i22.2798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Laudadio I, Manfroid I, Achouri Y, et al. A feedback loop between the liver-enriched transcription factor network and miR-122 controls hepatocyte differentiation. Gastroenterology. 2012;142:119–129. doi: 10.1053/j.gastro.2011.09.001. [DOI] [PubMed] [Google Scholar]
- 101.Nakao K, Miyaaki H, Ichikawa T. Antitumor function of microRNA-122 against hepatocellular carcinoma. J Gastroenterol. 2014 doi: 10.1007/s00535-014-0932-4. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 102.Gottwein E. Roles of microRNAs in the life cycles of mammalian viruses. Curr Top Microbiol Immunol. 2013;371:201–227. doi: 10.1007/978-3-642-37765-5_8. [DOI] [PubMed] [Google Scholar]
- 103.Fernández-Hernando C, Ramírez CM, Goedeke L, Suárez Y. MicroRNAs in metabolic disease. Arterioscler Thromb Vasc Biol. 2013;33:178–185. doi: 10.1161/ATVBAHA.112.300144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.McDaniel K, Herrera L, Zhou T, et al. The functional role of microRNAs in alcoholic liver injury. J Cell Mol Biol. 2014;18:197–207. doi: 10.1111/jcmm.12223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ong MM, Latchoumycandane C, Boelsterli UA. Troglitazone-induced hepatic necrosis in an animal model of silent genetic mitochondrial abnormalities. Toxicol Sci. 2007;97:205–213. doi: 10.1093/toxsci/kfl180. [DOI] [PubMed] [Google Scholar]
- 106.Harrill AH, Ross PK, Gatti DM, et al. Population-based discovery of toxicogenomics biomarkers for hepatotoxicity using a laboratory rodent strain diversity panel. Toxicol Sci. 2009;110:235–243. doi: 10.1093/toxsci/kfp096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ng W, Lobach AR, Zhu X, et al. Animal models of idiosyncratic drug reactions. Adv Pharmacol. 2012;63:81–135. doi: 10.1016/B978-0-12-398339-8.00003-3. [DOI] [PubMed] [Google Scholar]
- 108.Metushi IG, Uetrecht J. Isoniazid-induced liver injury and immune response in mice. J Immunotoxicol. 2013 doi: 10.3109/1547691X.2013.860644. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 109.Fujimoto K, Kumagai K, Ito K, et al. Sensitivity of liver injury in heterozygous Sod2 knockout mice treated with troglitazone or acetaminophen. Toxicol Pathol. 2009;37:193–200. doi: 10.1177/0192623308329282. [DOI] [PubMed] [Google Scholar]
- 110.McGill MR, Williams CD, Xie Y, et al. Acetaminophen-induced liver injury in rats and mice: comparison of protein adducts, mitochondrial dysfunction, and oxidative stress in the mechanism of toxicity. Toxicol Appl Pharmacol. 2012;264:387–394. doi: 10.1016/j.taap.2012.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Donato MT, Lahoz A, Castell JV, Gόmez-Lechόn MJ. Cell lines: a tool for in vitro drug metabolism studies. Curr Drug Metab. 2008;9:1–11. doi: 10.2174/138920008783331086. [DOI] [PubMed] [Google Scholar]
- 112.Hart SN, Li Y, Nakamoto K, et al. A comparison of whole genome gene expression profiles of HepaRG cells and HepG2 cells to primary human hepatocytes and human liver tissues. Drug Metab Dispos. 2010;38:988–994. doi: 10.1124/dmd.109.031831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lin J, Schyschka L, Mühl-Benninghaus R, et al. Comparative analysis of phase I and II enzyme activities in 5 hepatic cell lines identifies Huh-7 and HCC-T cells with the highest potential to study drug metabolism. Arch Toxicol. 2012;86:87–95. doi: 10.1007/s00204-011-0733-y. [DOI] [PubMed] [Google Scholar]
- 114.Guillouzo A, Corlu A, Aninat C, et al. The human hepatoma HepaRG cells: a highly differentiated model for studies of liver metabolism and toxicity of xenobiotics. Chem Biol Interact. 2007;168:66–73. doi: 10.1016/j.cbi.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 115.Godoy P, Hewitt NJ, Albrecht U, et al. Recent advances in 2D and 3D in vitro systems using primary hepatocytes, alternative hepatocyte sources and non-parenchymal liver cells and their use in investigating mechanisms of hepatotoxicity, cell signaling and ADME. Arch Toxicol. 2013;87:1315–1530. doi: 10.1007/s00204-013-1078-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Morel F, Beaune PH, Ratanasavanh D, et al. Expression of cytochrome P-450 enzymes in cultured human hepatocytes. Eur J Biochem. 1990;191:437–444. doi: 10.1111/j.1432-1033.1990.tb19140.x. [DOI] [PubMed] [Google Scholar]
- 117.Anthérieu S, Chesné C, Li R, et al. Stable expression, activity, and inducibility of cytochromes P450 in differentiated HepaRG cells. Drug Metab Dispos. 2010;38:516–525. doi: 10.1124/dmd.109.030197. [DOI] [PubMed] [Google Scholar]
- 118.Marshall KD, Edwards MA, Krenz M, et al. Proteomic mapping of proteins released during necrosis and apoptosis from cultured neonatal cardiac myocytes. Am J Physiol Cell Physiol. 2014;306:C639–C547. doi: 10.1152/ajpcell.00167.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Royo F, Schlangen K, Palomo L, et al. Transcriptome of extracellular vesicles released by hepatocytes. PLoS One. 2013;8:e68693. doi: 10.1371/journal.pone.0068693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Henderson NC, Pollock KJ, Frew J, et al. Critical role of c-Jun (NH2) terminal kinase in paracetamol-induced acute liver failure. Gut. 2007;56:982–990. doi: 10.1136/gut.2006.104372. [DOI] [PMC free article] [PubMed] [Google Scholar]