Abstract
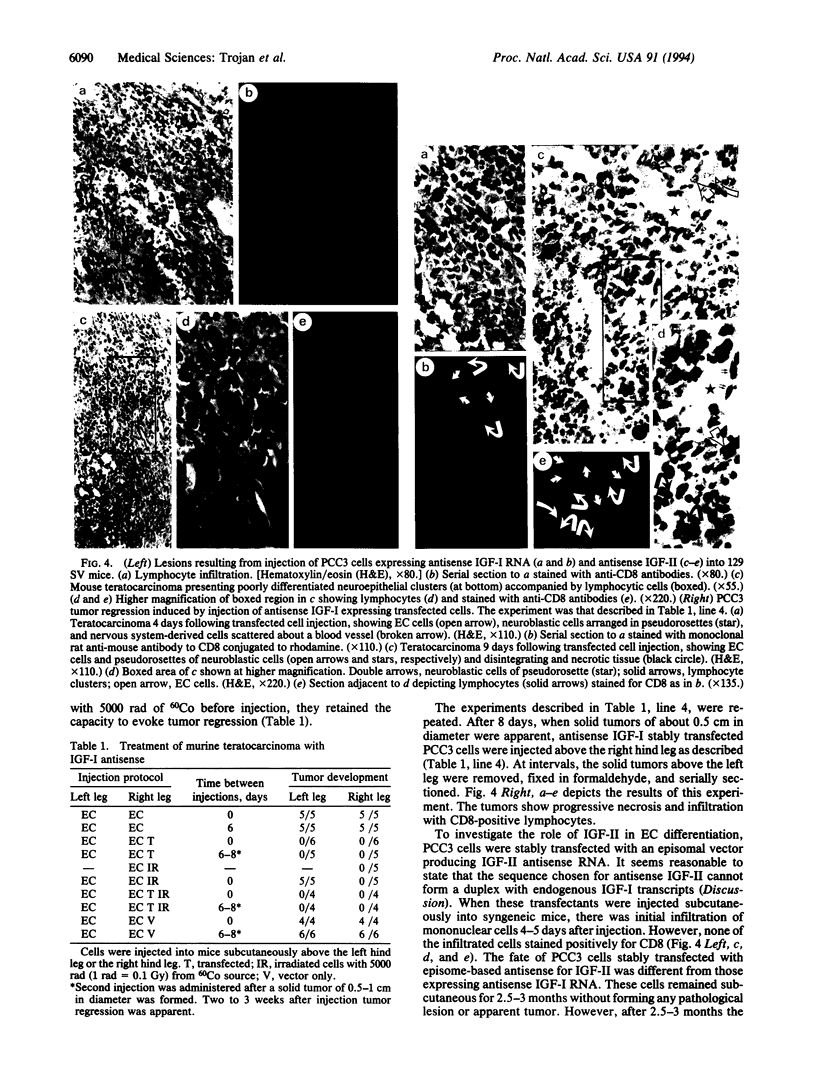
Teratocarcinoma is a germ-line carcinoma giving rise to an embryoid tumor with structures derived from the three embryonic layers: mesoderm, endoderm, and ectoderm. Teratocarcinoma is widely used as an in vitro model system to study regulation of cell determination and differentiation during mammalian embryogenesis. Murine embryonic carcinoma (EC) PCC3 cells express insulin-like growth factor I(IGF-I) and its receptor, while all derivative tumor structures express IGF-I and IGF-II and their receptors. Therefore the system lends itself to dissect the role of these two growth factors during EC differentiation. With an episomal antisense strategy, we define a role for IGF-I in tumorigenicity and evasion of immune surveillance. Antisense IGF-I EC transfectants are shown to elicit a curative anti-tumor immune response with tumor regression at distal sites. In contrast, IGF-II is shown to drive determination and differentiation in EC cells. Since IGF-I and IGF-II bind to type I receptor and antisense sequence used for IGF-II cannot form duplex with endogenous IGF-I transcripts, it follows that this receptor is not involved in determination and differentiation.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Antoniades H. N., Galanopoulos T., Neville-Golden J., Maxwell M. Expression of insulin-like growth factors I and II and their receptor mRNAs in primary human astrocytomas and meningiomas; in vivo studies using in situ hybridization and immunocytochemistry. Int J Cancer. 1992 Jan 21;50(2):215–222. doi: 10.1002/ijc.2910500210. [DOI] [PubMed] [Google Scholar]
- Beck F., Samani N. J., Byrne S., Morgan K., Gebhard R., Brammar W. J. Histochemical localization of IGF-I and IGF-II mRNA in the rat between birth and adulthood. Development. 1988 Sep;104(1):29–39. doi: 10.1242/dev.104.1.29. [DOI] [PubMed] [Google Scholar]
- Benda P., Lightbody J., Sato G., Levine L., Sweet W. Differentiated rat glial cell strain in tissue culture. Science. 1968 Jul 26;161(3839):370–371. doi: 10.1126/science.161.3839.370. [DOI] [PubMed] [Google Scholar]
- Chen L., Ashe S., Brady W. A., Hellström I., Hellström K. E., Ledbetter J. A., McGowan P., Linsley P. S. Costimulation of antitumor immunity by the B7 counterreceptor for the T lymphocyte molecules CD28 and CTLA-4. Cell. 1992 Dec 24;71(7):1093–1102. doi: 10.1016/s0092-8674(05)80059-5. [DOI] [PubMed] [Google Scholar]
- Culouscou J. M., Remacle-Bonnet M., Garrouste F., Marvaldi J., Pommier G. Simultaneous production of IGF-I and EGF competing growth factors by HT-29 human colon cancer line. Int J Cancer. 1987 Nov 15;40(5):646–652. doi: 10.1002/ijc.2910400513. [DOI] [PubMed] [Google Scholar]
- Damjanov I., Solter D. Experimental teratoma. Curr Top Pathol. 1974;59:69–130. doi: 10.1007/978-3-642-65857-0_2. [DOI] [PubMed] [Google Scholar]
- Damjanov I. Teratocarcinoma stem cells. Cancer Surv. 1990;9(2):303–319. [PubMed] [Google Scholar]
- Foekens J. A., Portengen H., Janssen M., Klijn J. G. Insulin-like growth factor-1 receptors and insulin-like growth factor-1-like activity in human primary breast cancer. Cancer. 1989 Jun 1;63(11):2139–2147. doi: 10.1002/1097-0142(19890601)63:11<2139::aid-cncr2820631112>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- Fox N., Damjanov I., Knowles B. B., Solter D. Immunohistochemical localization of the mouse stage-specific embryonic antigen 1 in human tissues and tumors. Cancer Res. 1983 Feb;43(2):669–678. [PubMed] [Google Scholar]
- Fu X. X., Su C. Y., Lee Y., Hintz R., Biempica L., Snyder R., Rogler C. E. Insulinlike growth factor II expression and oval cell proliferation associated with hepatocarcinogenesis in woodchuck hepatitis virus carriers. J Virol. 1988 Sep;62(9):3422–3430. doi: 10.1128/jvi.62.9.3422-3430.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gansler T., Allen K. D., Burant C. F., Inabnett T., Scott A., Buse M. G., Sens D. A., Garvin A. J. Detection of type 1 insulinlike growth factor (IGF) receptors in Wilms' tumors. Am J Pathol. 1988 Mar;130(3):431–435. [PMC free article] [PubMed] [Google Scholar]
- Glick R. P., Unterman T. G., Van der Woude M., Blaydes L. Z. Insulin and insulin-like growth factors in central nervous system tumors. Part V: Production of insulin-like growth factors I and II in vitro. J Neurosurg. 1992 Sep;77(3):445–450. doi: 10.3171/jns.1992.77.3.0445. [DOI] [PubMed] [Google Scholar]
- Golumbek P. T., Lazenby A. J., Levitsky H. I., Jaffee L. M., Karasuyama H., Baker M., Pardoll D. M. Treatment of established renal cancer by tumor cells engineered to secrete interleukin-4. Science. 1991 Nov 1;254(5032):713–716. doi: 10.1126/science.1948050. [DOI] [PubMed] [Google Scholar]
- Hambor J. E., Hauer C. A., Shu H. K., Groger R. K., Kaplan D. R., Tykocinski M. L. Use of an Epstein-Barr virus episomal replicon for anti-sense RNA-mediated gene inhibition in a human cytotoxic T-cell clone. Proc Natl Acad Sci U S A. 1988 Jun;85(11):4010–4014. doi: 10.1073/pnas.85.11.4010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath J. K., Shi W. K. Developmentally regulated expression of insulin-like growth factors by differentiated murine teratocarcinomas and extraembryonic mesoderm. J Embryol Exp Morphol. 1986 Jun;95:193–212. [PubMed] [Google Scholar]
- Heldin C. H., Westermark B. Growth factors as transforming proteins. Eur J Biochem. 1989 Oct 1;184(3):487–496. doi: 10.1111/j.1432-1033.1989.tb15041.x. [DOI] [PubMed] [Google Scholar]
- Jing N. H., Shiurba R., Kitani H., Kawakatsu H., Tomooka Y., Sakakura T. Secretion of polypeptides related to epidermal growth factor and insulinlike growth factor I by a human teratocarcinoma cell line. In Vitro Cell Dev Biol. 1991 Nov;27A(11):864–872. doi: 10.1007/BF02630989. [DOI] [PubMed] [Google Scholar]
- Macaulay V. M., Everard M. J., Teale J. D., Trott P. A., Van Wyk J. J., Smith I. E., Millar J. L. Autocrine function for insulin-like growth factor I in human small cell lung cancer cell lines and fresh tumor cells. Cancer Res. 1990 Apr 15;50(8):2511–2517. [PubMed] [Google Scholar]
- Martin G. R., Evans M. J. Differentiation of clonal lines of teratocarcinoma cells: formation of embryoid bodies in vitro. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1441–1445. doi: 10.1073/pnas.72.4.1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin G. R. Teratocarcinomas and mammalian embryogenesis. Science. 1980 Aug 15;209(4458):768–776. doi: 10.1126/science.6250214. [DOI] [PubMed] [Google Scholar]
- Meinkoth J., Wahl G. Hybridization of nucleic acids immobilized on solid supports. Anal Biochem. 1984 May 1;138(2):267–284. doi: 10.1016/0003-2697(84)90808-x. [DOI] [PubMed] [Google Scholar]
- Mintz B., Illmensee K. Normal genetically mosaic mice produced from malignant teratocarcinoma cells. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3585–3589. doi: 10.1073/pnas.72.9.3585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montarras D., Pinset C., Pérez M. C., Ilan J., Gros F. Muscle differentiation: insulin-like growth factors as positive modulators of myogenic regulatory genes? C R Acad Sci III. 1993 Sep;316(9):1025–1031. [PubMed] [Google Scholar]
- Nagarajan L., Anderson W. B., Nissley S. P., Rechler M. M., Jetten A. M. Production of insulin-like growth factor-II (MSA) by endoderm-like cells derived from embryonal carcinoma cells: possible mediator of embryonic cell growth. J Cell Physiol. 1985 Aug;124(2):199–206. doi: 10.1002/jcp.1041240205. [DOI] [PubMed] [Google Scholar]
- Nicolas J. F., Avner P., Gaillard J., Guenet J. L., Jakob H., Jacob F. Cell lines derived from teratocarcinomas. Cancer Res. 1976 Nov;36(11 Pt 2):4224–4231. [PubMed] [Google Scholar]
- Palmiter R. D., Norstedt G., Gelinas R. E., Hammer R. E., Brinster R. L. Metallothionein-human GH fusion genes stimulate growth of mice. Science. 1983 Nov 18;222(4625):809–814. doi: 10.1126/science.6356363. [DOI] [PubMed] [Google Scholar]
- Pierce G. B. Teratocarcinoma: model for a developmental concept of cancer. Curr Top Dev Biol. 1967;2:223–246. doi: 10.1016/s0070-2153(08)60289-6. [DOI] [PubMed] [Google Scholar]
- Plautz G. E., Yang Z. Y., Wu B. Y., Gao X., Huang L., Nabel G. J. Immunotherapy of malignancy by in vivo gene transfer into tumors. Proc Natl Acad Sci U S A. 1993 May 15;90(10):4645–4649. doi: 10.1073/pnas.90.10.4645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roholl P. J., Skottner A., Prinsen I., Lips C. J., Den Otter W., Van Unnik J. A. Expression of insulin-like growth factor 1 in sarcomas. Histopathology. 1990 May;16(5):455–460. doi: 10.1111/j.1365-2559.1990.tb01544.x. [DOI] [PubMed] [Google Scholar]
- Shen S. J., Daimon M., Wang C. Y., Jansen M., Ilan J. Isolation of an insulin-like growth factor II cDNA with a unique 5' untranslated region from human placenta. Proc Natl Acad Sci U S A. 1988 Mar;85(6):1947–1951. doi: 10.1073/pnas.85.6.1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend S. E., Allison J. P. Tumor rejection after direct costimulation of CD8+ T cells by B7-transfected melanoma cells. Science. 1993 Jan 15;259(5093):368–370. doi: 10.1126/science.7678351. [DOI] [PubMed] [Google Scholar]
- Trojan J., Blossey B. K., Johnson T. R., Rudin S. D., Tykocinski M., Ilan J., Ilan J. Loss of tumorigenicity of rat glioblastoma directed by episome-based antisense cDNA transcription of insulin-like growth factor I. Proc Natl Acad Sci U S A. 1992 Jun 1;89(11):4874–4878. doi: 10.1073/pnas.89.11.4874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trojan J., Johnson T. R., Rudin S. D., Ilan J., Tykocinski M. L., Ilan J. Treatment and prevention of rat glioblastoma by immunogenic C6 cells expressing antisense insulin-like growth factor I RNA. Science. 1993 Jan 1;259(5091):94–97. doi: 10.1126/science.8418502. [DOI] [PubMed] [Google Scholar]
- Williams D. W., Williams E. D., Wynford-Thomas D. Evidence for autocrine production of IGF-1 in human thyroid adenomas. Mol Cell Endocrinol. 1989 Jan;61(1):139–143. doi: 10.1016/0303-7207(89)90199-8. [DOI] [PubMed] [Google Scholar]
- Yang D. Y., Rogler C. E. Analysis of insulin-like growth factor II (IGF-II) expression in neoplastic nodules and hepatocellular carcinomas of woodchucks utilizing in situ hybridization and immunocytochemistry. Carcinogenesis. 1991 Oct;12(10):1893–1901. doi: 10.1093/carcin/12.10.1893. [DOI] [PubMed] [Google Scholar]
- van Zoelen E. J., Ward-van Oostwaard T. M., Nieuwland R., van der Burg B., van den Eijnden-van Raaij A. J., Mummery C. L., De Laat S. W. Identification and characterization of polypeptide growth factors secreted by murine embryonal carcinoma cells. Dev Biol. 1989 May;133(1):272–283. doi: 10.1016/0012-1606(89)90318-7. [DOI] [PubMed] [Google Scholar]