Abstract
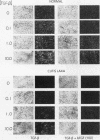
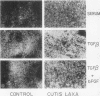
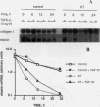
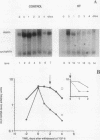
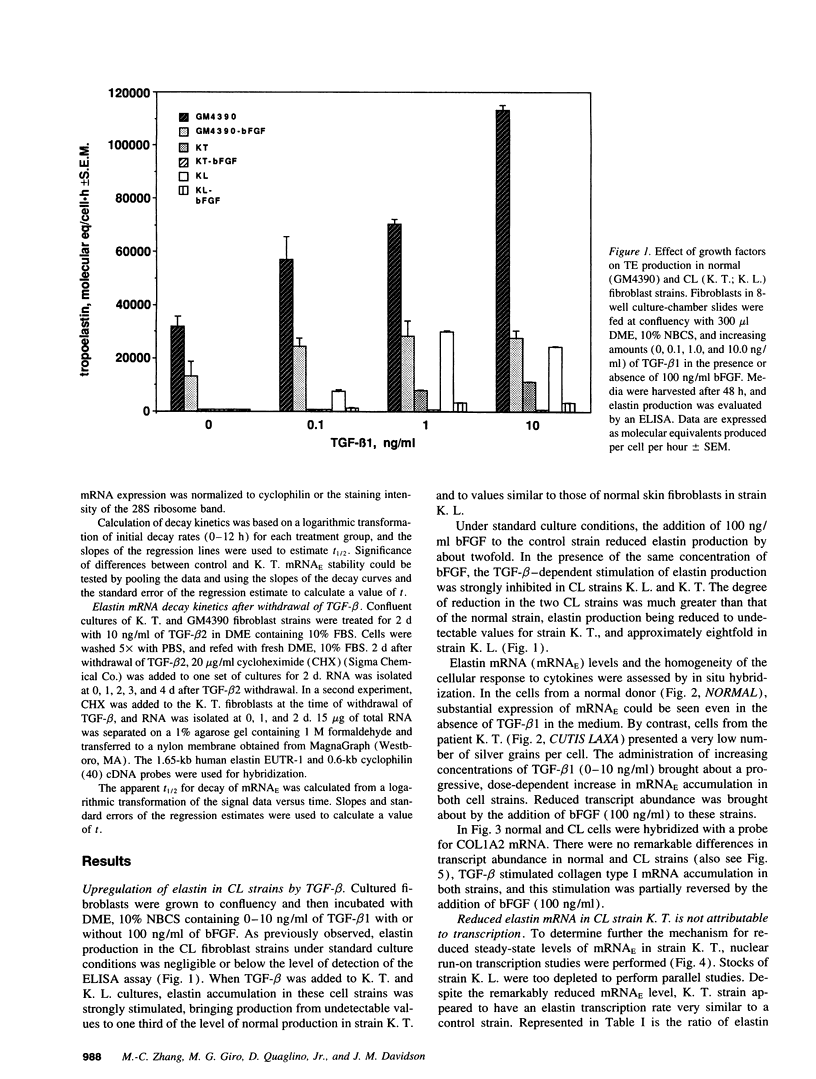
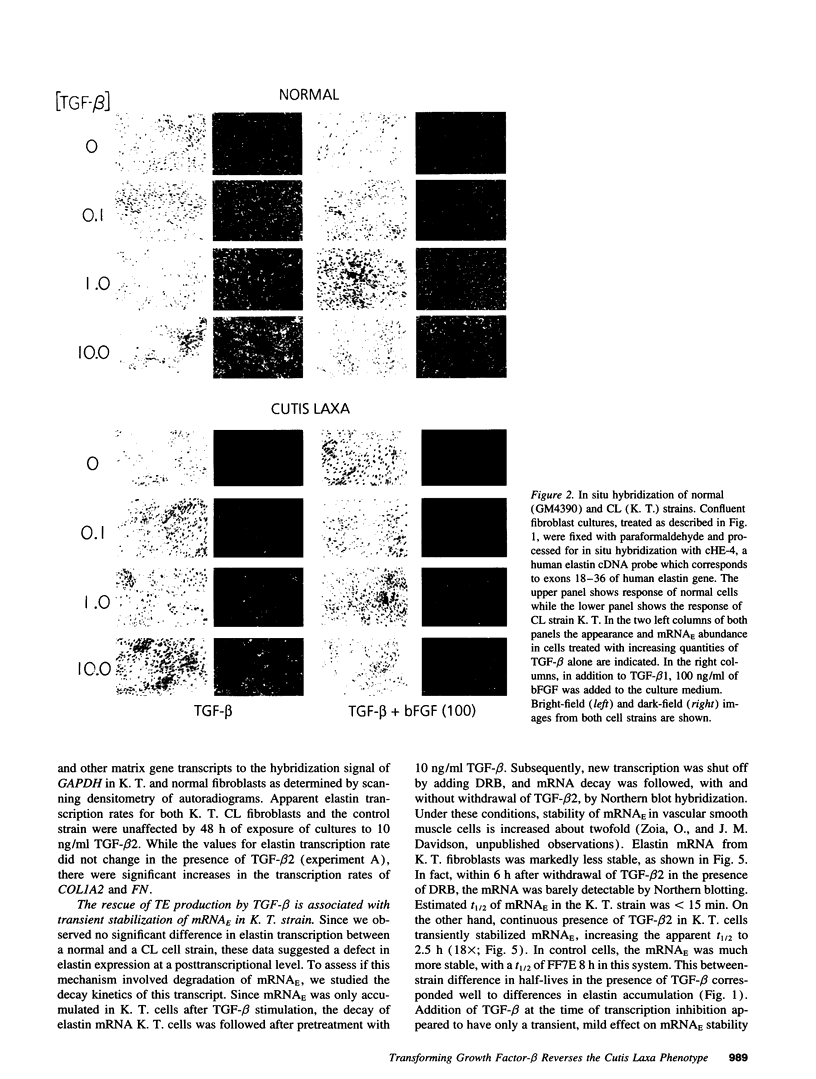
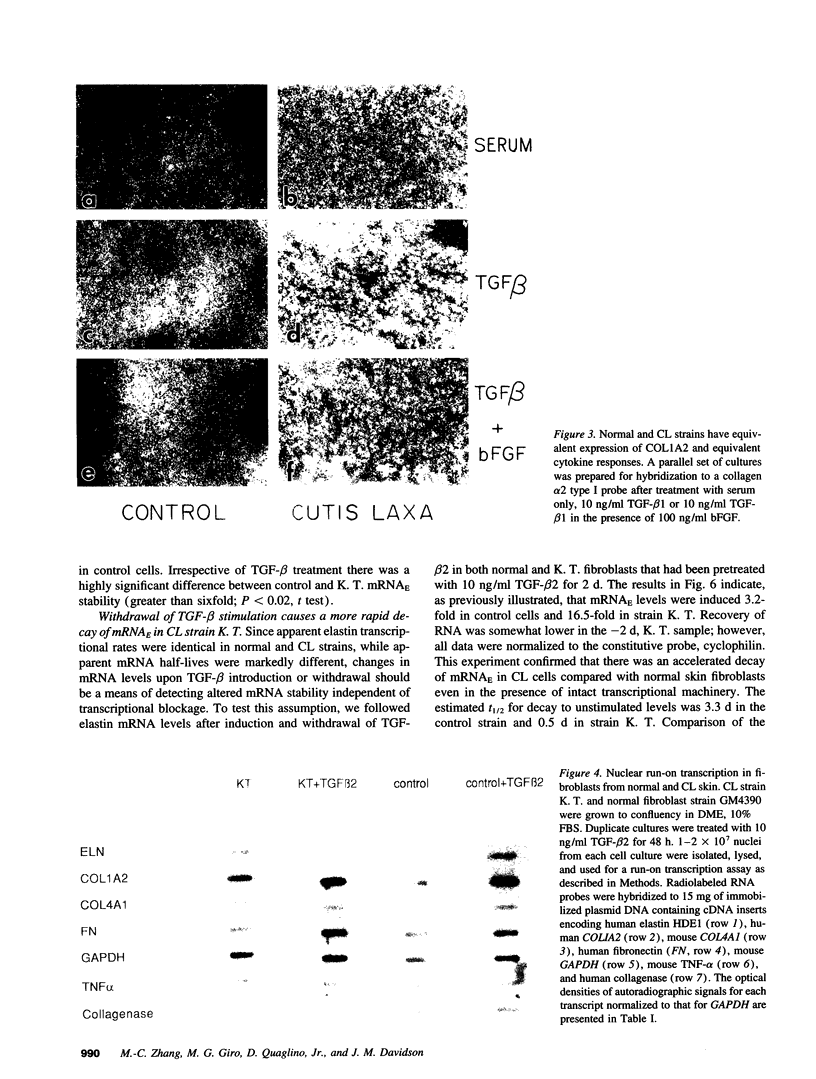
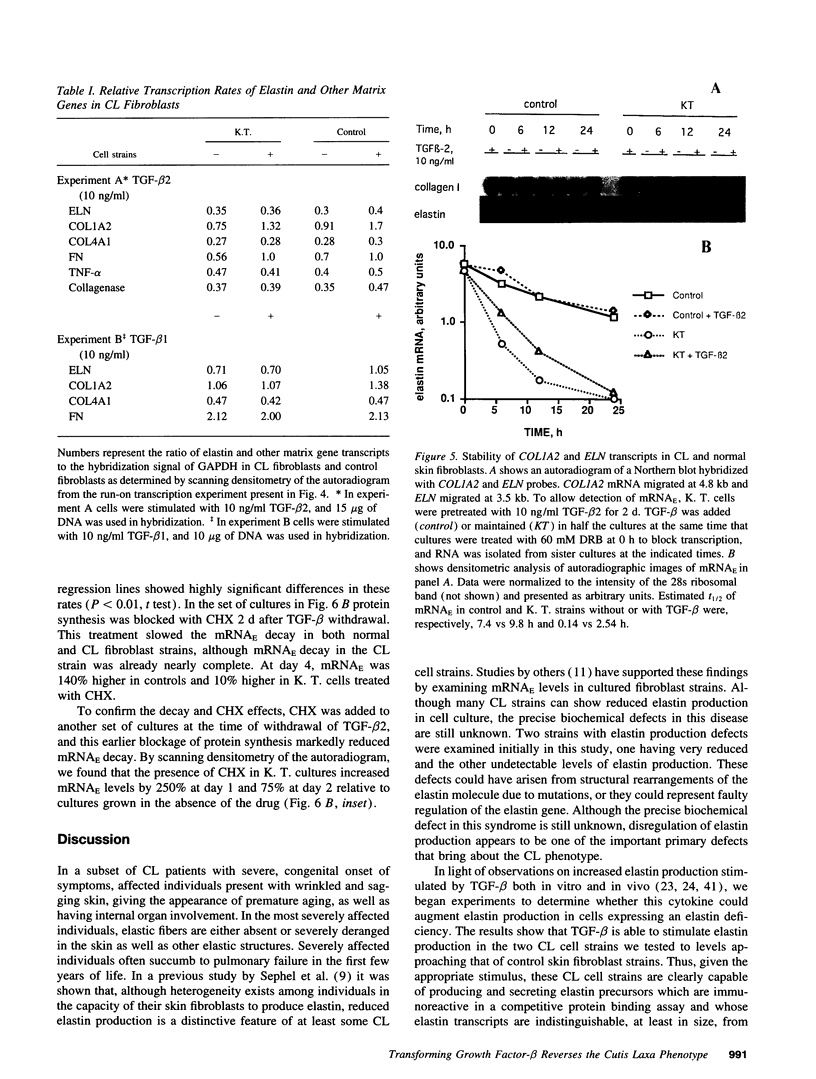
Skin fibroblasts from two cases of autosomal recessive cutis laxa (CL), having insignificant elastin production and mRNA levels, were challenged with transforming growth factor beta-1 (TGF-beta 1). Elastin production was brought from undetectable values to amounts typical of normal human skin fibroblasts in a dose-dependent fashion. Basic fibroblast growth factor (100 ng/ml) alone or in combination with TGF-beta 1 reduced elastin production and mRNA expression in CL skin fibroblasts more extensively than in normal cells. In situ hybridization showed that these effects were at the transcript level. One of the CL strains was examined in detail. Transcription rates for elastin were similar in normal and CL and unchanged by TGF-beta 1 or TGF-beta 2 (10 ng/ml), while in CL elastin mRNA half-life was increased > 10-fold by TGF-beta 2 and reduced 6-fold after TGF-beta 2 withdrawal, as compared with a control strain. Cycloheximide partially reversed elastin mRNA instability. These data are consistent with a defect in elastin mRNA stability that requires synthesis of labile factors or intact translational machinery, resulting in an extremely low steady state level of mRNA present in this strain of CL. Furthermore, TGF-beta can relieve elastin mRNA instability in at least one CL strain and elastin production defects in both CL strains.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Angel P., Baumann I., Stein B., Delius H., Rahmsdorf H. J., Herrlich P. 12-O-tetradecanoyl-phorbol-13-acetate induction of the human collagenase gene is mediated by an inducible enhancer element located in the 5'-flanking region. Mol Cell Biol. 1987 Jun;7(6):2256–2266. doi: 10.1128/mcb.7.6.2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beighton P. The dominant and recessive forms of cutis laxa. J Med Genet. 1972 Jun;9(2):216–221. doi: 10.1136/jmg.9.2.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard M. P., Kolbe M., Weil D., Chu M. L. Human cellular fibronectin: comparison of the carboxyl-terminal portion with rat identifies primary structural domains separated by hypervariable regions. Biochemistry. 1985 May 21;24(11):2698–2704. doi: 10.1021/bi00332a016. [DOI] [PubMed] [Google Scholar]
- Bernard M. P., Myers J. C., Chu M. L., Ramirez F., Eikenberry E. F., Prockop D. J. Structure of a cDNA for the pro alpha 2 chain of human type I procollagen. Comparison with chick cDNA for pro alpha 2(I) identifies structurally conserved features of the protein and the gene. Biochemistry. 1983 Mar 1;22(5):1139–1145. doi: 10.1021/bi00274a023. [DOI] [PubMed] [Google Scholar]
- Braverman I. M., Fonferko E. Studies in cutaneous aging: I. The elastic fiber network. J Invest Dermatol. 1982 May;78(5):434–443. doi: 10.1111/1523-1747.ep12507866. [DOI] [PubMed] [Google Scholar]
- Brettell L. M., McGowan S. E. Basic fibroblast growth factor decreases elastin production by neonatal rat lung fibroblasts. Am J Respir Cell Mol Biol. 1994 Mar;10(3):306–315. doi: 10.1165/ajrcmb.10.3.8117449. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Curran M. E., Atkinson D. L., Ewart A. K., Morris C. A., Leppert M. F., Keating M. T. The elastin gene is disrupted by a translocation associated with supravalvular aortic stenosis. Cell. 1993 Apr 9;73(1):159–168. doi: 10.1016/0092-8674(93)90168-p. [DOI] [PubMed] [Google Scholar]
- Danielson P. E., Forss-Petter S., Brow M. A., Calavetta L., Douglass J., Milner R. J., Sutcliffe J. G. p1B15: a cDNA clone of the rat mRNA encoding cyclophilin. DNA. 1988 May;7(4):261–267. doi: 10.1089/dna.1988.7.261. [DOI] [PubMed] [Google Scholar]
- Davidson J. M., Zoia O., Liu J. M. Modulation of transforming growth factor-beta 1 stimulated elastin and collagen production and proliferation in porcine vascular smooth muscle cells and skin fibroblasts by basic fibroblast growth factor, transforming growth factor-alpha, and insulin-like growth factor-I. J Cell Physiol. 1993 Apr;155(1):149–156. doi: 10.1002/jcp.1041550119. [DOI] [PubMed] [Google Scholar]
- Ewart A. K., Morris C. A., Atkinson D., Jin W., Sternes K., Spallone P., Stock A. D., Leppert M., Keating M. T. Hemizygosity at the elastin locus in a developmental disorder, Williams syndrome. Nat Genet. 1993 Sep;5(1):11–16. doi: 10.1038/ng0993-11. [DOI] [PubMed] [Google Scholar]
- Fafeur V., Terman B. I., Blum J., Böhlen P. Basic FGF treatment of endothelial cells down-regulates the 85-KDa TGF beta receptor subtype and decreases the growth inhibitory response to TGF-beta 1. Growth Factors. 1990;3(3):237–245. doi: 10.3109/08977199009043908. [DOI] [PubMed] [Google Scholar]
- Fazio M. J., Olsen D. R., Kuivaniemi H., Chu M. L., Davidson J. M., Rosenbloom J., Uitto J. Isolation and characterization of human elastin cDNAs, and age-associated variation in elastin gene expression in cultured skin fibroblasts. Lab Invest. 1988 Mar;58(3):270–277. [PubMed] [Google Scholar]
- Foster J., Rich C. B., Florini J. R. Insulin-like growth factor I, somatomedin C, induces the synthesis of tropoelastin in aortic tissue. Coll Relat Res. 1987 Aug;7(3):161–169. doi: 10.1016/s0174-173x(87)80007-9. [DOI] [PubMed] [Google Scholar]
- Giro M. G., Duvic M., Smith L. T., Kennedy R., Rapini R., Arnett F. C., Davidson J. M. Buschke-Ollendorff syndrome associated with elevated elastin production by affected skin fibroblasts in culture. J Invest Dermatol. 1992 Aug;99(2):129–137. doi: 10.1111/1523-1747.ep12616769. [DOI] [PubMed] [Google Scholar]
- Giro M. G., Oikarinen A. I., Oikarinen H., Sephel G., Uitto J., Davidson J. M. Demonstration of elastin gene expression in human skin fibroblast cultures and reduced tropoelastin production by cells from a patient with atrophoderma. J Clin Invest. 1985 Feb;75(2):672–678. doi: 10.1172/JCI111746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giro M., Davidson J. M. Familial co-segregation of the elastin phenotype in skin fibroblasts from Hutchinson-Gilford progeria. Mech Ageing Dev. 1993 Aug 15;70(3):163–136. doi: 10.1016/0047-6374(93)90046-t. [DOI] [PubMed] [Google Scholar]
- Horton W. E., Jr, Higginbotham J. D., Chandrasekhar S. Transforming growth factor-beta and fibroblast growth factor act synergistically to inhibit collagen II synthesis through a mechanism involving regulatory DNA sequences. J Cell Physiol. 1989 Oct;141(1):8–15. doi: 10.1002/jcp.1041410103. [DOI] [PubMed] [Google Scholar]
- Katchman S. D., Hsu-Wong S., Ledo I., Wu M., Uitto J. Transforming growth factor-beta up-regulates human elastin promoter activity in transgenic mice. Biochem Biophys Res Commun. 1994 Aug 30;203(1):485–490. doi: 10.1006/bbrc.1994.2208. [DOI] [PubMed] [Google Scholar]
- Kähäri V. M., Chen Y. Q., Bashir M. M., Rosenbloom J., Uitto J. Tumor necrosis factor-alpha down-regulates human elastin gene expression. Evidence for the role of AP-1 in the suppression of promoter activity. J Biol Chem. 1992 Dec 25;267(36):26134–26141. [PubMed] [Google Scholar]
- Kähäri V. M., Olsen D. R., Rhudy R. W., Carrillo P., Chen Y. Q., Uitto J. Transforming growth factor-beta up-regulates elastin gene expression in human skin fibroblasts. Evidence for post-transcriptional modulation. Lab Invest. 1992 May;66(5):580–588. [PubMed] [Google Scholar]
- Labarca C., Paigen K. A simple, rapid, and sensitive DNA assay procedure. Anal Biochem. 1980 Mar 1;102(2):344–352. doi: 10.1016/0003-2697(80)90165-7. [DOI] [PubMed] [Google Scholar]
- Lewis P. G., Hood A. F., Barnett N. K., Holbrook K. A. Postinflammatory elastolysis and cutis laxa. A case report. J Am Acad Dermatol. 1990 Jan;22(1):40–48. doi: 10.1016/0190-9622(90)70005-3. [DOI] [PubMed] [Google Scholar]
- Liu J. M., Davidson J. M. The elastogenic effect of recombinant transforming growth factor-beta on porcine aortic smooth muscle cells. Biochem Biophys Res Commun. 1988 Aug 15;154(3):895–901. doi: 10.1016/0006-291x(88)90224-0. [DOI] [PubMed] [Google Scholar]
- Marchase P., Holbrook K., Pinnell S. R. A familial cutis laxa syndrome with ultrastructural abnormalities of collagen and elastin. J Invest Dermatol. 1980 Nov;75(5):399–403. doi: 10.1111/1523-1747.ep12523655. [DOI] [PubMed] [Google Scholar]
- Marigo V., Volpin D., Bressan G. M. Regulation of the human elastin promoter in chick embryo cells. Tissue-specific effect of TGF-beta. Biochim Biophys Acta. 1993 Feb 20;1172(1-2):31–36. doi: 10.1016/0167-4781(93)90265-f. [DOI] [PubMed] [Google Scholar]
- Marigo V., Volpin D., Vitale G., Bressan G. M. Identification of a TGF-beta responsive element in the human elastin promoter. Biochem Biophys Res Commun. 1994 Mar 15;199(2):1049–1056. doi: 10.1006/bbrc.1994.1335. [DOI] [PubMed] [Google Scholar]
- Mecham R. P., Morris S. L., Levy B. D., Wrenn D. S. Glucocorticoids stimulate elastin production in differentiated bovine ligament fibroblasts but do not induce elastin synthesis in undifferentiated cells. J Biol Chem. 1984 Oct 25;259(20):12414–12418. [PubMed] [Google Scholar]
- Mehregan A. H., Lee S. C., Nabai H. Cutis laxa (generalized elastolysis). A report of four cases with autopsy findings. J Cutan Pathol. 1978 Jun;5(3):116–126. doi: 10.1111/j.1600-0560.1978.tb00948.x. [DOI] [PubMed] [Google Scholar]
- Muthukumaran G., Blumberg B., Kurkinen M. The complete primary structure for the alpha 1-chain of mouse collagen IV. Differential evolution of collagen IV domains. J Biol Chem. 1989 Apr 15;264(11):6310–6317. [PubMed] [Google Scholar]
- Olsen D. R., Fazio M. J., Shamban A. T., Rosenbloom J., Uitto J. Cutis laxa: reduced elastin gene expression in skin fibroblast cultures as determined by hybridizations with a homologous cDNA and an exon 1-specific oligonucleotide. J Biol Chem. 1988 May 15;263(14):6465–6467. [PubMed] [Google Scholar]
- Parks W. C., Kolodziej M. E., Pierce R. A. Phorbol ester-mediated downregulation of tropoelastin expression is controlled by a posttranscriptional mechanism. Biochemistry. 1992 Jul 28;31(29):6639–6645. doi: 10.1021/bi00144a003. [DOI] [PubMed] [Google Scholar]
- Pennica D., Hayflick J. S., Bringman T. S., Palladino M. A., Goeddel D. V. Cloning and expression in Escherichia coli of the cDNA for murine tumor necrosis factor. Proc Natl Acad Sci U S A. 1985 Sep;82(18):6060–6064. doi: 10.1073/pnas.82.18.6060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quaglino D., Jr, Nanney L. B., Ditesheim J. A., Davidson J. M. Transforming growth factor-beta stimulates wound healing and modulates extracellular matrix gene expression in pig skin: incisional wound model. J Invest Dermatol. 1991 Jul;97(1):34–42. [PubMed] [Google Scholar]
- Quaglino D., Jr, Nanney L. B., Kennedy R., Davidson J. M. Transforming growth factor-beta stimulates wound healing and modulates extracellular matrix gene expression in pig skin. I. Excisional wound model. Lab Invest. 1990 Sep;63(3):307–319. [PubMed] [Google Scholar]
- Reed W. B., Horowitz R. E., Beighton P. Acquired cutis laxa. Primary generalized elastolysis. Arch Dermatol. 1971 Jun;103(6):661–669. [PubMed] [Google Scholar]
- Rosenbloom J. Elastin: relation of protein and gene structure to disease. Lab Invest. 1984 Dec;51(6):605–623. [PubMed] [Google Scholar]
- Saksela O., Moscatelli D., Rifkin D. B. The opposing effects of basic fibroblast growth factor and transforming growth factor beta on the regulation of plasminogen activator activity in capillary endothelial cells. J Cell Biol. 1987 Aug;105(2):957–963. doi: 10.1083/jcb.105.2.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sephel G. C., Byers P. H., Holbrook K. A., Davidson J. M. Heterogeneity of elastin expression in cutis laxa fibroblast strains. J Invest Dermatol. 1989 Jul;93(1):147–153. doi: 10.1111/1523-1747.ep12277389. [DOI] [PubMed] [Google Scholar]
- Uitto J., Shamban A. Heritable skin diseases with molecular defects in collagen or elastin. Dermatol Clin. 1987 Jan;5(1):63–84. [PubMed] [Google Scholar]
- West D. C., Sattar A., Kumar S. A simplified in situ solubilization procedure for the determination of DNA and cell number in tissue cultured mammalian cells. Anal Biochem. 1985 Jun;147(2):289–295. doi: 10.1016/0003-2697(85)90274-x. [DOI] [PubMed] [Google Scholar]