Abstract
Colorectal cancer is the third major cause of cancer-related mortality in both men and women worldwide. The beneficial role of n-3 polyunsaturated fatty acids (PUFA) in preventing colon cancer is substantiated by experimental, epidemiological, and clinical data. From a mechanistic perspective, n-3 PUFA are pleiotropic and multifaceted with respect to their molecular mechanisms of action. For example, this class of dietary lipid uniquely modulates membrane and nuclear receptors, sensors/ion channels, and membrane structure/cytoskeletal function, thereby regulating signaling processes that influence patterns of gene expression and cell phenotype. In addition, n-3 PUFA can synergize with other potential chemoprotective agents known to reprogram the chromatin landscape, such as the fermentable fiber product, butyrate. Nutri-epigenomics is an emerging field of research that is focused on the interaction between nutrition and epigenetics. Epigenetics refers to a group of heterogeneous processes that regulate transcription without changing the DNA coding sequence, ranging from DNA methylation, to histone tail modifications and transcription factor activity. One implication of the nutri-epigenome is that it may be possible to reprogram epigenetic marks that are associated with increased disease risk by nutritional or lifestyle interventions. This review will focus on the nutri-epigenomic role of n-3 PUFA, particularly DHA, as well as the combinatorial effects of n-3 PUFA and fermentable fiber in relation to colon cancer.
Keywords: Fish oil, colon cancer, nutri-epigenomics, chemoprevention, fermentable fiber, docosahexaenoic acid
Introduction
Over the past 25 years, hundreds of published papers have described the effects of polyunsaturated fatty acids (PUFA) on normal and cancer cell types, including differences between n-6 and n-3 PUFA with respect to their mechanisms of action [1–4]. From this body of work, there is now mounting evidence that n-3 PUFA, namely, docosahexaenoic acid (DHA, 22:6n-3) and eicosapentaenoic acid (EPA, 20:5n-3) found in fish and algal oils, exert anti-inflammatory properties in the colon, enhance the efficacy of chemotherapeutic drugs, suppress chronic inflammatory biomarkers associated with obesity/diabetes, and reduce colon cancer risk [5–10]. The actions of n-3 PUFA appear to involve multiple mechanisms that link the cell membrane, cytosol, and the nucleus [4]*, [11]. For example, n-3 PUFA modulate membrane and nuclear receptors, and sensors/ion channels, thereby regulating signaling processes that influence patterns of gene expression. These effects appear to be mediated, in part, via the incorporation of n-3 PUFA into cell membranes [4]*, [12]. Moreover, these changes in membrane composition can affect membrane order, the formation of lipid rafts, and intracellular signaling processes [2].
With respect to the cell nucleus, nutri-epigenomics is an emerging field of research that is focused on the interaction between nutrition and the epigenome. Epigenetics refers to a group of heterogeneous processes that regulate transcription without changing the DNA coding sequence. These changes include covalent histone modifications, principally acetylation and methylation of lysine residues but also phosphorylation and ubiquitination, DNA methylation, transcriptional machinery and noncoding RNA activities [13–15]. Epigenetic marks can exhibit plasticity throughout the life course, albeit to varying degrees, and can be modified by environmental factors including diet [16]. One implication of the interaction between the diet and the epigenome is that it may be possible to reprogram epigenetic marks that are associated with increased disease risk by nutritional or lifestyle interventions. This review will focus on the nutri-epigenomic role of n-3 PUFA, particularly DHA, in relation to colon cancer.
Direct n-3 PUFA interaction with nuclear receptors
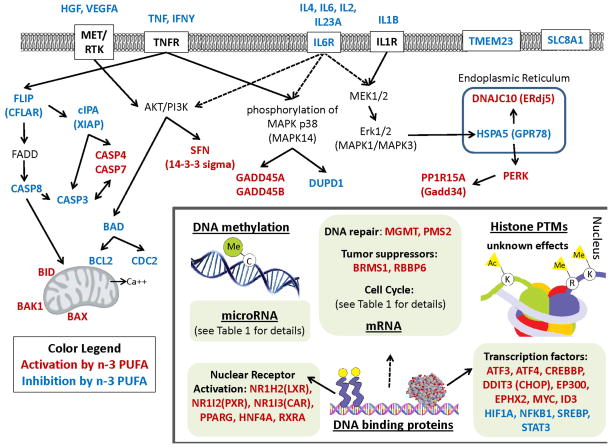
DHA and EPA and their oxidative metabolites have been shown to interact with specific ligand dependent nuclear receptors including CAR, HNF4A, PPARG, PXR and RXRA (Figure 1) [17]. In this fashion, n-3 PUFA regulate the function of nuclear receptors and their impact on transcriptional processes. For example, DHA bound PPARG can be transported to the nucleus where it controls energy balance by regulating fatty acid homeostasis in part via enhancing the expression of genes associated with membrane-bound fatty acid transporting proteins, and β-oxidation of fatty acids in peroxisomes and mitochondria [18]. Interestingly, impaired expression and function of PPARG is associated with inflammatory bowel diseases (IBD) and colon cancer [19, 20]. RXRA, which is implicated in cancer chemoprevention, also preferentially binds to n-3 PUFA in colonocytes [21]. Activation of PPARG as well as heterodimers formed with RXR play an important role in the antitumor effects of n-3 PUFAs [19].
Figure 1. Epigenetic effects of n-3 PUFA in the colon.
Intestinal genes that are up or down regulated by n-3 PUFA at the mRNA and protein levels. Red font represents gene up-regulation and blue font indicates gene down-regulation. Epigenetic levels of regulation in the nucleus are underlined. Nuclear genes are grouped by classification.
LXRs are transcriptional regulators of cholesterol metabolism that control cholesterol uptake into cells, catabolism, and efflux [22]. This is noteworthy, because cholesterol can control cell proliferation; and disruptions in cholesterol metabolism have been associated with the development of colon cancer [23–25]. LXRs also function by heterodimerizing with RXRA and binding to direct repeats with four nucleotide spacers (DR4 elements), termed LXR response elements (LXREs), in the promoter regions of target genes [22]. Interestingly, n-3 PUFA activated LXRα blocks proliferation of human colorectal cancer cells and slows the growth of xenograft tumors in mice [26].
PXR (NR1I2) has been shown to regulate the expression of genes involved in the oxidation, conjugation, and the transport of xenobiotics, and promotes the metabolism, elimination and detoxification of chemotherapeutic agents [27]. The transcription of PXR increases in the presence of n-3 PUFA [28]. This is noteworthy, because PXR can suppress the proliferation and tumorigenicity of colon cancer cells [29]. CAR (NR1I3) is likewise transcriptionally increased by n-3 PUFAs in epithelial colorectal adenocarcinoma cells and similarly regulates genes involved in xenobiotic detoxification and energy homeostasis [28].
HNF4A maintains epithelial cell function and normal colon physiology via regulation of the balance between proliferation and differentiation, immune function, ion transport, epithelial barrier function and oxidative stress [30, 31]. P1-, but not P2-HNF4A, expression is lost in colorectal carcinomas in humans and it is predicted that treatments that increase nuclear P1-HNF4α protein levels, such as n-3 PUFA, could help slow colon cancer progression [32, 33].
Indirect DHA regulation of transcription factors
Since the original description of dietary fat as a regulator of gene expression over a decade ago, many transcription factors have been identified as prospective indirect targets for n-3 PUFA regulation. For example, DHA can increase the activity of CREBBP, EP300, and MYC and decrease activity of NF-kB (NFKB1), and STAT3 [17]. However, DHA does not directly bind to this class of transcription factors. With respect to colon cancer, DHA exhibits a protective suppressive effect against hyperactivated STAT3 and may reestablish the equilibrium between STAT3 and PPARG [34]. The ability to decrease STAT3 activity may be associated with the ability of n-3 PUFA ligands to trigger PPARG-RXR heterodimers to localize at their cognate PPAR response elements (PPREs) and exchange corepressors for coactivators such as cyclic AMP response element binding protein (CREB) and p300 [20].
The cytotoxic effects of DHA are also associated with signaling pathways involving lipid metabolism and endoplasmic reticulum (ER) stress. DHA induced depletion of free cholesterol in the ER can lead to ER stress, resulting in the growth arrest/apoptosis of metastatic tumor cells [35]. It has been suggested that these alterations in the sterol content of the ER by DHA mediate growth reduction partly by down regulating nuclear SREBP, an important manager of lipid homeostasis and cell growth regulation [36]. Induction of ER stress mediators by DHA also promotes expression of the kinase PERK, which in turn promotes translation of transcription factors ATF3, 4 and 6 [35]. Furthermore, an elevation in PERK activity can increase levels of ER protein GADD34 (PPP1R15A) and the proapoptotic transcription factor DDIT3 (CHOP) along with its downstream target TRIB3 [37]. The experimental details associated with differentially expressed target genes are described in Table 1.
Table 1.
Epigenetic Studies Examining the Effects of n-3 PUFA and Fermentable Fiber on Colonic Gene Expression.
| Studies Documenting the Effects of n-3 PUFA | ||||||
|---|---|---|---|---|---|---|
| Up regulated | ||||||
| Gene symbols | Reference | Assay type | Carcinogenesis method | Treatment method | Dose | Organism |
| FOXO3A,FAF1,BCL10,DFFA,TNFRSF1A,GADD45A, CASP7,MOAP1,DAP3,TNFRSF10B,GADD45B,CASP4, CIP1/P21/CDKN1A,CCNG2,SFN/14–3-3, PPP1R15A/GADD34,TRIB3 | Slagsvold et al., 2010 [37] | Genome Arrays | colon adenocarcinoma cell line, SW620 | In vitro | DHA 70 umol/L | Human |
| ATF6,GCLC,OSBP,CAPN7,NPC2,VCP,SOD1,VLDLR, NRF2,XBP1,HSP47,CAPN2,CAMLG,ATF4,PERK,CASP7,PSMD1/RPN2,IP3R1,LDLR,TXNRD1,CASP4,GCLM, GADD34,ATF3,DNAJB1,NPC1,BAG3,HSPA1B,TRIB3, SQSTM1, HSPA1A/B,HMOX1 | Jakobsen et al., 2008 [35] | Genome Arrays | colon adenocarcinoma cell line, SW620 | In vitro | DHA 70 umol/L | Human |
| ERDJ5,PERK | Fasano et al., 2012 [49] | Western Blot | colon adenocarcinoma cell line, SW480 | In vitro | DHA 30 umol/L | Human |
| BID,BAK,BAX | Giros et al., 2009 [50] | Western Blot | colon adenocarcinoma cell line, HT-29 and Caco-2 | In vitro | DHA 60 umol/L | Human |
| MYC | Calviello et al., 2005 [43] | Western Blot | colon adenocarcinoma cell line, HT-29 and LS-174 | In vitro | DHA 10 umol/L | Human |
| miR-18a, miR-27b, miR-93, miR-200c, miR-497 | Shah et al., 2011 [11] | Low-density array | Azoxymethane (AOM) injection (15 mg/kg bw) | Diet | 11.5% fish oil (ad-libitum) | Rat distal colon |
| miR-30c, miR-141 | Gil-Zamorano et al., 2014 [91] | q-PCR | colon adenocarcinoma cell line, Caco-2 | In vitro | DHA 200 umol/L | Human |
| Down regulated | ||||||
| Gene symbols | Reference | Assay type | Carcinogenesis method | Treatment method | Dose | Organism |
| CCND1,CCND3,CDK2,CDC42,CDC25C,CDC45L,CDC20, CDK4,E2F1,CENPE,AKT1/PKB,BAD,CCNA2,CCNF, CDC25B,TNFRSF1B,CDK2AP,AURKA,BUB1,CDC7, PCNA,BIK,BIRC5,BIRC5,UNG,CCNA2,CCNB2,STMN1, CDC2/CDK1,AURKB,PLK1,NFKB,CHOP | Slagsvold et al., 2010 [37] | Genome Arrays | colon adenocarcinoma cell line, SW620 | In vitro | 70 umol/L | Human |
| FDPS,CAT,CAV1,DHCR7,DHCR24,PMVK,TM7SF2, CCND1,HMGCR,SREBP2 | Jakobsen et al., 2008 [35] | Genome Arrays | colon adenocarcinoma cell line, SW621 | In vitro | DHA 70 umol/L | Human |
| GRP78 | Fasano et al., 2012 [49] | Western Blot | colon adenocarcinoma cell line, SW480 | In vitro | DHA 30 umol/L | Human |
| IL2,IL4,IFNG,TNF,IL6,IL1B | Purasiri et al., 1994 [62] | ELISA | CRC patient serum | Supplement | 50% n-3 PUFA supplement | Human |
| XIAP,FLIP,BAD,BCL2,COX2 | Giros et al., 2009 [50] | Western Blot | colon adenocarcinoma cell line, HT-29 and Caco-2 | In vitro | DHA 60 umol/L | Human |
| BIRC5,CTNNB1, MMP7,PPARD,VEGF | Calviello et al., 2007 [67] | Western Blot | colon adenocarcinoma cell line, HCT116 and SW480 | In vitro | DHA 10 umol/L | Human |
| miR-21 | Shah et al., 2011 [11] | Low-density array | Azoxymethane (AOM) injection (15 mg/kg bw) | Diet | 11.5% fish oil (ad-libitum) | Rat distal colon |
| Studies Documenting the Effects of n-3 PUFA - Fermentable Fiber Combination | ||||||
| Up regulated | ||||||
| Gene symbols | Reference | Assay type | Carcinogenesis method | Treatment method | Dose | Organism |
| RBBP6,ID3,BRMS1,MTMR4,MGMT,PMS2 | Cho et al., 2011 [80] | Genome Arrays | Azoxymethane (AOM) injection (15 mg/kg bw) | Diet | 11.5% fish oil, 6% pectin (ad-libitum) | Rat distal colon |
| miR-19b, miR-26b, miR-203 | Shah et al., 2011 [11] | Low-density array | Azoxymethane (AOM) injection (15 mg/kg bw) | Diet | 11.5% fish oil, 6% pectin (ad-libitum) | Rat distal colon |
| Down regulated | ||||||
| Gene symbols | Reference | Assay type | Carcinogenesis method | Treatment method | Dose | Organism |
| HIPK2,FEM1B,SLC8A1,PTHR2,DUPD1,IL6R,MFN1, SMOC1,TMEM23,HGF,IL23A,STX1A,ADAM3,PPP1R7, CYP2S1,NRN1,MMP2,SNIP | Cho et al., 2011 [80] | Genome Arrays | Azoxymethane (AOM) injection (15 mg/kg bw) | Diet | 11.5% fish oil (ad-libitum) | Rat distal colon |
| PGES2,CTNNB1,PPARD | Vanamala et al., 2008 [57] | Western Blot | Azoxymethane (AOM) injection (15 mg/kg bw) | Diet | 11.5% fish oil (ad-libitum) | Ratl colon |
Colon adenocarcinomas exhibit defective expression of the adenomatous polyposis coli (APC) gene, which is a critical regulator of the Wnt signaling pathway. This and other developmental pathways play an important role in both genetic (familial) and sporadic epithelial cancers [38]. From a chemoprevention perspective, in vivo studies demonstrate that fish oil-derived n-3 PUFA suppress the formation of intestinal tumors in mice and humans with a defective APC allele [39, 40]. The downstream APC signaling oncogene, MYC, is an important regulator of cell proliferation, and the lack of MYC expression is associated with a reduced number of intestinal adenomas [41]. Interestingly, patients with an amplified MYC gene and wild type p53 have a greater response to anticancer therapies [42]. In colon cancer cells, DHA increases the level of MYC, which is believed to induce a chemoprotective, proapoptotic phenotype [43].
NF-kB activity can be inhibited by DHA [44]. This is relevant because NF-kB mediates signaling pathways that control the transcriptional activation of genes important for the regulation of many cellular processes and is aberrantly activated in many types of cancer [45, 46]. n-3 PUFA treatment inhibits the expression and activity of NF-kB in many cell types, however, the exact mechanism is not fully understood [37]. This has implications in chronic disease management because the DHA - mediated decrease in NF-kB activity has been shown to sensitize tumor cells to gamma-irradiation and promote the induction of apoptosis [19].
DHA-mediated modulation of apoptosis regulatory pathways
It has been demonstrated that DHA contributes to the down-regulation of BCL2, a well-known antiapoptotic molecule [47], which can block lipid peroxidation and thus apoptosis induction. Additionally, DHA induces caspase-dependent apoptosis in colon adenocarcinoma cells and adenoma cells [48]. There is also evidence of upregulation of CASP4 and CASP7 [35] along with increased activation of the intrinsic apoptotic pathway as demonstrated by CASP9 and Bid cleavage [48]. CASP4 activation may also be linked to augmented expression of ER resident factor ERdj5 and downregulation of antiapoptotic GRP78 [49]. The major involvement of the intrinsic apoptotic pathway following DHA treatment is through increased expression and activation of BAX and BAK [37], depolarization of the mitochondrial membrane, and the subsequent release of cytochrome c and Smac/Diablo into the cytosol [50]. Once these factors are released from mitochondria, apoptosis is accelerated [51]. These findings have been confirmed both in vitro [52] as well as in vivo [53].
n-3 PUFA can also act as efficient modulators of both the level and activity of endogenous caspase inhibitors. For example, DHA and EPA decrease XIAP (an X-linked inhibitor of apoptosis protein) at both the protein and mRNA levels, which may in part explain their antineoplastic effects [50]. High XIAP expression correlates with poor clinical outcome, resistance to chemotherapy and radiotherapy in different colon cancer cell lines [50]. DHA also down-regulates mRNA and protein levels of two other inhibitors of apoptosis, survivin (BIRC5) and livin (BIRC7) in cancer cells [37]. Furthermore, the immediate and dramatic down-regulation of FLIP (CFLAR), a potent inhibitor of caspase-8 (CASP8) activation, appears to be linked to the induction of apoptosis in colon cancer cells following DHA and EPA supplementation [50].
DHA can inhibit the expression of antioxidant enzymes or deplete cells of antioxidants [54]. It has also been suggested that DHA may have anti-inflammatory/proapoptotic effects in colon cancer cell lines by inhibiting the expression and activity of a key rate-limiting cyclooxygenase enzyme, COX-2 [55]. This is noteworthy, because COX-2 is often overexpressed in colon tumors and is able to confer a pro-inflammatory niche, which contributes to epithelial cell resistance to apoptosis [56, 57]. Activation of NF-kB and the PPAR-BCL2 feedback loop may control the life-death continuum in colon cells and has been associated with the expression of COX-2 [56]. Chemoprotective suppression of the activation of NF-kB by DHA reduces the production of pro-proliferative eicosanoids produced by COX-2 [58]. Moreover, DHA may suppress tumor cell growth directly by inhibition of the COX-2 derived metabolite, PGE2, which stimulates cell proliferation and suppresses apoptosis [57]. However, it is possible that DHA may also act via mechanisms independent of COX-2 inhibition [59], because suppression of tumor growth also occurs in cell lines that do not express COX at the protein level. Moreover, the growth of these cells in culture and in nude mice is not affected by overexpression of COX-1 or COX-2 [60]. Additional DHA-dependent proapoptotic mechanisms impacting colon adenocarcinomas include the upregulation of several growth arrest DNA-damage-inducible proteins such as GADD445A and GADD45B, likely through the stimulation of p38 MAPK phosphorylation [37].
Modulation of cytokines and growth factors
Cytokines, including IL1β, IL2, IL4, IFNγ, and TNFα increase in the early stages of carcinogenesis. n-3 PUFA suppression of NF-kB activity is at least partly responsible for the reduction in cytokine levels, including IL2, IL4, IFNγ, TNFα, IL6, and IL1β [61, 62]. These cytokines (IL1β, TGFβ, TNFα and IL6) further regulate transcription factor, e.g., HNF4A, function through modulation of proteosomal degradation, DNA binding affinity, transcriptional activity and cofactor interaction [63]*. Thus, n-3 PUFA cytokine regulatory control can extend to ion transport, epithelial barrier function and oxidative stress via effects on this transcription factor.
The protective role of n-3 PUFA can also be attributed to an increase in the expression of TGFβ through inhibition of the Akt pathway in intestinal epithelial cells [64] and fat-1 transgenic mice [65]. This is noteworthy, because the reduction of TGFβ expression increases chemical-induced colon carcinogenesis [66]. Furthermore, both EPA and DHA decrease the growth of colon tumors by reducing VEGF and TNFα expression through inhibition of ERK1/2 phosphorylation and hypoxia-induced factor HIF1α protein expression [67].
Effects of DHA on the cell cycle
There is some evidence that DHA has a selective dose dependent growth inhibitory effect on colon cancer but not normal colonic cells [68]. Several key genes involved in the regulation of both the G1 and G2 phases of the cell cycle are affected by DHA treatment in colon cancer. Generally, molecules involved in cell cycle progression, such as Cdc25c, Cdc25b, Cdc20, CDK1, CDK2, and cyclin D, A, and B, are down-regulated [37] by DHA incubation as compared to control. In comparison, genes involved in cell cycle arrest such as cyclin-dependent kinase inhibitors (CDKN1A, CDKN1B, CDKN1C, CDKN2A) and stratifin are up-regulated by DHA [69]. Some studies additionally show that activated PXR inhibits the proliferation and tumorigenicity of colon cancer cells by targeting the cell cycle at the G(0)/G(1) cell phase via modulation of the p21(WAF1/CIP1) and E2F/Rb signaling pathways [29]. In addition, in some cell contexts, DHA induces cell cycle arrest and down-regulates the nuclear form of sterol regulatory element-binding proteins (SREBP1 and 2) in colon cancer cell lines, indicating a possible relationship between disturbances in lipid homeostasis and cell cycle arrest [35, 36, 43]. While a large number of mechanisms are linked to DHA anti-proliferative effects in cancer, several reports have focused on whether p53 protein plays a role in DHA-induced growth inhibition. DHA inhibits the growth of p53-wildtype colon cell lines as well as of those with inactivating p53 mutations; thus its action does not seem to be dependent on p53 status [43].
Optimal chemoprevention: Interaction of DHA with butyrate
It has been proposed by us and others that n-3 PUFA and butyrate (fiber fermentation product) interact in the colon to profoundly suppress colon cancer [70–72]. Interaction of dietary fiber-derived compounds in the colonic lumen can have a substantial impact on the metabolism and kinetics of the colon epithelial cell population and suppress inflammation and neoplasia [73–75]. For example, butyrate, a four-carbon short-chain fatty acid, is produced during anaerobic fermentation of dietary fiber by endogenous bacteria present in the colon. This agent has pleiotropic effects in the colon [76, 77]. It acts as a principal energy source and a survival factor for normal colon cells, whereas it exerts anti-proliferative, differentiation- and apoptosis-inducing effects in cancer cells [78]. In addition to the regulation of basic cytokinetic processes, butyrate has also been shown to affect cell adhesion, morphology, invasiveness, metastasis, oxidative metabolism, angiogenesis, and the activity of different enzymes and transcription factors. These effects are linked in part to butyrate’s function as a histone deacetylase inhibitor, which mechanistically links it to gene expression [79].
Studies published by our group describe the protective effects of fish oil containing DHA, compared to corn oil and its interaction with fiber using rat and mouse model colon carcinogenesis models [2, 6]. These data demonstrate that the combination of n-3 PUFA and butyrate (fermentable fiber) treatment maximally enhances cell cycle arrest, by inhibiting expression of cell cycle genes (Table 1), shifting the balance between differentiation and apoptosis depending on the cell transformation status of the model [75, 80, 81]. These findings demonstrate that dietary n-3 PUFA and fermentable fiber can act synergistically to protect against colon carcinogenesis primarily by enhancing the deletion of DNA damaged cells [57, 71, 72, 82, 83].
Temporal gene expression profiles from exfoliated rat colonocytes have revealed at the cancer initiation stage that fish oil plus fermentable fiber (FO/F) downregulates the expression of genes involved with cell adhesion and enhances apoptosis compared to the non-chemoprotective control of corn oil plus cellulose (CO/C) [80]. In addition, at the cancer progression stage, the expression of genes involved in cell cycle promotion are downregulated while DNA mismatch repair genes, MGMT and PMS2, are upregulated. FO/F also increases apoptosis and the expression of genes that promote apoptosis at the tumor stage [80]. The chemoprotective gene profiles at the tumor stage include the up-regulation of the proapoptotic inhibitor of DNA binding ID3 and tumor suppressors BRMS1 and RBBP6, and also downregulation of antiapoptotic genes HGF and TMMEM23, and down-regulation of cytokine signaling, IL23A and receptor IL6RA [80]. Signal transduction related genes such as MAPK, DUPD1 and PPP1R7, and calcium signaling receptor SLC8A1 were also downregulated [80]. In addition, the chemotherapeutic effect of the FO/F dietary extends to translational activation of the xenobiotic metabolizing phase I enzyme EPHX2 and tumor suppressor retinoblastoma-associated protein RB1. These novel findings demonstrate that the effects of the chemotherapeutic (FO/F) diet on epithelial cell gene expression can be monitored noninvasively throughout the tumorigenic process by analysis of exfoliated colonocytes.
Combinatorial effects of n-3 PUFA and fermentable fiber on non-coding microRNAs
High throughput micro-RNA (miRNA) profiling studies have linked aberrant expression of miRNAs to the development of colon cancer [84, 85]. Dysregulation of miRNA editing has been linked to aberrant epidermal growth factor receptor (EGFR) signaling, which interacts with argonaute 2 (AGO2), thereby perturbing miRNA processing from precursor to mature miRNAs [86, 87]. The fact that DHA antagonizes EGFR in cancer cells by increasing receptor internalization and degradation [88], implicates a potential regulatory molecular mechanism involving fish oil and miRNAs. Further study is needed to validate this epigenetic mechanism of action.
Recently, the effects of colon carcinogen and the combination of dietary fish oil and fermentable fiber (pectin) on rodent microRNA expression during the early stages of colon tumorigenesis have been examined [11, 89]. miRNAs modulated by fish oil in colon cancer in both human and rodent models have also been reported [11, 89] [90]*. Specific miRNAs influenced by fish oil treatment or the highly chemoprotective combination of fish oil and pectin diet are summarized with respect to their validated mRNA target genes in Table 1. miR-18a, miR-27b, miR-30c, miR-93, miR-141, miR-200c, and miR-497 were increased by fish oil feeding, while miR-21 was decreased compared to control diet [91]. This is noteworthy, because miR-21 is well-known “oncogenic” miRNA and its validated targets PDCD4, and PTEN are known tumor suppressor genes [92–94]. In comparison, miR-19b, miR-26b, and miR-203 were increased by fish oil and pectin combination feeding compared to control diet (corn oil plus cellulose diet) [11]. This is noteworthy, because their validated targets, HIPK3, ARID4B, ARPC3, LEF1, RUNX1, CXCL12, TRP63 and ZFP281, are known to promote tumorigenesis.
Conclusion
n-3 PUFA are an ideal colon cancer chemotherapeutic because (1) they are toxicologically innocuous and free of safety problems intrinsic to drugs administered over long periods of time, (2) are relatively inexpensive, and (3) provide additional health benefits, such as reduction in mortality [40, 95, 96]. In addition, the ingestion of n-3 PUFA with other agents such as fermentable fiber and curcumin may improve their efficacy in colon cancer prevention/therapy [97–99].
From an epigenetic perspective, there is still much to be discovered in terms of the effects of n-3 PUFA in the colon at the chromatin state level. From a chemoprevention perspective, not only can dietary choices modify the epigenome, but intimate knowledge of the mechanisms involved could help tailor nutritional intervention to specific individuals. Along these lines, recent work has begun to focus on n-3 PUFA effects on DNA methylation with respect to colon cancer risk [100, 101]*. Although a substantial body of work exists regarding the effects of n-3 PUFA on cytokines and the resolution of chronic inflammation, studies addressing the specifics of these effects in terms of colon cancer cells are limited [61, 62] [64] [67]. In the future, personalized chemoprevention will be based on individual nutritional requirements and susceptibility to disease, including anatomical considerations such as differences in proximal versus distal colonic tumorigenesis [15, 102].
As discussed in this review, the breadth of n-3 PUFA effects on epigenetic regulation in colon cancer is wide and complex. As described in Table 1 and furthermore illustrated in Figure 1, a wide array of potential pathways, molecular interactions, and mechanisms are modulated by n-3 PUFA. An interesting future frontier will be the pursuit of epigenetic molecular complexes targeted by chemoprotective n-3 PUFA in combination with fiber.
Acknowledgments
This work was supported in part by an NIH Research Supplement to Promote Diversity in Health-Related Research (CA129444), P30ES023512 and the American Institute for Cancer Research. The authors wish to thank Nadia Ponce for assistance with figure design.
Footnotes
Conflict of Interest
Karen Triff, Eunjoo Kim and Robert S. Chapkin declare that they have no conflict of interest.
Compliance with Ethics Guidelines
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
References
Papers of particular interest, published recently, have been highlighted as:
* Of importance
- 1.Chapkin RS, McMurray DN, Davidson LA, et al. Bioactive dietary long-chain fatty acids: emerging mechanisms of action. Br J Nutr. 2008;100:1152–1157. doi: 10.1017/S0007114508992576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chapkin RS, Seo J, McMurray DN, Lupton JR. Mechanisms by which docosahexaenoic acid and related fatty acids reduce colon cancer risk and inflammatory disorders of the intestine. Chem Phys Lipids. 2008;153:14–23. doi: 10.1016/j.chemphyslip.2008.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reddy BS, Burill C, Rigotty J. Effect of diets high in omega-3 and omega-6 fatty acids on initiation and postinitiation stages of colon carcinogenesis. Cancer Res. 1991;51:487–491. [PubMed] [Google Scholar]
- 4*.Turk HF, Chapkin RS. Membrane lipid raft organization is uniquely modified by n-3 polyunsaturated fatty acids. Prostaglandins Leukot Essent Fatty Acids. 2013;88:43–47. doi: 10.1016/j.plefa.2012.03.008. Highlights recent work demonstrating that enrichment of n-3 PUFA in the plasma membrane alters the lateral organization of membrane signaling assemblies. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pardini RS. Nutritional intervention with omega-3 fatty acids enhances tumor response to anti-neoplastic agents. Chem Biol Interact. 2006;162:89–105. doi: 10.1016/j.cbi.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 6.Kim W, McMurray DN, Chapkin RS. Chemotherapeutic Properties of n-3 Polyunsaturated Fatty Acids - Old Concepts and New Insights. Immunol Endocr Metab Agents Med Chem. 2009;9:38–44. doi: 10.2174/187152209788009841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gomez Candela C, Bermejo Lopez LM, Loria Kohen V. Importance of a balanced omega 6/omega 3 ratio for the maintenance of health: nutritional recommendations. Nutr Hosp. 2011;26:323–329. doi: 10.1590/S0212-16112011000200013. [DOI] [PubMed] [Google Scholar]
- 8.West NJ, Clark SK, Phillips RKS, et al. Eicosapentaenoic acid reduces rectal polyp number and size in familial adenomatous polyposis. Gut. 2010;59:918–925. doi: 10.1136/gut.2009.200642. [DOI] [PubMed] [Google Scholar]
- 9.Moosheer SM, Waldschutz W, Itariu BK, Brath H, Stulnig TM. A protein-enriched low glycemic index diet with omega-3 polyunsaturated fatty acid supplementation exerts beneficial effects on metabolic control in type 2 diabetes. Prim Care Diabetes. 2014 doi: 10.1016/j.pcd.2014.02.004. [DOI] [PubMed] [Google Scholar]
- 10.van Heusden GP, Bos K, Raetz CR, Wirtz KW. Chinese hamster ovary cells deficient in peroxisomes lack the nonspecific lipid transfer protein (sterol carrier protein 2) J Biol Chem. 1990;265:4105–4110. [PubMed] [Google Scholar]
- 11.Shah MS, Schwartz SL, Zhao C, et al. Integrated microRNA and mRNA expression profiling in a rat colon carcinogenesis model: effect of a chemo-protective diet. Physiol Genomics. 2011;43:640–654. doi: 10.1152/physiolgenomics.00213.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hou TY, Monk JM, Fan YY, et al. n-3 polyunsaturated fatty acids suppress phosphatidylinositol 4,5-bisphosphate-dependent actin remodelling during CD4+ T-cell activation. Biochem J. 2012;443:27–37. doi: 10.1042/BJ20111589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goldberg AD, Allis CD, Bernstein E. Epigenetics: a landscape takes shape. Cell. 2007;128:635–638. doi: 10.1016/j.cell.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 14.Skinner MK. Environmental epigenomics and disease susceptibility. EMBO Rep. 2011;12:620–622. doi: 10.1038/embor.2011.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burdge GC, Hoile SP, Lillycrop KA. Epigenetics: are there implications for personalised nutrition? Curr Opin Clin Nutr Metab Care. 2012;15:442–447. doi: 10.1097/MCO.0b013e3283567dd2. [DOI] [PubMed] [Google Scholar]
- 16.Burdge GC, Lillycrop KA. Nutrition, epigenetics, and developmental plasticity: implications for understanding human disease. Annu Rev Nutr. 2010;30:315–339. doi: 10.1146/annurev.nutr.012809.104751. [DOI] [PubMed] [Google Scholar]
- 17.Pegorier JP, Le May C, Girard J. Control of gene expression by fatty acids. J Nutr. 2004;134:2444S–2449S. doi: 10.1093/jn/134.9.2444S. [DOI] [PubMed] [Google Scholar]
- 18.Ponferrada A, Caso JR, Alou L, et al. The role of PPARgamma on restoration of colonic homeostasis after experimental stress-induced inflammation and dysfunction. Gastroenterology. 2007;132:1791–1803. doi: 10.1053/j.gastro.2007.02.032. [DOI] [PubMed] [Google Scholar]
- 19.Zand H, Rahimipour A, Salimi S, Shafiee SM. Docosahexaenoic acid sensitizes Ramos cells to Gamma-irradiation-induced apoptosis through involvement of PPAR-gamma activation and NF-kappaB suppression. Mol Cell Biochem. 2008;317:113–120. doi: 10.1007/s11010-008-9838-x. [DOI] [PubMed] [Google Scholar]
- 20.Edwards IJ, O’Flaherty JT. Omega-3 Fatty Acids and PPAR gamma in Cancer. Ppar Research. 2008 doi: 10.1155/2008/358052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fan YY, Spencer TE, Wang N, Moyer MP, Chapkin RS. Chemopreventive n-3 fatty acids activate RXRalpha in colonocytes. Carcinogenesis. 2003;24:1541–1548. doi: 10.1093/carcin/bgg110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sampath H, Ntambi JM. Polyunsaturated fatty acid regulation of genes of lipid metabolism. Annu Rev Nutr. 2005;25:317–340. doi: 10.1146/annurev.nutr.25.051804.101917. [DOI] [PubMed] [Google Scholar]
- 23.Possidonio AC, Miranda M, Gregoracci GB, et al. Cholesterol depletion induces transcriptional changes during skeletal muscle differentiation. BMC Genomics. 2014;15:544. doi: 10.1186/1471-2164-15-544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Robbins D, Chen T. Tissue-specific regulation of pregnane X receptor in cancer development and therapy. Cell Biosci. 2014;4:17. doi: 10.1186/2045-3701-4-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang X, Zhao XW, Liu DB, et al. Lipid levels in serum and cancerous tissues of colorectal cancer patients. World J Gastroenterol. 2014;20:8646–8652. doi: 10.3748/wjg.v20.i26.8646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lo Sasso G, Bovenga F, Murzilli S, et al. Liver X receptors inhibit proliferation of human colorectal cancer cells and growth of intestinal tumors in mice. Gastroenterology. 2013;144:1497–1507. 1507 e1491–1413. doi: 10.1053/j.gastro.2013.02.005. [DOI] [PubMed] [Google Scholar]
- 27.Qiao EQ, Ji MH, Wu JZ, et al. Expression of the PXR gene in various types of cancer and drug resistance (Review) Oncology Letters. 2013;5:1093–1100. doi: 10.3892/ol.2013.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kuan CY, Walker TH, Luo PG, Chen CF. Long-chain polyunsaturated fatty acids promote paclitaxel cytotoxicity via inhibition of the MDR1 gene in the human colon cancer Caco-2 cell line. J Am Coll Nutr. 2011;30:265–273. doi: 10.1080/07315724.2011.10719969. [DOI] [PubMed] [Google Scholar]
- 29.Ouyang N, Ke S, Eagleton N, et al. Pregnane X receptor suppresses proliferation and tumourigenicity of colon cancer cells. Br J Cancer. 2010;102:1753–1761. doi: 10.1038/sj.bjc.6605677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ahn SH, Shah YM, Inoue J, et al. Hepatocyte nuclear factor 4alpha in the intestinal epithelial cells protects against inflammatory bowel disease. Inflamm Bowel Dis. 2008;14:908–920. doi: 10.1002/ibd.20413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cattin AL, Le Beyec J, Barreau F, et al. Hepatocyte nuclear factor 4alpha, a key factor for homeostasis, cell architecture, and barrier function of the adult intestinal epithelium. Mol Cell Biol. 2009;29:6294–6308. doi: 10.1128/MCB.00939-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oshima T, Kawasaki T, Ohashi R, et al. Downregulated P1 promoter-driven hepatocyte nuclear factor-4alpha expression in human colorectal carcinoma is a new prognostic factor against liver metastasis. Pathol Int. 2007;57:82–90. doi: 10.1111/j.1440-1827.2006.02061.x. [DOI] [PubMed] [Google Scholar]
- 33.Chellappa K, Robertson GR, Sladek FM. HNF4alpha: a new biomarker in colon cancer? Biomark Med. 2012;6:297–300. doi: 10.2217/bmm.12.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.D’Archivio M, Scazzocchio B, Giammarioli S, et al. omega3-PUFAs exert anti-inflammatory activity in visceral adipocytes from colorectal cancer patients. PLoS One. 2013;8:e77432. doi: 10.1371/journal.pone.0077432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jakobsen CH, Storvold GL, Bremseth H, et al. DHA induces ER stress and growth arrest in human colon cancer cells: associations with cholesterol and calcium homeostasis. J Lipid Res. 2008;49:2089–2100. doi: 10.1194/jlr.M700389-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schonberg SA, Lundemo AG, Fladvad T, et al. Closely related colon cancer cell lines display different sensitivity to polyunsaturated fatty acids, accumulate different lipid classes and downregulate sterol regulatory element-binding protein 1. FEBS J. 2006;273:2749–2765. doi: 10.1111/j.1742-4658.2006.05292.x. [DOI] [PubMed] [Google Scholar]
- 37.Slagsvold JE, Pettersen CH, Storvold GL, et al. DHA alters expression of target proteins of cancer therapy in chemotherapy resistant SW620 colon cancer cells. Nutr Cancer. 2010;62:611–621. doi: 10.1080/01635580903532366. [DOI] [PubMed] [Google Scholar]
- 38.Gerner EW, Ignatenko NA, Lance P, Hurley LH. A comprehensive strategy to combat colon cancer targeting the adenomatous polyposis coli tumor suppressor gene. Ann N Y Acad Sci. 2005;1059:97–105. doi: 10.1196/annals.1339.033. [DOI] [PubMed] [Google Scholar]
- 39.West NJ, Clark SK, Phillips RK, et al. Eicosapentaenoic acid reduces rectal polyp number and size in familial adenomatous polyposis. Gut. 2010;59:918–925. doi: 10.1136/gut.2009.200642. [DOI] [PubMed] [Google Scholar]
- 40.Cockbain AJ, Toogood GJ, Hull MA. Omega-3 polyunsaturated fatty acids for the treatment and prevention of colorectal cancer. Gut. 2012;61:135–149. doi: 10.1136/gut.2010.233718. [DOI] [PubMed] [Google Scholar]
- 41.Paulsen JE, Elvsaas IK, Steffensen IL, Alexander J. A fish oil derived concentrate enriched in eicosapentaenoic and docosahexaenoic acid as ethyl ester suppresses the formation and growth of intestinal polyps in the Min mouse. Carcinogenesis. 1997;18:1905–1910. doi: 10.1093/carcin/18.10.1905. [DOI] [PubMed] [Google Scholar]
- 42.Arango D, Corner GA, Wadler S, Catalano PJ, Augenlicht LH. c-myc/p53 interaction determines sensitivity of human colon carcinoma cells to 5-fluorouracil in vitro and in vivo. Cancer Res. 2001;61:4910–4915. [PubMed] [Google Scholar]
- 43.Calviello G, Di Nicuolo F, Serini S, et al. Docosahexaenoic acid enhances the susceptibility of human colorectal cancer cells to 5-fluorouracil. Cancer Chemother Pharmacol. 2005;55:12–20. doi: 10.1007/s00280-004-0846-6. [DOI] [PubMed] [Google Scholar]
- 44.Weber C, Erl W, Pietsch A, Danesch U, Weber PC. Docosahexaenoic acid selectively attenuates induction of vascular cell adhesion molecule-1 and subsequent monocytic cell adhesion to human endothelial cells stimulated by tumor necrosis factor-alpha. Arterioscler Thromb Vasc Biol. 1995;15:622–628. doi: 10.1161/01.atv.15.5.622. [DOI] [PubMed] [Google Scholar]
- 45.Mishra A, Chaudhary A, Sethi S. Oxidized omega-3 fatty acids inhibit NF-kappa B activation via a PPAR alpha-dependent pathway. Arteriosclerosis Thrombosis and Vascular Biology. 2004;24:1621–1627. doi: 10.1161/01.ATV.0000137191.02577.86. [DOI] [PubMed] [Google Scholar]
- 46.Martinez-Augustin O, Lopez-Posadas R, Gonzalez R, et al. Genomic analysis of sulfasalazine effect in experimental colitis is consistent primarily with the modulation of NF-kappaB but not PPAR-gamma signaling. Pharmacogenet Genomics. 2009;19:363–372. doi: 10.1097/FPC.0b013e3283299a73. [DOI] [PubMed] [Google Scholar]
- 47.Chapkin RS, Wang N, Fan YY, Lupton JR, Prior IA. Docosahexaenoic acid alters the size and distribution of cell surface microdomains. Biochim Biophys Acta. 2008;1778:466–471. doi: 10.1016/j.bbamem.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Habermann N, Schon A, Lund EK, Glei M. Fish fatty acids alter markers of apoptosis in colorectal adenoma and adenocarcinoma cell lines but fish consumption has no impact on apoptosis-induction ex vivo. Apoptosis. 2010;15:621–630. doi: 10.1007/s10495-010-0459-y. [DOI] [PubMed] [Google Scholar]
- 49.Fasano E, Serini S, Piccioni E, et al. DHA induces apoptosis by altering the expression and cellular location of GRP78 in colon cancer cell lines. Biochim Biophys Acta. 2012;1822:1762–1772. doi: 10.1016/j.bbadis.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 50.Giros A, Grzybowski M, Sohn VR, et al. Regulation of colorectal cancer cell apoptosis by the n-3 polyunsaturated fatty acids Docosahexaenoic and Eicosapentaenoic. Cancer Prev Res (Phila) 2009;2:732–742. doi: 10.1158/1940-6207.CAPR-08-0197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kagan VE, Bayir HA, Belikova NA, et al. Cytochrome c/cardiolipin relations in mitochondria: a kiss of death. Free Radic Biol Med. 2009;46:1439–1453. doi: 10.1016/j.freeradbiomed.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Watkins SM, Carter LC, German JB. Docosahexaenoic acid accumulates in cardiolipin and enhances HT-29 cell oxidant production. J Lipid Res. 1998;39:1583–1588. [PubMed] [Google Scholar]
- 53.Hong MY, Chapkin RS, Barhoumi R, et al. Fish oil increases mitochondrial phospholipid unsaturation, upregulating reactive oxygen species and apoptosis in rat colonocytes. Carcinogenesis. 2002;23:1919–1925. doi: 10.1093/carcin/23.11.1919. [DOI] [PubMed] [Google Scholar]
- 54.Siddiqui RA, Harvey K, Stillwell W. Anticancer properties of oxidation products of docosahexaenoic acid. Chem Phys Lipids. 2008;153:47–56. doi: 10.1016/j.chemphyslip.2008.02.009. [DOI] [PubMed] [Google Scholar]
- 55.Swamy MV, Cooma I, Patlolla JM, et al. Modulation of cyclooxygenase-2 activities by the combined action of celecoxib and decosahexaenoic acid: novel strategies for colon cancer prevention and treatment. Mol Cancer Ther. 2004;3:215–221. [PubMed] [Google Scholar]
- 56.Yang WL, Frucht H. Activation of the PPAR pathway induces apoptosis and COX-2 inhibition in HT-29 human colon cancer cells. Carcinogenesis. 2001;22:1379–1383. doi: 10.1093/carcin/22.9.1379. [DOI] [PubMed] [Google Scholar]
- 57.Vanamala J, Glagolenko A, Yang P, et al. Dietary fish oil and pectin enhance colonocyte apoptosis in part through suppression of PPARdelta/PGE2 and elevation of PGE3. Carcinogenesis. 2008;29:790–796. doi: 10.1093/carcin/bgm256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hardman WE, Moyer MP, Cameron IL. Consumption of an omega-3 fatty acids product, INCELL AAFA, reduced side-effects of CPT-11 (irinotecan) in mice. Br J Cancer. 2002;86:983–988. doi: 10.1038/sj.bjc.6600175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Agarwal B, Swaroop P, Protiva P, et al. Cox-2 is needed but not sufficient for apoptosis induced by Cox-2 selective inhibitors in colon cancer cells. Apoptosis. 2003;8:649–654. doi: 10.1023/A:1026199929747. [DOI] [PubMed] [Google Scholar]
- 60.Boudreau MD, Sohn KH, Rhee SH, et al. Suppression of tumor cell growth both in nude mice and in culture by n-3 polyunsaturated fatty acids: mediation through cyclooxygenase-independent pathways. Cancer Res. 2001;61:1386–1391. [PubMed] [Google Scholar]
- 61.Jho DH, Cole SM, Lee EM, Espat NJ. Role of omega-3 fatty acid supplementation in inflammation and malignancy. Integr Cancer Ther. 2004;3:98–111. doi: 10.1177/1534735404264736. [DOI] [PubMed] [Google Scholar]
- 62.Purasiri P, Murray A, Richardson S, et al. Modulation of cytokine production in vivo by dietary essential fatty acids in patients with colorectal cancer. Clin Sci (Lond) 1994;87:711–717. doi: 10.1042/cs0870711. [DOI] [PubMed] [Google Scholar]
- 63*.Babeu JP, Boudreau F. Hepatocyte nuclear factor 4-alpha involvement in liver and intestinal inflammatory networks. World J Gastroenterol. 2014;20:22–30. doi: 10.3748/wjg.v20.i1.22. Presents potential functional roles for HNF4-α isoforms in protecting the intestinal mucosa from chronic pathological inflammation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cao Y, Deng C, Townsend CM, Jr, Ko TC. TGF-beta inhibits Akt-induced transformation in intestinal epithelial cells. Surgery. 2006;140:322–329. doi: 10.1016/j.surg.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 65.Nowak J, Weylandt KH, Habbel P, et al. Colitis-associated colon tumorigenesis is suppressed in transgenic mice rich in endogenous n-3 fatty acids. Carcinogenesis. 2007;28:1991–1995. doi: 10.1093/carcin/bgm166. [DOI] [PubMed] [Google Scholar]
- 66.Tang B, Bottinger EP, Jakowlew SB, et al. Transforming growth factor-beta1 is a new form of tumor suppressor with true haploid insufficiency. Nat Med. 1998;4:802–807. doi: 10.1038/nm0798-802. [DOI] [PubMed] [Google Scholar]
- 67.Calviello G, Resci F, Serini S, et al. Docosahexaenoic acid induces proteasome-dependent degradation of beta-catenin, down-regulation of survivin and apoptosis in human colorectal cancer cells not expressing COX-2. Carcinogenesis. 2007;28:1202–1209. doi: 10.1093/carcin/bgl254. [DOI] [PubMed] [Google Scholar]
- 68.Toit-Kohn JL, Louw L, Engelbrecht AM. Docosahexaenoic acid induces apoptosis in colorectal carcinoma cells by modulating the PI3 kinase and p38 MAPK pathways. J Nutr Biochem. 2009;20:106–114. doi: 10.1016/j.jnutbio.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 69.Davidson LA, Nguyen DV, Hokanson RM, et al. Chemopreventive n-3 polyunsaturated fatty acids reprogram genetic signatures during colon cancer initiation and progression in the rat. Cancer Res. 2004;64:6797–6804. doi: 10.1158/0008-5472.CAN-04-1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chapkin RS, McMurray DN, Lupton JR. Colon cancer, fatty acids and anti-inflammatory compounds. Curr Opin Gastroenterol. 2007;23:48–54. doi: 10.1097/MOG.0b013e32801145d7. [DOI] [PubMed] [Google Scholar]
- 71.Kolar SS, Barhoumi R, Lupton JR, Chapkin RS. Docosahexaenoic acid and butyrate synergistically induce colonocyte apoptosis by enhancing mitochondrial Ca2+ accumulation. Cancer Res. 2007;67:5561–5568. doi: 10.1158/0008-5472.CAN-06-4716. [DOI] [PubMed] [Google Scholar]
- 72.Kolar SS, Barhoumi R, Callaway ES, et al. Synergy between docosahexaenoic acid and butyrate elicits p53-independent apoptosis via mitochondrial Ca(2+) accumulation in colonocytes. Am J Physiol Gastrointest Liver Physiol. 2007;293:G935–943. doi: 10.1152/ajpgi.00312.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chapkin RS, Clark AE, Davidson LA, et al. Dietary fiber differentially alters cellular fatty acid-binding protein expression in exfoliated colonocytes during tumor development. Nutr Cancer. 1998;32:107–112. doi: 10.1080/01635589809514727. [DOI] [PubMed] [Google Scholar]
- 74.Kolar S, Barhoumi R, Jones CK, et al. Interactive effects of fatty acid and butyrate-induced mitochondrial Ca(2)(+) loading and apoptosis in colonocytes. Cancer. 2011;117:5294–5303. doi: 10.1002/cncr.26205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Turk HF, Kolar SS, Fan YY, et al. Linoleic acid and butyrate synergize to increase Bcl-2 levels in colonocytes. Int J Cancer. 2011;128:63–71. doi: 10.1002/ijc.25323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hofmanova J, Hyrslova Vaculova A, Kozubik A. Regulation of the metabolism of polyunsaturated Fatty acids and butyrate in colon cancer cells. Curr Pharm Biotechnol. 2013;14:274–288. doi: 10.2174/1389201011314030004. [DOI] [PubMed] [Google Scholar]
- 77.Donohoe DR, Garge N, Zhang X, et al. The microbiome and butyrate regulate energy metabolism and autophagy in the mammalian colon. Cell Metab. 2011;13:517–526. doi: 10.1016/j.cmet.2011.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Canani RB, Costanzo MD, Leone L, et al. Potential beneficial effects of butyrate in intestinal and extraintestinal diseases. World J Gastroenterol. 2011;17:1519–1528. doi: 10.3748/wjg.v17.i12.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Scharlau D, Borowicki A, Habermann N, et al. Mechanisms of primary cancer prevention by butyrate and other products formed during gut flora-mediated fermentation of dietary fibre. Mutat Res. 2009;682:39–53. doi: 10.1016/j.mrrev.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 80.Cho Y, Kim H, Turner ND, et al. A chemoprotective fish oil- and pectin-containing diet temporally alters gene expression profiles in exfoliated rat colonocytes throughout oncogenesis. J Nutr. 2011;141:1029–1035. doi: 10.3945/jn.110.134973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kachroo P, Ivanov I, Davidson LA, et al. Classification of diet-modulated gene signatures at the colon cancer initiation and progression stages. Dig Dis Sci. 2011;56:2595–2604. doi: 10.1007/s10620-011-1652-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chang WL, Chapkin RS, Lupton JR. Fish oil blocks azoxymethane-induced rat colon tumorigenesis by increasing cell differentiation and apoptosis rather than decreasing cell proliferation. J Nutr. 1998;128:491–497. doi: 10.1093/jn/128.3.491. [DOI] [PubMed] [Google Scholar]
- 83.Crim KC, Sanders LM, Hong MY, et al. Upregulation of p21Waf1/Cip1 expression in vivo by butyrate administration can be chemoprotective or chemopromotive depending on the lipid component of the diet. Carcinogenesis. 2008;29:1415–1420. doi: 10.1093/carcin/bgn144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Piepoli A, Tavano F, Copetti M, et al. Mirna expression profiles identify drivers in colorectal and pancreatic cancers. PLoS One. 2012;7:e33663. doi: 10.1371/journal.pone.0033663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Slaby O, Svoboda M, Michalek J, Vyzula R. MicroRNAs in colorectal cancer: translation of molecular biology into clinical application. Mol Cancer. 2009;8:102. doi: 10.1186/1476-4598-8-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Diederichs S, Haber DA. Dual role for argonautes in microRNA processing and posttranscriptional regulation of microRNA expression. Cell. 2007;131:1097–1108. doi: 10.1016/j.cell.2007.10.032. [DOI] [PubMed] [Google Scholar]
- 87.Shen J, Xia W, Khotskaya YB, et al. EGFR modulates microRNA maturation in response to hypoxia through phosphorylation of AGO2. Nature. 2013;497:383–387. doi: 10.1038/nature12080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Turk HF, Barhoumi R, Chapkin RS. Alteration of EGFR spatiotemporal dynamics suppresses signal transduction. PLoS One. 2012;7:e39682. doi: 10.1371/journal.pone.0039682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Davidson LA, Wang N, Shah MS, et al. n-3 Polyunsaturated fatty acids modulate carcinogen-directed non-coding microRNA signatures in rat colon. Carcinogenesis. 2009;30:2077–2084. doi: 10.1093/carcin/bgp245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90*.Chapkin RS, DeClercq V, Kim E, Fuentes RN, Fan YY. Mechanisms by Which Pleiotropic Amphiphilic n–3 PUFA Reduce Colon Cancer Risk. Current Colorectal Cancer Reports. 2014 doi: 10.1007/s11888-014-0241-6. Summarizes recent non-epigenetic mechanisms of action linking n-3 PUFA intake, membrane alterations and effects on obesity associated colon cancer risk. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gil-Zamorano J, Martin R, Daimiel L, et al. Docosahexaenoic acid modulates the enterocyte Caco-2 cell expression of microRNAs involved in lipid metabolism. J Nutr. 2014;144:575–585. doi: 10.3945/jn.113.189050. [DOI] [PubMed] [Google Scholar]
- 92.Li X, Xin S, He Z, et al. MicroRNA-21 (miR-21) post-transcriptionally downregulates tumor suppressor PDCD4 and promotes cell transformation, proliferation, and metastasis in renal cell carcinoma. Cell Physiol Biochem. 2014;33:1631–1642. doi: 10.1159/000362946. [DOI] [PubMed] [Google Scholar]
- 93.Asangani IA, Rasheed SA, Nikolova DA, et al. MicroRNA-21 (miR-21) post-transcriptionally downregulates tumor suppressor Pdcd4 and stimulates invasion, intravasation and metastasis in colorectal cancer. Oncogene. 2008;27:2128–2136. doi: 10.1038/sj.onc.1210856. [DOI] [PubMed] [Google Scholar]
- 94.Meng F, Henson R, Wehbe-Janek H, et al. MicroRNA-21 regulates expression of the PTEN tumor suppressor gene in human hepatocellular cancer. Gastroenterology. 2007;133:647–658. doi: 10.1053/j.gastro.2007.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lien EL. Toxicology and safety of DHA. Prostaglandins Leukot Essent Fatty Acids. 2009;81:125–132. doi: 10.1016/j.plefa.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 96.Bell GA, Kantor ED, Lampe JW, et al. Intake of long-chain omega-3 fatty acids from diet and supplements in relation to mortality. Am J Epidemiol. 2014;179:710–720. doi: 10.1093/aje/kwt326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Davidson LA, Wang N, Ivanov I, et al. Identification of actively translated mRNA transcripts in a rat model of early-stage colon carcinogenesis. Cancer Prev Res (Phila) 2009;2:984–994. doi: 10.1158/1940-6207.CAPR-09-0144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Jia Q, Ivanov I, Zlatev ZZ, et al. Dietary fish oil and curcumin combine to modulate colonic cytokinetics and gene expression in dextran sodium sulphate-treated mice. Br J Nutr. 2011;106:519–529. doi: 10.1017/S0007114511000390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Fenton JI, McCaskey SJ. Curcumin and docosahexaenoic acid block insulin-induced colon carcinoma cell proliferation. Prostaglandins Leukot Essent Fatty Acids. 2013;88:219–226. doi: 10.1016/j.plefa.2012.11.010. [DOI] [PubMed] [Google Scholar]
- 100*.Cho Y, Turner ND, Davidson LA, et al. Colon cancer cell apoptosis is induced by combined exposure to the n-3 fatty acid docosahexaenoic acid and butyrate through promoter methylation. Exp Biol Med (Maywood) 2014;239:302–310. doi: 10.1177/1535370213514927. This body of work descibes the effect of n-3 PUFA plus butyrate combination on DNA methylation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Cho Y, Turner ND, Davidson LA, et al. A chemoprotective fish oil/pectin diet enhances apoptosis via Bcl-2 promoter methylation in rat azoxymethane-induced carcinomas. Exp Biol Med (Maywood) 2012;237:1387–1393. doi: 10.1258/ebm.2012.012244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Triff K, Konganti K, Gaddis S, et al. Genome-wide analysis of the rat colon reveals proximal-distal differences in histone modifications and proto-oncogene expression. Physiol Genomics. 2013;45:1229–1243. doi: 10.1152/physiolgenomics.00136.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]