Abstract
We develop an institutional pediatric stroke database at West Virginia University to support the classification and description of clinical and radiographic characteristics of children with stroke in West Virginia.
Methods
A custom-made database was developed using Microsoft Access to include specific query forms for data retrieval. Data were collected retrospectively from electronic medical record of pediatric patients with ischemic and hemorrhagic stroke, with emphasis on clinical presentation, risk factors and neuroimaging studies, between 2000 and 2012.
Results
In the children group cardiac disease was over-represented over vasculitis and hypercoagulable disorders. Neonates diagnosed with acute stroke were almost exclusively very sick or symptomatic patents.
Conclusion
Paediatric stroke in rural areas might be under-detected, particularly in neonates or in patients with mild or transient neurological signs. Patients with very high risk for stroke were over-represented in our registry; this support the need for increased awareness about paediatric stroke among practitioners and emergency room doctors in rural locations.
Keywords: Paediatric stroke, Neonatal Stroke, Risk Factors
Introduction
Stroke has a reported incidence of 1.3 to 13 per 100,000 children per year (1–5) and it is one of the top 10 causes of death among children. Paediatric stroke including acute ischemic stroke (AIS), haemorrhagic stroke (HS), and cerebral sinovenous thrombosis (CSVT) results in significant long-term morbidity in this population (6–7). Stroke in children has a multifactorial aetiology (8–9) with congenital cardiac disorders, vascular disorders, pro-thrombotic states and head and neck infections being the most common risk factors (10–12). Early identification of risk factors allows for rapid diagnosis, implementation of treatment, and preventive strategies.
The goal of the study was to retrospectively determine the most common causes of paediatric stroke in patient referred to West Virginia University Hospitals from the state of West Virginia, western Maryland, South-western Pennsylvania and eastern Ohio, and to compare our population with published data.
Methods
This is retrospective chart review of all pediatric stroke patients admitted to West Virginia University Hospital from 2000 to 2011. Patients referred to our institution from the state of West Virginia, western Maryland, Southwestern Pennsylvania and Eastern Ohio. Patients were identified by using ICD-9 codes for arterial ischemic stroke, CSVT or hemorrhagic stroke. Data were collected retrospectively from electronic medical records, with particular emphasis on patient’s clinical presentation, risk factors, and neuroimaging studies.
Inclusion Criteria
Paediatric patients 0 to 18 years old with AIS, CSVT, or hemorrhagic stroke confirmed by head CT, MRI or ultrasound.
Exclusion criteria
All patients with no neuroimaging confirmation were excluded from the study. Neonates (0 to 28 days) with intraventricular hemorrhage (IVH) and children identified to have subdural or epidural hematomas resulting from head trauma were excluded.
Data collection
A custom-made database was developed using Microsoft Access, including specific query forms for data retrieval. Data was collected retrospectively from patients’ admission charts. Data entered in the database included patient demographics, type of stroke, clinical presentation, radiological investigation, and identified risk factors. Clinical presentation was classified into focal signs (hemiparesis, visual field defect, speech defect, and sensory defect), or diffuse signs including level of consciousness, headache, nausea and vomiting (children), and difficulty maintaining respiration or feeding, lethargy or abnormal level of consciousness (neonates). Seizures as time of presentation, and thromboembolic events preceding or concurrent with the time of the stroke were identified.
Medical charts were reviewed for identification of the following risk factors: 1) cardiac disorders, including congenital heart disease, acquired heart disease, previous cardiac surgeries for congenital malformations (with stroke occurring more than or less than 72 hours after cardiac surgery), and isolated PFO; 2) Infections, including head and neck infection such as otitis media, sinusitis, encephalitis and meningitis, sepsis, varicella and herpes zoster; 3) vascular disorders, including arterial dissection, vasculitis, sickle-cell arteriopathy, and vascular malformations; 4) hypercoagulability, including MTHFR mutation, prothrombin, acquired thrombophilia, protein S deficiency, protein C deficiency, DIC, antithrombin III, antiphospholipd syndrome and anti-cardiolipin antibody.; and 5) chronic systemic conditions such as sickle cell diseases, anemia, hematological malignancies, connective tissue disorder, brain tumors and hypertension.
Neuroimaging data included information regarding the initial, confirmatory diagnostic test (CT, MRI, or ultrasound). Details included the vascular territory of infarction such as anterior, posterior distribution, and information regarding the side of involvement.
Results
Population Characteristics
We identified 187 patients with an ICD-9 diagnosis of ischemic or hemorrhagic stroke or CVST. Of these, 133 patients were confirmed to have AIS, CVST, or hemorrhagic stroke by at least one neuroradiologic investigation. Of these 133 patients, 31 (23%) were neonates and 102 (77%) were children; 65% of children and 68% of neonates were males.
Clinical Findings and Stroke Type
Table 1 summarizes all clinical findings at stroke onset. Eighty-five percent of children presented with focal neurological signs. Forty-nine percent had hemiparesis (left, n = 21; right, n = 14), and three had bilateral weakness; in six cases the side of the deficit could not be confirmed by chart review. Twenty-six children (30%) had visual field defect (left, n = 4; right, n = 2; bilateral, n = 9; unclear, n = 11). Speech deficit (aphasia, n = 5; non-specified, n = 6) was noted in 11 children (13%). Seven children (8%) reported to have sensory deficit (left, n = 5, right n = 2).
Table 1.
Clinical symptoms in neonates and children.
| Neonates, n (%) | Children, n (%) | |
|---|---|---|
| Clinical Symptoms | ||
| Seizures | 29 (93) | 44 (43) |
| Fever | 2 | 13 (13) |
| Headache | 0 | 31 (30) |
| Pneumonia | 0 | 9 |
| Dehydration & hypernatremia | 4 | 6 |
| Focal Clinical Signs | 14 (48) | 87 (85) |
| Hemiparesis | 5 | 46 (53) |
| Left | 0 | 21 |
| Right | 3 | 14 |
| Bilateral weakness | 2 | 3 |
| Unclear | 1 | 6 |
| Visual field deficit | 4 | 26 (30) |
| Left | 1 | 4 |
| Right | 0 | 2 |
| Bilateral | 1 | 9 |
| Unclear | 2 | 11 |
| Speech deficit | 0 | 16 (18) |
| Aphasia | 0 | 5 |
| Unclear | 0 | 11 |
| Sensory deficit | 1 | 7 |
| Left | 1 | 5 |
| Right | 0 | 2 |
43% of children had a seizure at onset; 13% presented with fever at the time of diagnosis, and 30% had an associated headache. Six children out of 102 (6%) were found to have dehydration and hypernatremia.
Within neonate group, ten patients (32%) presented with focal neurological signs. Five neonates (16%) presented with hemiparesis (right, n = 3), and two had bilateral weakness; in four neonates, the side of the deficit could not be confirmed by chart review. Four neonates (13%) were reported to have visual field defect (left, n = 1; bilateral, n = 1; unclear, n = 2). One patient was reported to have sensory deficit.
Twenty-nine neonates (93%) had a history of seizure at the time of diagnosis. 20% had fever at the time of diagnosis. All neonates with CSVT presented with seizures and had acute systemic symptoms (dehydration and hypernatremia) at the time of diagnosis.
Figure 1 and table 2 summarize the type of stroke that was identified by neuroimaging modalities. Sixty-seven percent of children presented with AIS; 20% presented with intracerebral hemorrhage (ICH); 9% had CSVT. Two children had both AIS and CSVT, one child had both ICH and CSVT, and two had AIS and ICH.
Figure 1.
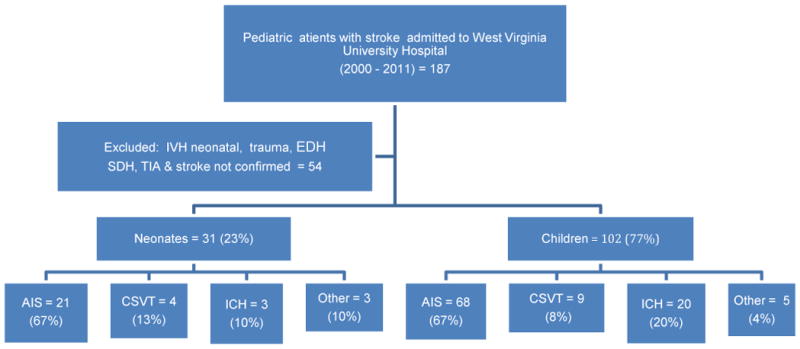
Flow chart showing summary of all patients. AIS = Acute ischemic stroke, CSVT = Cerebral Sinovenous Thrombosis, IVH = Intraventricular Hemorrhage, SDH= Subdural Hematoma, EDH=Epidural Hematoma, TIA =Transient Ischemic Attack.
Table 2.
Stroke types in neonates and children.
| Type of stroke | Neonates, n (%) | Children, n (%) |
|---|---|---|
| AIS | 21 (67) | 68 (67) |
| CSVT | 4 (13) | 9 (9) |
| AIS and CSVT | 2 | 2 |
| AIS and SAH | 1 | 0 |
| ICH | 3 (10) | 20 (20) |
| ICH and CSVT | 0 | 1 |
| ICH and AIS | 0 | 2 |
AIS = Acute ischemic stroke; CSVT = Cerebral Sinovenous Thrombosis; ICH = Intracerebral Hemorrhage; SAH = Subarchnoid Hemorrhage.
Twenty-one neonates (67%) presented with AIS; four neonates (13%) had CSVT, three neonates (10%) presented with intra-cerebral hemorrhage (ICH), two neonates had both CSVT and infarction, and one neonate had AIS with associated sub-arachnoid hemorrhage.
Neuroimaging
CT scan was obtained as the initial neuroimaging modality in 56% of children; head MRI was obtained as the initial imaging study in 44% of cases. Head ultrasound was the initial study in three neonates (10%); CT scan was the initial, diagnostic study in 60% of neonates, while MRI was the study in the remaining 30%.
Table 3 summarizes neuroimaging finding regarding vascular territory distribution.
Table 3.
Vascular territory by neuroimaging investigations.
| Acute Ischemic Stroke | Haemorrhagic Stroke | |||
|---|---|---|---|---|
| Neonates n (%) | Children n (%) | Neonates n (%) | Children n (%) | |
| Distribution | ||||
| Anterior | 11 (52) | 46 (68) | 8 (40) | |
| Posterior | 5 (24) | 16 (23) | 1 | 5 (25) |
| Both | 5 (24) | 6 (9) | 2 | 7(35) |
| Laterality | ||||
| Left | 8 (38) | 23 (34) | - | 6 (30) |
| Right | 7 (33) | 20 (29) | - | 4 (20) |
| Bilateral | 6 (29) | 25 (37) | 3 | 10 (50) |
Fifty- two percent of neonates had anterior circulation involvements, 24% had lesions in the posterior circulation and 24% had multiple lesions with involvement of both anterior and posterior circulation territory. In regard to side of lesion, 38% had left-sided infarction, 33% had right-side involvement, and 29%) of patients had bilateral lesions.
Among children with AIS, 68% had anterior circulation involvements, 23% had lesions in the posterior circulation, 9% had multiple lesions with involvement of both anterior and posterior vascular territories. In relation to side of the lesion, 34% of children had left-sided infarction, 29% had right-side involvement, and 37% had bilateral lesions
In the hemorrhagic group, 40% of children had anterior fossa involvement, 25% had posterior fossa hemorrhage, and 35% had multiple hemorrhages. In relation to side of the lesion, 30% of children had left-sided involvement, 20% had right-side involvement, and 50% had bilateral lesions.
Three neonates presented with hemorrhagic stroke. One-third had posterior fossa hemorrhage, and two-third had multiple bleeds. In relation to side of the lesion, all three neonates with hemorrhagic stroke had bilateral involvement.
Risk Factors
Several risk factors - summarized in Table 4 - were identified in our patients.
Table 4.
Identified risk factors in neonates and children with stroke.
| Risk factors | Neonates n (%) | Children n (%) |
|---|---|---|
| Cardiac disorders | 13(42) | 32 (31) |
| Congenital heart diseases | 13 | 26 |
| Acquired heart diseases | 0 | 6 |
| Isolated Patent Foramen Ovale | 2 | 0 |
| Previously (>72hrs) operated for CHD | 2 | 10 |
| Stroke at cardiac surgery (<72 hrs) | 1 | 1 |
| Vascular diseases | 1 | 15 (14) |
| Moyamoya | 0 | 3 |
| Dissection (2 carotid, 1 vertebral) | 0 | 3 |
| Cerebral aneurysm rupture | 0 | 2 |
| Multiple cavernous hemangioma | 0 | 1 |
| A–V malformation | 1 | 6 |
| Hypercoagulability/malignancy | 1 | 14 (13) |
| MTHFR mutation | 0 | 1 |
| Unspecified prothrombotic state | 0 | 6 |
| Factor V Leiden mutation | 0 | 1 |
| Antithrombin 3 | 0 | 1 |
| Low protein S level | 0 | 1 |
| Antiphospholipid syndrome | 0 | 1 |
| DIC | 1 | 1 |
| Anti cardiolipin antibody | 0 | 1 |
| Hematological malignancy | 0 | 1 |
| Infection | ||
| Head/neck (OM, Meningitis, Enceph) | 5 (16) | 24 (23) |
| Herpes Zoster | 0 | 2 |
| Varicella | 0 | 2 |
| Sepsis | 1 | 5 |
| Hypertension | 0 | 1 |
| Brain Tumor | 1 | 7 |
| Connective tissue disease | 0 | 1 |
| Undetermined causes | 8 (26) | 14 (14) |
We were able to identify a clear risk factor in 109 patients, including 88 children and 23 neonates. At least one risk factor was identified in 71% of neonates and 86% of children.
Thirty-four patients had cardiac disorders including 13 neonates (42%) with congenital heart disease and 32 children. In the children group 25.5% had congenital heart disease, and 6% presented with acquired heart diseases. Eleven children and three neonates developed stroke following surgical repair of cardiac malformation; only one child and one neonate were diagnosed within 72 hours of surgery.
Sixteen patients (11%, 15 children and one neonate) had vascular disorders including Moyamoya disease (n=3), dissection (n=3; 2 in external carotid artery, one in the vertebral artery), cerebral aneurysm rupture (n=2), multiple cavernous hemangiomas (n=1) and A-V malformation (n= 6).
Twenty-nine patients presented with infections including 24 (23%) children and five neonates (16%) with head and neck infection (otitis media, meningitis and encephalitis). Six patients (5 children and one neonate) had sepsis. Two children had varicella infection, and two had herpes zoster infection.
Fifteen patients (11.5%) were found to have hypercoagulability including MTHFR mutation (n=1), factor V Leiden mutation (n=1), antithrombin III (n=1), low protein S level (n=1), antiphospholipid syndrome (n=1), DIC (n=1), anti-cardiolipin antibody (n=1), and unspecified prothrombotic state (n=5); one child was found to have hematological malignancy.
Discussion
AIS incidence ranges from 1.2 to 8 per 100,000 children per year (1–6) with hemorrhagic stroke accounting for about half of these cases (12–14); stroke is more common in males and African Americans (4, 5, 12), possibly in relation to the high incidence of sickle cell disease in this population. The incidence of CSVT in children varies between 0.4 and 0.7 per 100,000 per year (15–17) with more than 40% of cases occurring within the neonatal period, with an incidence for this age group of 2.6 per 100,000 per year (6,18).
We are reporting the first collection of cases of pediatric stroke in the state of West Virginia with particular focus on identification of risk factors, and a comparison with existing literature in order to identify similarities or differences in our Appalachian population.
Sixty-seven percent of our patients (67% of children and 68% of neonates) presented with AIS. The reason for this high prevalence of ischemic lesions - higher then the one reported in the literature-, as opposed to hemorrhagic strokes, remains unclear. This difference could potentially be related to preferential transfer of patients with ICH to a different regional hospital. In the neonates, the exclusion IVH grade I-IV could be the reason for this unusual finding.
Neonatal AIS, which occurs in 1 per 4000 term births, (19–21) presents with seizures in up to 72% of cases (19). In our population 93% of neonates with AIS had seizures at onset. This finding raises a concern that only neonates with clear neurologic symptoms like seizures were correctly identified around the time of birth.
Our findings in neonates with CSVT are similar to published data, with the majority of neonates (81%) presenting within the first week of life (18). However, in our population all neonates with CSVT presented with seizures, which are the most common clinical presentation (~80% of cases) along with lethargy, irritability, poor feeding, apnea, jitteriness, changes in muscle tone or diffuse neurologic signs (18, 22). This finding again suggests that only neonates with clear neurologic symptoms were timely identified. Published data demonstrated that between 61% and 84% of neonates with CVST have associated acute systemic illnesses at the time of diagnosis (22, 23). However, all our neonates with CVST had an associated systemic illness at the time of diagnosis, particularly dehydration and electrolytes imbalances. This suggests a preferential identification of CVST only in the sickest neonates. Under-diagnosed CSVT in the least severe group could have been related to limited diagnostic investigations, or to delayed imaging with occurrence of recanalization.
50% of children with CSVT presented with seizure, and 63% had an acute systemic illness at the time of diagnosis; these data is similar to the findings documented in published literature (24).
Congenital heart disease is responsible for up to one third of pediatric strokes (9). Stroke associated with cardiac disorders was the most common finding in our patients as 42% of neonates and 31% of children had a stroke related to cardiac disorders. Stroke related to cardiac disease in our children population was similar to value reported by a recent large study by the IPSS group, where cardiac disease was second most common risk factor for pediatric stroke (9). The higher association with cardiac disease in our population of neonates could be a relative over-representation due to lack of identification of other causative factors (i.e. vasculopathy), or be related to a higher incidence of patients with congenital heart disease at our institution, which is the main referral center in the state for pediatric cardiovascular surgery.
Only 16% of our cases were identified to have a vasculopathy. This number is substantially lower than the reported incidence of arteriopathies in a large recent multicentric study (53%) (9). This finding might suggests arteriopathies were under-detected in our population. However, it should be noticed that only a handful of patients with sickle cell disease are followed at our institution, therefore eliminating a significant population of patients with arteriopathy. Nevertheless, the importance of complete vascular studies, if necessary with different modalities (CTA, or MRA) for identification of arterial lesions should not be undermined.
No identifiable risk factor was found in 16% of our patients, as compared 9% of children with no identifiable risk factors reported in the literature (9). This suggests incomplete investigation for identification of risk factors in our population.
The most common causes of hemorrhagic stroke in children are vascular malformations including artero-venous malformations (AVMs), aneurysms, and cavernous malformations. All together these lesions cause 5% 29% of cerebral hemorrhages in children (13, 14, 25, 26). Other important causes of HS in pediatrics are hematological disorders, such as thrombocytopenia or hemophilia, or neoplasms (14, 27). In our population, AVM was the most common cause of hemorrhagic stroke. While generally AVMs are diagnosed in patients between 20 and 40 years, about 20% of patients can become symptomatic during childhood; therefore, as identification of these lesions plays important role in preventive care, high index of suspicion is mandatory.
CT scan was the initial neuroimaging study in 56% of our patients. This is significantly lower when compared to large studies reporting that 96% of children with symptoms concerning for stroke had brain CT scan as initial neuroimaging study (9, 27). MRI was the initial neuroimaging study in 30% of our neonates, which is higher that reported (9). This high percentage of MRI as first diagnostic test is possibly related to identification of stroke in neonates who were admitted to our hospital severe medical conditions. This again, suggests that stroke could have been preferentially diagnosed only in the sickest neonates who were transferred from rural locations to our facility.
CT scan was the first imaging choice in emergency setting both in our institution of before transfer from other rural hospital when stroke was suspected in neonates. Given the known risk resulting from radiation, particularly in young children, this finding is concerning and suggest the need to encourage rural institution to pursue rapid transfer to larger hospitals with availability of MR scans.
Limitations of this study include its retrospective nature, which resulted in missing data in a number of patients. Additionally, retrospective identification of patients by discharge ICD-9 code it is known to be highly inaccurate, with reported accuracy ranging from 37 to 88% (28).
Our data suggest that in rural areas paediatric stroke might be under-detected, particularly in neonates or in patients with mild or transient neurological signs. This support the need for increased awareness among general practitioners and emergency room doctors working in rural locations. As paediatric stroke can results from multiple pathogenetic mechanisms, a multidisciplinary competence is required and complete investigation of potential risk factor is necessary for identification of potentially treatable causes and prevention of recurrence. Radiological confirmation is mandatory, and it should be obtained when the child is first seen, for early diagnostic verification.
References
- 1.Lynch JK, Hirtz DG, DeVeber G, et al. Report of the National Institute of Neurological Disorders and Stroke Workshop on Perinatal and Childhood Stroke. Pediatrics. 2002;109:116–123. doi: 10.1542/peds.109.1.116. [DOI] [PubMed] [Google Scholar]
- 2.Lynch JK, Han CJ. Pediatric stroke: what do we know and what do we need to know? Seminars in Neurology. 2005;25(4):410–423. doi: 10.1055/s-2005-923535. [DOI] [PubMed] [Google Scholar]
- 3.Giroud M, Lemesle M, Gouyon JB, et al. Cerebrovascular disease in children under 16 years of age in the city of Dijon, France: A study of incidence and clinical features from 1985 to 1993. J Clin Epidemiol. 1995;48:1343–1348. doi: 10.1016/0895-4356(95)00039-9. [DOI] [PubMed] [Google Scholar]
- 4.Earley CJ, Kittner SJ, Feeser BR, et al. Stroke in children and sickle-cell disease. Baltimore-Washington Cooperative Young Stroke Study. Neurology. 1998;51:169–176. doi: 10.1212/wnl.51.1.169. [DOI] [PubMed] [Google Scholar]
- 5.Fullerton HJ, Wu YW, Zhao S, Johnston SC. Risk of stroke in children: Ethnic and gender disparities. Neurology. 2003;61:189–194. doi: 10.1212/01.wnl.0000078894.79866.95. [DOI] [PubMed] [Google Scholar]
- 6.Schoenberg BS, Mellinger JF, Schoenberg DG. Cerebrovascular disease in infants and children: A study of incidence, clinical features, and survival. Neurology. 1978;28:763–768. doi: 10.1212/wnl.28.8.763. [DOI] [PubMed] [Google Scholar]
- 7.Eeg-Olofsson O, Ringheim Y. Stroke in children. Clinical characteristics and prognosis. Acta Paediatr Scand. 1983;72:391–395. doi: 10.1111/j.1651-2227.1983.tb09734.x. [DOI] [PubMed] [Google Scholar]
- 8.Ganesan V, Prengler M, McShane MA, et al. Investigation of risk factors in children with arterial ischemic stroke. Ann Neurol. 2003;53:167–173. doi: 10.1002/ana.10423. [DOI] [PubMed] [Google Scholar]
- 9.Mackay MT, Wiznitzer M, Benedict SL, et al. Arterial ischemic stroke risk factors: the International Pediatric Stroke Study. Ann Neurol. 2011;69:130–140. doi: 10.1002/ana.22224. [DOI] [PubMed] [Google Scholar]
- 10.Lo W, Stephens J, Fernandez S. Pediatric stroke in the United States and the impact of risk factors. Journal of Child Neurology. 2009;24(2):194–202. doi: 10.1177/0883073808322665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bernard TJ, Goldenberg NA. Pediatric arterial ischemic stroke. Hematology/Oncology Clinics of North America. 2010;24(1):167–180. doi: 10.1016/j.hoc.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 12.Broderick J, Talbot GT, Prenger E, et al. Stroke in children within a major metropolitan area: the surprising importance of intracerebral hemorrhage. J Child Neurol. 1993;8:250–255. doi: 10.1177/088307389300800308. [DOI] [PubMed] [Google Scholar]
- 13.Fullerton HJ, Achrol AS, Johnston SC, et al. Long-term hemorrhage risk in children versus adults with brain arteriovenous malformations. Stroke. 2005;36:2099–2104. doi: 10.1161/01.STR.0000181746.77149.2b. [DOI] [PubMed] [Google Scholar]
- 14.Lo WD, Lee J, Rusin J, et al. Intracranial hemorrhage in children: an evolving spectrum. Archives of Neurology. 2008;65(12):1629–163. doi: 10.1001/archneurol.2008.502. [DOI] [PubMed] [Google Scholar]
- 15.DeVeber G, Andrew M, Adams C, et al. Canadian Pediatric Ischemic Stroke Study Group. Cerebral sinovenous thrombosis in children. N Engl J Med. 2001 Aug 9;345(6):417–23. doi: 10.1056/NEJM200108093450604. [DOI] [PubMed] [Google Scholar]
- 16.Dlamin N, Billinghurst FJ, Kirkham L. Cerebral venous sinus (sinovenous) thrombosis in children. Neurosurg Clin N Am. 2010 Jul;21(3):511–27. doi: 10.1016/j.nec.2010.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grunt S, Wingeier K, Wehrli E, et al. Cerebral sinus venous thrombosis in Swiss children. Dev Med Child Neurol. 2010 Dec;52(12):1145–50. doi: 10.1111/j.1469-8749.2010.03722.x. [DOI] [PubMed] [Google Scholar]
- 18.Jordan JC, Rafay MF, Smith SE, et al. International Pediatric Stroke Study Group. Antithrombotic treatment in neonatal cerebral sinovenous thrombosis: results of the International Pediatric Stroke Study. J Pediatr. 2010 May;156(5):704–10. doi: 10.1016/j.jpeds.2009.11.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kirton A, Armstrong-Wells J, et al. Symptomatic neonatal arterial ischemic stroke: the International Pediatric Stroke Study, International Pediatric Stroke Study Investigators. Pediatrics. 2011;128(6):1402–10. doi: 10.1542/peds.2011-1148. [DOI] [PubMed] [Google Scholar]
- 20.Laugesaar R, Kolk A, Tomberg T, et al. Acutely and retrospectively diagnosed perinatal stroke: a population-based study. Stroke. 2007;38(8):2234–2240. doi: 10.1161/STROKEAHA.107.483743. [DOI] [PubMed] [Google Scholar]
- 21.Ramenghi LA, Bassi L, Fumagalli M, et al. Neonatal stroke. Minerva Pediatr. 2010 Jun;62(3 Suppl 1):177–9. Review. [PubMed] [Google Scholar]
- 22.Jordan LC, Rafay MF, Smith SE, et al. Antithrombotic treatment in neonatal cerebral sinovenous thrombosis: results of the International Pediatric Stroke Study. J Pediatr. 2010 May;156(5):704–10. doi: 10.1016/j.jpeds.2009.11.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Berfelo FJ, Kersbergen KJ, van Ommen CH, et al. Neonatal cerebral sinovenous thrombosis from symptom to outcome. Stroke. 2010 Jul;41(7):1382–8. doi: 10.1161/STROKEAHA.110.583542. [DOI] [PubMed] [Google Scholar]
- 24.Dlamini N, Billinghurst L, Kirkham FJ. Cerebral venous sinus (sinovenous) thrombosis in children. Neurosurg Clin N Am. 2010 Jul;21(3):511–27. doi: 10.1016/j.nec.2010.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meyer-Heim AD, Boltshauser E. Spontaneous intracranial haemorrhage in children: aetiology, presentation and outcome. Brain and Development. 2003;25(6):416–421. doi: 10.1016/s0387-7604(03)00029-9. [DOI] [PubMed] [Google Scholar]
- 26.Jordan LC, Hillis AE. Hemorrhagic stroke in children. Pediatr Neurol. 2007 Feb;36(2):73–80. doi: 10.1016/j.pediatrneurol.2006.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yock-Corrales A, Barnett P. The Role of Imaging Studies for Evaluation of Stroke in Children. Pediatric Emergency Care. 2011;10(27):966–974. doi: 10.1097/PEC.0b013e318230a002. [DOI] [PubMed] [Google Scholar]
- 28.Golomb MR, Garg BP, Saha C, et al. Accuracy and yield of ICD-9 codes for identifying children with ischemic stroke. Neurology. 2006;67(11):2053–2067. doi: 10.1212/01.wnl.0000247281.98094.e2. [DOI] [PubMed] [Google Scholar]


