Abstract
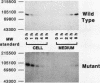
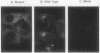
We studied the defect responsible for deficiency of the b subunit for factor XIII in the first known case of this condition. The patient is a compound heterozygote of two genetic defects: deletion of A-4161 at the acceptor splice junction of intron A, resulting in a loss of the obligatory AG splicing sequence; and, replacement of G-11499 by T in exon VIII, resulting in an amino acid substitution of Cys430 by Phe. To determine how the latter mutation impaired b subunit synthesis, recombinant b subunit bearing the mutation was expressed in BHK cells. The mutant as well as wild-type b subunit was synthesized by the cells. However, the apparent molecular weight of the mutant was slightly higher than those of the wild-type and plasma b subunits under nonreducing conditions, probably because of destruction of a disulfide bond. The mutant b subunit was secreted from the cells much less effectively than the wild type and remained susceptible to endoglycosidase H, indicating that it was not transported from the endoplasmic reticulum to the Golgi apparatus where the processing of oligosaccharides occurs. Immunofluorescence study suggested that the mutant protein was retained in the endoplasmic reticulum. These studies demonstrate that a Cys430-Phe mutation does not prevent the de novo synthesis of the b subunit, but alters the conformation of the mutant protein sufficiently to impair its intracellular transport, resulting in its deficiency in this patient.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Board P., Coggan M., Miloszewski K. Identification of a point mutation in factor XIII A subunit deficiency. Blood. 1992 Aug 15;80(4):937–941. [PubMed] [Google Scholar]
- Bottenus R. E., Ichinose A., Davie E. W. Nucleotide sequence of the gene for the b subunit of human factor XIII. Biochemistry. 1990 Dec 25;29(51):11195–11209. doi: 10.1021/bi00503a007. [DOI] [PubMed] [Google Scholar]
- Brantly M., Courtney M., Crystal R. G. Repair of the secretion defect in the Z form of alpha 1-antitrypsin by addition of a second mutation. Science. 1988 Dec 23;242(4886):1700–1702. doi: 10.1126/science.2904702. [DOI] [PubMed] [Google Scholar]
- Busby S. J., Mulvihill E., Rao D., Kumar A. A., Lioubin P., Heipel M., Sprecher C., Halfpap L., Prunkard D., Gambee J. Expression of recombinant human plasminogen in mammalian cells is augmented by suppression of plasmin activity. J Biol Chem. 1991 Aug 15;266(23):15286–15292. [PubMed] [Google Scholar]
- Capellato M. G., Lazzaro A. R., Marafioti F., Polato G., Girolami A. A new family with congenital factor XIII deficiency showing a deficit of both subunit A and B. Type I factor XIII deficiency. Haematologia (Budap) 1987;20(3):179–187. [PubMed] [Google Scholar]
- Carrell N. A., Erickson H. P., McDonagh J. Electron microscopy and hydrodynamic properties of factor XIII subunits. J Biol Chem. 1989 Jan 5;264(1):551–556. [PubMed] [Google Scholar]
- Cheng S. H., Gregory R. J., Marshall J., Paul S., Souza D. W., White G. A., O'Riordan C. R., Smith A. E. Defective intracellular transport and processing of CFTR is the molecular basis of most cystic fibrosis. Cell. 1990 Nov 16;63(4):827–834. doi: 10.1016/0092-8674(90)90148-8. [DOI] [PubMed] [Google Scholar]
- Curiel D. T., Chytil A., Courtney M., Crystal R. G. Serum alpha 1-antitrypsin deficiency associated with the common S-type (Glu264----Val) mutation results from intracellular degradation of alpha 1-antitrypsin prior to secretion. J Biol Chem. 1989 Jun 25;264(18):10477–10486. [PubMed] [Google Scholar]
- Curiel D. T., Holmes M. D., Okayama H., Brantly M. L., Vogelmeier C., Travis W. D., Stier L. E., Perks W. H., Crystal R. G. Molecular basis of the liver and lung disease associated with the alpha 1-antitrypsin deficiency allele Mmalton. J Biol Chem. 1989 Aug 15;264(23):13938–13945. [PubMed] [Google Scholar]
- Curiel D. T., Vogelmeier C., Hubbard R. C., Stier L. E., Crystal R. G. Molecular basis of alpha 1-antitrypsin deficiency and emphysema associated with the alpha 1-antitrypsin Mmineral springs allele. Mol Cell Biol. 1990 Jan;10(1):47–56. doi: 10.1128/mcb.10.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlbäck B., Smith C. A., Müller-Eberhard H. J. Visualization of human C4b-binding protein and its complexes with vitamin K-dependent protein S and complement protein C4b. Proc Natl Acad Sci U S A. 1983 Jun;80(11):3461–3465. doi: 10.1073/pnas.80.11.3461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girolami A., Burul A., Fabris F., Cappellato G., Betterle C. Studies on factor XIII antigen in congenital factor XIII deficiency. A tentative classification of the disease in two groups. Folia Haematol Int Mag Klin Morphol Blutforsch. 1978;105(1):131–141. [PubMed] [Google Scholar]
- Gross V., Andus T., Tran-Thi T. A., Schwarz R. T., Decker K., Heinrich P. C. 1-deoxynojirimycin impairs oligosaccharide processing of alpha 1-proteinase inhibitor and inhibits its secretion in primary cultures of rat hepatocytes. J Biol Chem. 1983 Oct 25;258(20):12203–12209. [PubMed] [Google Scholar]
- Hashiguchi T., Saito M., Morishita E., Matsuda T., Ichinose A. Two genetic defects in a patient with complete deficiency of the b-subunit for coagulation factor XIII. Blood. 1993 Jul 1;82(1):145–150. [PubMed] [Google Scholar]
- Hurtley S. M., Helenius A. Protein oligomerization in the endoplasmic reticulum. Annu Rev Cell Biol. 1989;5:277–307. doi: 10.1146/annurev.cb.05.110189.001425. [DOI] [PubMed] [Google Scholar]
- Ichinose A., Bottenus R. E., Davie E. W. Structure of transglutaminases. J Biol Chem. 1990 Aug 15;265(23):13411–13414. [PubMed] [Google Scholar]
- Ichinose A., Davie E. W. Characterization of the gene for the a subunit of human factor XIII (plasma transglutaminase), a blood coagulation factor. Proc Natl Acad Sci U S A. 1988 Aug;85(16):5829–5833. doi: 10.1073/pnas.85.16.5829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichinose A., Hendrickson L. E., Fujikawa K., Davie E. W. Amino acid sequence of the a subunit of human factor XIII. Biochemistry. 1986 Nov 4;25(22):6900–6906. doi: 10.1021/bi00370a025. [DOI] [PubMed] [Google Scholar]
- Ichinose A., Kaetsu H. Molecular approach to structure-function relationship of human coagulation factor XIII. Methods Enzymol. 1993;222:36–51. doi: 10.1016/0076-6879(93)22006-2. [DOI] [PubMed] [Google Scholar]
- Ichinose A., McMullen B. A., Fujikawa K., Davie E. W. Amino acid sequence of the b subunit of human factor XIII, a protein composed of ten repetitive segments. Biochemistry. 1986 Aug 12;25(16):4633–4638. doi: 10.1021/bi00364a027. [DOI] [PubMed] [Google Scholar]
- Janatova J., Reid K. B., Willis A. C. Disulfide bonds are localized within the short consensus repeat units of complement regulatory proteins: C4b-binding protein. Biochemistry. 1989 May 30;28(11):4754–4761. doi: 10.1021/bi00437a036. [DOI] [PubMed] [Google Scholar]
- Kamura T., Okamura T., Murakawa M., Tsuda H., Teshima T., Shibuya T., Harada M., Niho Y. Deficiency of coagulation factor XIII A subunit caused by the dinucleotide deletion at the 5' end of exon III. J Clin Invest. 1992 Aug;90(2):315–319. doi: 10.1172/JCI115864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato H., Enjyoji K. Amino acid sequence and location of the disulfide bonds in bovine beta 2 glycoprotein I: the presence of five Sushi domains. Biochemistry. 1991 Dec 17;30(50):11687–11694. doi: 10.1021/bi00114a012. [DOI] [PubMed] [Google Scholar]
- Klausner R. D., Sitia R. Protein degradation in the endoplasmic reticulum. Cell. 1990 Aug 24;62(4):611–614. doi: 10.1016/0092-8674(90)90104-m. [DOI] [PubMed] [Google Scholar]
- Kornfeld R., Kornfeld S. Assembly of asparagine-linked oligosaccharides. Annu Rev Biochem. 1985;54:631–664. doi: 10.1146/annurev.bi.54.070185.003215. [DOI] [PubMed] [Google Scholar]
- Lorand L., Jeong J. M., Radek J. T., Wilson J. Human plasma factor XIII: subunit interactions and activation of zymogen. Methods Enzymol. 1993;222:22–35. doi: 10.1016/0076-6879(93)22005-z. [DOI] [PubMed] [Google Scholar]
- Lorand L., Losowsky M. S., Miloszewski K. J. Human factor XIII: fibrin-stabilizing factor. Prog Hemost Thromb. 1980;5:245–290. [PubMed] [Google Scholar]
- Lyons S. E., Bruck M. E., Bowie E. J., Ginsburg D. Impaired intracellular transport produced by a subset of type IIA von Willebrand disease mutations. J Biol Chem. 1992 Mar 5;267(7):4424–4430. [PubMed] [Google Scholar]
- Miura O., Sugahara Y., Aoki N. Hereditary alpha 2-plasmin inhibitor deficiency caused by a transport-deficient mutation (alpha 2-PI-Okinawa). Deletion of Glu137 by a trinucleotide deletion blocks intracellular transport. J Biol Chem. 1989 Oct 25;264(30):18213–18219. [PubMed] [Google Scholar]
- Pelham H. R. Control of protein exit from the endoplasmic reticulum. Annu Rev Cell Biol. 1989;5:1–23. doi: 10.1146/annurev.cb.05.110189.000245. [DOI] [PubMed] [Google Scholar]
- Perkins S. J., Haris P. I., Sim R. B., Chapman D. A study of the structure of human complement component factor H by Fourier transform infrared spectroscopy and secondary structure averaging methods. Biochemistry. 1988 May 31;27(11):4004–4012. doi: 10.1021/bi00411a017. [DOI] [PubMed] [Google Scholar]
- Poncz M., Rifat S., Coller B. S., Newman P. J., Shattil S. J., Parrella T., Fortina P., Bennett J. S. Glanzmann thrombasthenia secondary to a Gly273-->Asp mutation adjacent to the first calcium-binding domain of platelet glycoprotein IIb. J Clin Invest. 1994 Jan;93(1):172–179. doi: 10.1172/JCI116942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodeghiero F., Tosetto A., Di Bona E., Castaman G. Clinical pharmacokinetics of a placenta-derived factor XIII concentrate in type I and type II factor XIII deficiency. Am J Hematol. 1991 Jan;36(1):30–34. doi: 10.1002/ajh.2830360107. [DOI] [PubMed] [Google Scholar]
- Saito M., Asakura H., Yoshida T., Ito K., Okafuji K., Yoshida T., Matsuda T. A familial factor XIII subunit B deficiency. Br J Haematol. 1990 Mar;74(3):290–294. doi: 10.1111/j.1365-2141.1990.tb02585.x. [DOI] [PubMed] [Google Scholar]
- Standen G. R., Bowen D. J. Factor XIII ABristol 1: detection of a nonsense mutation (Arg171-->stop codon) in factor XIII A subunit deficiency. Br J Haematol. 1993 Dec;85(4):769–772. doi: 10.1111/j.1365-2141.1993.tb03221.x. [DOI] [PubMed] [Google Scholar]
- Taylor J. W., Schmidt W., Cosstick R., Okruszek A., Eckstein F. The use of phosphorothioate-modified DNA in restriction enzyme reactions to prepare nicked DNA. Nucleic Acids Res. 1985 Dec 20;13(24):8749–8764. doi: 10.1093/nar/13.24.8749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolleshaug H., Hobgood K. K., Brown M. S., Goldstein J. L. The LDL receptor locus in familial hypercholesterolemia: multiple mutations disrupt transport and processing of a membrane receptor. Cell. 1983 Mar;32(3):941–951. doi: 10.1016/0092-8674(83)90079-x. [DOI] [PubMed] [Google Scholar]
- Tsuji E., Misumi Y., Fujiwara T., Takami N., Ogata S., Ikehara Y. An active-site mutation (Gly633-->Arg) of dipeptidyl peptidase IV causes its retention and rapid degradation in the endoplasmic reticulum. Biochemistry. 1992 Dec 1;31(47):11921–11927. doi: 10.1021/bi00162a035. [DOI] [PubMed] [Google Scholar]
- Verbanac K. M., Heath E. C. Biosynthesis, processing, and secretion of M and Z variant human alpha 1-antitrypsin. J Biol Chem. 1986 Jul 25;261(21):9979–9989. [PubMed] [Google Scholar]