Abstract
Objective
The idea that distinct psychosocial factors may underlie specific patterns of neuroendocrine stress responses has been a topic of recurrent debate. We examined a recent contribution to this debate, the Social Self Preservation Theory, which predicts that stressors involving social evaluative threat (SET) characteristically activate the hypothalamic-pituitary-adrenal (HPA) axis.
Methods
Sixty-one healthy university students (31 females) performed a challenging speech task in one of three conditions that aimed to impose increasing levels of SET: performing the task alone (no social evaluation), with 1 evaluating observer, or with 4 evaluating observers. Indices of sympathetic (pre-ejection period) and parasympathetic (heart rate variability) cardiac drive were obtained by impedance- and electrocardiography. Salivary cortisol was used to index HPA activity. Questionnaires assessed affective responses.
Results
Affective responses (shame/embarrassment, anxiety, negative affect, and self-esteem), cortisol, heart rate, sympathetic, and parasympathetic activation all differentiated evaluative from non-evaluative task conditions (p<.001). The largest effect-sizes were observed for cardiac autonomic responses. Physiological reactivity increased in parallel with increasing audience size (p<.001). A rise in cortisol was predicted by sympathetic activation during the task (p<.001), but not by affective responses.
Conclusion
It would appear that SET determines the magnitude, rather than the pattern, of physiological activation. This potential to broadly perturb multiple physiological systems may help explain why social stress has been associated with a range of health outcomes. We propose a threshold-activation model as a physiological explanation for why engaging stressors, such as those involving social evaluation or uncontrollability, may appear to selectively induce cortisol release.
Keywords: social evaluation, autonomic reactivity, HPA-axis, response specificity, shame, self-esteem, psychological stress
Introduction
Response specificity, the idea that particular characteristics of a stimulus or an individual are associated with distinct neuro-endocrine and physiological response patterns, is one of the fundamental assumptions in psychophysiology and a dominant hypothesis in bio-behavioral medicine (1-5). To most stress researchers this notion is intuitively obvious, buttressed by the evolutionary argument that different physiological response patterns are required to cope adaptively with different threats (6). Response specificity may also explain why certain types of stress have a more profound health impact than others, or why some stressors appear associated with specific pathologies (c.f., (4, 7, 8).
Given the central role of the hypothalamic-pituitary-adrenal (HPA) axis in the stress response and its important physiological functions (9, 10), substantial research has been directed at identifying the psychological determinants of its activation (11, 12). A guiding, but sometimes implicit, assumption is that the psychological determinants of HPA activation can be distinguished from those that activate other stress-response systems (e.g., sympathetic-adrenal-medullary (SAM) system or parasympathetic nervous system) (7, 11, 13, 14). For example, it has been proposed that stressors involving novelty, lack of control, or loss/harm-appraisals preferentially activate the HPA axis, whereas factors like effort, arousal, or challenge-appraisals drive SAM activation (7, 11, 13-15). However, the status of such models does not always appear to be matched by the strength of their empirical support; much of the supportive evidence takes the form of extrapolations from non-human studies, and the human data remain somewhat inconclusive (11). It is perhaps hardly surprising, then, that the specific psychological determinants of HPA activation continue to be a topic of research and debate.
The Social Self Preservation Theory is the most recent contribution to this debate (16, 17). This theory, formulated by Kemeny and co-workers, predicts that “… threats to the social self, or situations which threaten to demean one's social image or standing, engender a specific set of psychological and physiological reactions” (17). These specific reactions are proposed to be feelings of low social worth, accompanied by self-conscious emotions such as embarrassment and shame and, in the physiological domain, increases in hypothalamic-pituitary-adrenal (HPA) activation (16, 17). Key support for the theory was adduced from a meta-analytic review of 208 acute laboratory stress studies, which demonstrated that stress exposure paradigms characterized by social-evaluative threat, such as stressful situations in which an evaluative audience was present or in which the participant was the target of a negative social comparison, led to greater cortisol reactivity than paradigms in which social-evaluative threat was absent or minimal (11). The authors concluded that this meta-analysis supported a “stressorphysiology specificity perspective” (11).
Subsequent empirical support for this specificity perspective was provided by Gruenewald et al. (17). In this study participants underwent the Trier Social Stress Test (TSST) protocol (18), which involves delivering an impromptu speech and performing a mental arithmetic task, in the presence or absence of evaluative others. As predicted by Social Self Preservation Theory, performing the TSST in the presence of an evaluative audience provoked larger increases in self-reported shame/embarrassment than performing the task alone. Further, cortisol increased only when the TSST was performed with the audience present, whereas the two task conditions elicited largely similar increases in heart rate and blood pressure. These findings suggest that the SAM system responds less sensitive to social evaluative threat than the HPA-axis, reinforcing the view that there may be distinctive psychological determinants of HPA activation. At the same time, however, the observation that cardiovascular and autonomic indices minimally differentiate between evaluative and non-evaluative conditions conflicts with the general finding in social facilitation and social anxiety research (19, 20). Such research has shown that tasks involving high evaluative threat (for example caused by the presence of an observer, the presence of a high status observer, or because of being evaluated on valued traits like intelligence) provokes much larger cardiovascular and autonomic reactions than situations of low evaluative threat (19, 21, 22). Indeed, evaluated performance tasks, such as speech performance, have become routinely employed in stress research exactly because of their robust activation of the autonomic and cardiovascular system (23-25).
Considering the theoretical importance of the aforementioned study by Gruenewald et al. (2004), providing the strongest empirical support for the social-self preservation theory to date, replication using a more detailed assessment of autonomic nervous system activity seemed appropriate. Two additional major modifications were made to their original study protocol. First, we noted that the TSST protocol utilized by Gruenewald and co-workers required participants to repeatedly change between sitting and standing posture and to walk between different areas of the laboratory (17). While cortisol release is relatively insensitive to modest physical activity (26), movement and posture changes substantially affect cardiovascular and autonomic measures (27-31). Recent evidence indeed confirms that somatic activity confounds cardiovascular and autonomic responses during the TSST (32). In order to prevent such confounding we controlled movement by keeping study participants in the same position (sitting) through-out the pre-task baseline and all experimental procedures. Second, while the present study similarly manipulated social-evaluative threat by performing a demanding task (impromptu speech delivery) alone or in front of an evaluating audience, we included two different audience sizes (one observer or four observers) to manipulate levels of social evaluation.
Three specific hypotheses were tested. First, on the basis of the findings by Gruenewald et al. (17), it was expected that social evaluation would enhance cortisol reactivity but not, or only modestly, autonomic and cardiovascular reactivity. Second, on the same basis we further expected that cortisol reactivity, but not autonomic and cardiovascular activity, would increase in parallel with the degree of social evaluative threat, i.e., show a dose-dependent relationship with audience size (33). Third, to further test the specificity of HPA responses during evaluative threat, it was predicted that HPA activity would increase independently of autonomic and cardiovascular responses, but would correlate with increases in shame/embarrassment.
Methods
Participants
Sixty-one university undergraduates (31 women) participated in the present study (mean age 20.3; SD = ± 1.09; range = 18–24). Participants were recruited via advertisements in lecture rooms and by posters on campus, and were given course credit hours or paid £5 for completion of the study. Exclusion criteria were: (1) suffering from an immune, cardiovascular, metabolic, or kidney disorder; (2) current cold or respiratory infection; (3) use of prescribed medication (excluding the contraceptive pill) during the previous month; (4) pregnancy or suspected pregnancy; (5) being a smoker. Participants were instructed not to consume food or caffeinated beverages 2 hr before testing, and to abstain from alcohol and strenuous exercise 12 hr prior to testing. Based on research showing that females in the follicular phase and those taking oral contraception show comparable cortisol responses to stress and ACTH infusion (34), female participants were tested between days 4 to 7 post-menses, or, when oral contraceptives were used, on a day the pill was taken. The study was approved by the ethical review committee of the School of Sport Exercise Sciences. Data were collected between November 2005 and March 2006.
Procedures
Task preparations
Participants were invited to complete a single 2-hour afternoon testing session, commencing between 1.00pm and 4:00pm. On arrival at the laboratory informed consent was obtained and electrodes for impedance cardiography (ICG) and electrocardiography (ECG) were attached. Participants were comfortably seated in a chair with arm rests, and were instructed to maintain a similar posture throughout and minimize movement. The latter was facilitated by the use of supportive pillows, and by the positioning of legs in a box, which did not physically restrict movement but limited the perimeter for movement. Participants were then provided with a standardized snack (1178kJ; 43.1g carbohydrate, of which 26.3g glucose; 10.9g fat; 2.6g protein) and a glass of water (250cc). During the subsequent 45 min baseline period participants completed a set of questionnaires and engaged in quiet reading. During the final six minutes of this baseline, while still engaged in quiet reading, cardiac activity was assessed unobtrusively. At the completion of this baseline measurement the first saliva sample (“baseline”) was taken, and a second set of questionnaires was administered (described in detail below). Subsequently the task was explained and initiated.
Stress task
The task consisted of two back-to-back speeches, each with 2 minutes of preparation and 4 minutes of speech delivery, as described elsewhere (35, 36). For the first speech, participants were required to argue convincingly they were wrongly accused of shoplifting (37). For the second speech task participants were asked to reveal and explain three of their best and three of their worst characteristics (38). The total task duration was 15-minutes, including instructions. Task instructions and timing were standardized by use of a DVD which was played on a TV screen. The experimenter left the room after starting the DVD and remained outside during the task. Participants were informed that the experimenter could still be contacted though a 2-way radio.
Conditions
Participants were randomly allocated to one of three conditions, structured to provoke increasing social evaluative threat; (1) a non-evaluative, or no-audience, condition during which nobody was present while the participant undertook the speech tasks; (2) a social evaluation condition whereby a single audience member (of opposite sex) was present, and; (3) a social evaluation condition whereby four people (two men and two women) were present. Participants in the social evaluative conditions were told that the audience would be evaluating their speech performance and ability to communicate ideas successfully in a social situation. Participants in the non-evaluative condition were informed that they would perform the task alone in the room and that their performance was under no form of evaluation. The 2-way radio was merely used to check if the speeches were on the correct topic (all participants followed speech instructions). The specifics of the task condition were only revealed at the point when task preparations were initiated to avoid baseline differences in anticipatory arousal. After the task was described, audience members entered the room (in the social evaluative conditions) and were seated in front of the participant. Audience members were approximately the same age as the participants and trained to adopt a non-accepting and critical manner as described by Gruenewald et al. (2004). If the participants stopped speaking for a period exceeding 20 seconds the experimenter would prompt by sounding an alert through the 2-way radio. This procedure was to ensure that the participants spoke for the full period in all three conditions.
Immediately after completion of the speech tasks the experimenter re-entered the room, collected the second saliva sample and issued the third set of questionnaires. At the same time the audience left the room. During the subsequent 45-minute recovery period a saliva sample and mood questionnaire data was obtained at 15-min intervals.
Questionnaires
Health behaviors were assessed by questionnaire and included assessments of exercise, alcohol and caffeine consumption, sleep, health complaints, use of non-prescribed medication, and menstrual cycle phase. The Test Anxiety Scale (39), is a 37-item (“true/false”) questionnaire that assesses test anxiety as a situation-specific personality trait (α = 0.82); the Fear of Negative Evaluation scale (40) is a 12-item questionnaire (4-point Likert scale; 1 = very little to 4 = much) that provides an index of social anxiety (α = 0.94). The State Self Esteem Scale (41) was administered pre- and post-task. For this 20-item scale, respondents had to rate their current thoughts regarding confidence, social self esteem and performance on a five point Likert scale (1 = Not at all to 5 = Extremely). For the current study only the performance and social subscales were used (Performance αpre = 0.82, αpost = 0.88; Social αpre = 0.86, αpost = 0.91). At both pre-task and post-task time points participants were also administered an extended version of the Affect Balance Scale (ABS; (42), which is a 43-item (Likert format; 1 = Not at all to 5 = Very strong) measure of positive and negative affect. Participant's rated the emotions experienced over ‘the preceding minutes’ (baseline, recovery) or ‘during the task’. For the present study we analyzed the negative affect subscale anxiety subscale (e.g., nervous, timid, anxious; αpre = 0.78, αpost = 0.85), and the shame/embarrassment subscale1 (items: embarrassed, self-conscious, ashamed, humiliated; αpre = 0.74, αpost = 0.87). The latter subscale is an extension of the ABS developed by Gruenewald et al. (17). The pre- and post task questionnaires were supplemented by 7 single-item questions (using a 7-point Likert scale) assessing difficulty, stressfulness, arousal, performance, embarrassment, confusion and engagement. Pre-task items were formulated to assess task expectations (e.g., “how difficult do you expect to find the task”).
Cardiac and autonomic measures
Assessment of cardiac responses focused on cardiac sympathetic and parasympathetic control (35, 43, 44). Indices of sympathetic and vagal drive were obtained though analyses of ECG and thoracic ICG signals. Signals were recorded continuously throughout the experiment through six Ag/AgCl spot electrodes (AMI type 1650-005, Medtronic, Mineapolis, USA) using the Vrije Universiteit Ambulatory Monitoring Device (VU-AMD; Vrije Universiteit, Amsterdam, The Netherlands) (45). ECG and ICG complexes were ensemble averaged with reference to the ECG R-wave across one-minute time points. From these 1-min ensembles, average levels were computed for heart rate (HR), pre-ejection period (PEP), the Root Mean Square of Successive Differences (RMSSD), respiratory frequency (RF), a respiratory depth (tidal volume, TV). These minute-by-minute means were averaged over the 6-min pre-task baseline, each 6-min stressor (2 min preparation plus 4 min speech), and a 6-min recovery (15-min post task) (36, 46). PEP was used as an index of cardiac sympathetic drive, and RMSSD as a measure of cardiac parasympathetic activity (47, 48).
Saliva Collection and cortisol assessment
Saliva was collected using Salivettes (Sartstead, Oxford, UK). For each collection participants were instructed to place the Salivette under the tongue for 3-minutes and not to chew. Once the 3-minutes had elapsed subjects returned the salivette into a sealed plastic tube. The samples were centrifuged at room temperature for 5 minutes at 3000 × g, and saliva was divided into 500μl aliquots and frozen at −20°C until assayed. Salivary cortisol was measured using a competitive Enzyme-Linked Immunosorbent Assay (ELISA), and analysis was carried out according to the manufacturers' instructions (R&D Systems, Minneapolis, USA; Sensitivity <0.7 ng/ml, intra-assay variability 9.2%).
Statistical Analysis
An initial comparison of baseline differences was performed using a series of univariate ANOVAs. The psychological and physiological responses were examined using repeated measures MANOVAs, which treated the different sampling times (pre-task, post-task for psychological variables; baseline, task 1, task 2, recovery for cardiovascular and autonomic variables; baseline, post-task, +15 min, +30 min, and +45 min recovery for cortisol) as a within-subject factor and the condition as a between-subject factor. Separate time × condition ANOVAs compared the responses during each conditions in a pair-wise fashion. Two subjects had baseline cortisol levels > 3.5 SD above the mean, and were excluded from the analyses. Eta-squared (η2) is reported as a measure of effect size. Heart rate variability (RMSSD) was log transformed [ln (RMSSD+1)] for statistical analyses and also presented in Figures. Occasional missing data are reflected in the slight variations in degrees of freedom. Data were analyzed using SPSS 15.01 for Windows (SPSS Inc, Chicago)
Results
Group differences
There were no differences among the three experimental groups in age, BMI or ethnicity (MANOVA F(2,58) = 1.43, p = .25, η2 = .037). Trait Fear of Negative Evaluation and Anxiety were also similar between groups (MANOVA F(2,58) = 2.40, p = .11, η2 = .046). As can be seen in Table 1, at baseline no significant group differences were observed in affective state (state anxiety, shame/embarrassment, social self-esteem, total negative affect) or task expectations (difficult, stressful, arousing, confusing, engaging, or embarrassing). Similarly, no baseline group differences were observed for physiological measures (viz., HR, PEP, RMSSD) or cortisol (MANOVA F(2,58) = 0.56, p = .576, η2 = .019). The number of participants that required prompting to continue speaking following a 20 second silence was approximately similar for each condition (3, 2, and 3 for the 0-, 1- and 4-audience conditions, respectively).
Table 1.
Mean (S.D.) baseline values in each condition.
| No Audience | 1-Audience | 4-Audience | |
|---|---|---|---|
|
|
|||
| Age | 20.6 (1.4) | 20.4 (1.0) | 20.0 (0.9) |
| BMI | 22.5 (2.2) | 23.0 (2.6) | 22.4 (1.7) |
|
| |||
| Fear of Negative evaluation | 25.8 (6.0) | 29.6 (8.6) | 24.8 (8.0) |
| Anxiety (ABS) | 1.4 (0.5) | 1.5 (0.6) | 1.5 (0.5) |
| Shame/Embarrassment (ABS) | 1.4 (0.5) | 1.6 (0.8) | 1.5 (0.8) |
| Negative affect (ABS) | 1.2 (0.2) | 1.3 (0.3) | 1.3 (0.4) |
| Social self-esteem | 3.9 (0.5) | 3.8 (0.6) | 3.9 (0.7) |
|
| |||
| Task expectations: | |||
| difficulty | 1.7 (0.9) | 1.7 (1.3) | 1.8 (1.4) |
| stressfulness | 1.8 (1.2) | 1.7 (1.3) | 1.9 (1.3) |
| arousing | 1.6 (1.2) | 1.7 (1.1) | 1.9 (1.2) |
| performance | 3.0 (0.7) | 3.4 (0.9) | 3.3 (0.9) |
| confusing | 1.9 (0.9) | 2.2 (1.2) | 2.1 (0.9) |
| engaging | 2.7 (0.9) | 3.5 (1.0) | 3.1 (1.1) |
| embarrassing | 1.9 (1.3) | 2.1 (1.5) | 1.9 (1.7) |
|
| |||
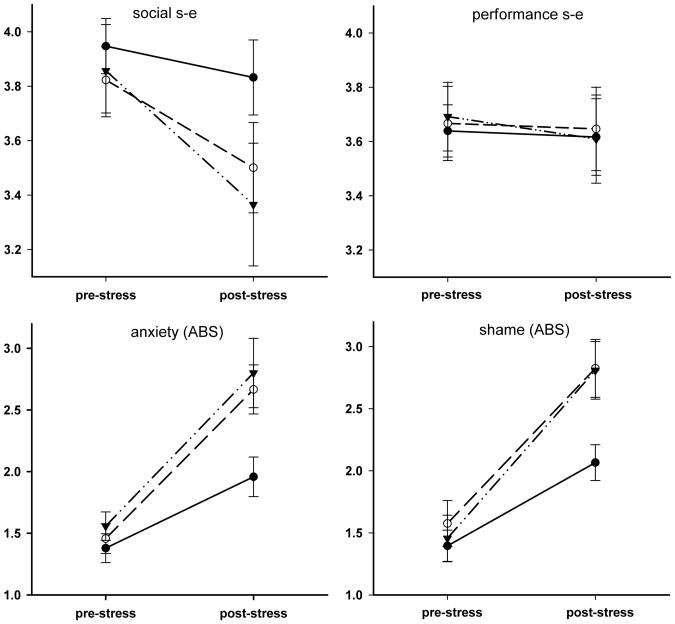
Affective Responses
Figure 1 presents an overview of affective responses during the tasks. Repeated measures ANOVA revealed significant effects of time for anxiety (F(1,58) = 79.76, p < .001, η2 = .579), shame/embarrassment (F(1,55) = 88.67, p < .001, η2 = .617), total negative affect (F(1,57) = 36.88, p < .001, η2 = .393), and social self esteem (F(1,57) = 14.11, p < .001, η2 = .198), but not for performance self esteem (F(1,58) = 0.21, p = .645, η2 = .004). Significant Time × Condition interactions were found for anxiety (F(2,58) = 3.96, p = .024, η2 = .120) and shame/embarrassment (F(2,55) = 3.26, p = .046, η2 = .106), and a marginal interaction effect emerged for total negative affect (F(2,57) = 2.90, p = .064, η2 = .092). Table 2 presents the results of subsequent pair-wise analyses of Time × Condition interactions (i.e., comparing affective responses in the no-audience with either the 1- or 4-audience conditions, as well as comparing the two audience conditions). These analyses revealed significantly greater increases in anxiety, shame, and negative affect in the audience conditions in comparison to the no-audience condition; these affective responses did not differ between the two audience conditions (see Table 2).
Figure 1.
Mean (±SEM) pre- and post-stressor scores on self-esteem (s-e), anxiety and shame/embarrassment in the No-Audience (filled diamonds), 1-Audience (open circles), and 4-Audience (filled triangles) conditions.
Table 2.
Univariate statistical analyses (repeated measures ANOVA) of changes in affect from baseline to post-task. Significant time by condition interactions are highlighted in bold.
| Anxiety | Shame | Negative affect | |
|---|---|---|---|
| Control vs. 1-Audience | F(1,39) = 2.30, p < .01, η2 = .172 | F(1,38) = 5.16, p < .05, η2 = .120 | F(1,39) = 4.63, p < .05, η2 = .106 |
| Control vs. 4-Audience | F(1, 38) = 4.83, p < .05, η2 = .113 | F(1, 36) = 4.80, p < .05, η2 = .117 | F(1, 37) = 5.80, p < .05, η2 = .135 |
| 1-Audience vs. 4-Audience | F(1,39) = 0.01, p = .915, η2 = .000 | F(1,36) = 0.01, p = .886, η2 = .001 | F(1,38) = 0.07, p = .798, η2 = .002 |
Physiological responses
Cardiac and Autonomic Responses
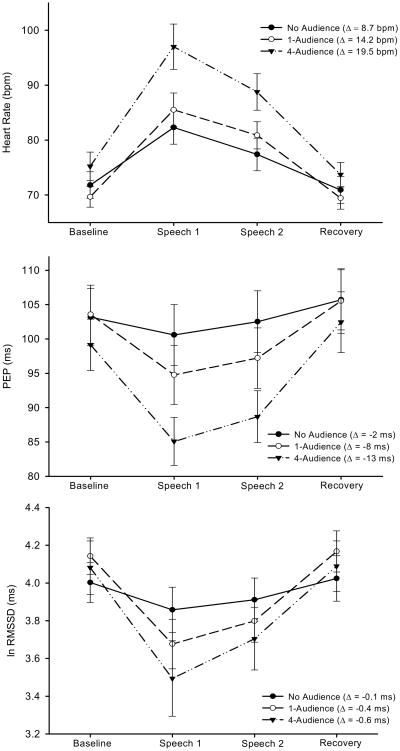
Figure 2 presents the summary data of cardiac autonomic responses. Repeated measures ANOVA yielded significant Time × Condition interactions for HR (F(6,162) = 5.45, p < .001, η2 = .168), PEP (F(6,162) = 9.74, p < .001, η2 = .265) and RMSSD (F(6,159) = 3.76, p = .002, η2 = .124). Subsequently, we again compared the responses for each condition in a pair-wise fashion, using repeated measures ANOVA (see Table 3). These analyses demonstrated that for HR and PEP both the 1- and 4-audience conditions induced significantly larger responses than the control condition (presented in Table 3). Further, the 4-audience condition elicited larger changes in HR and PEP than the 1-audience condition. RMSSD similarly showed larger responses in the 1- and 4-audience conditions than in the control condition, but responses to the 1- and 4-audience conditions were not significantly different (see Table 3). Essentially the same outcomes emerged when these analyses were repeated using only the 2 min preparation period, in order to exclude respiration artefacts caused by speaking. We also analysed respiratory patterns to further determine if effects on RMSSD could have been confounded by concomitant condition effects on respiration frequency and tidal volume (the 2-minute no-speech preparation period was used for these analyses). However, no significant Time × Condition interactions emerged for respiration (data not shown). The effects of condition on HR, PEP, and RMSDD remained unaltered following adjustment for baseline values, gender and BMI.
Figure 2.
Mean (± SEM) values in No Audience (filled circles), 1-audience (open circles), and 4-audience (filled triangles) stressor conditions across the session (Δ = Speech values minus Baseline values). Presented are: Heart Rate (upper graph); PEP (middle graph); RMSSD (lower graph).
Table 3.
Univariate statistical analysesa of changes in heart rate, PEP, and RMSSD (baseline, task-1, task-2, recovery) and cortisol (baseline, post-task, +15 min, +30 min, +45 min recovery).b
| Heart rate | PEP | RMSSD | Cortisol | |
|---|---|---|---|---|
| Control vs. 1-Audience | F(3,111) = 3.75, p < .05, η2 = .092 | F(3,111) = 10.64, p < .001, η2 = .223 | F(3,111) = 6.30, p < .01, η2 = .146 | F(4,140) = 2.25, p = .107, η2 = .060 |
| Control vs. 4-Audience | F(3,111) = 10.15, p < .001, η2= .215 | F(3,111) = 20.70, p < .001, η2 = .359 | F(3,111) = 9.45, p < .001, η2 = .203 | F(4,136) = 8.32, p < .01, η2 = .197 |
| 1-Audience vs. 4-Audience | F(3,114) = 3.58, p < .05, η2 = .086 | F(3,114) = 4.22, p < .01, η2 = .100 | F(3,114) = 1.06, p = .369, η2 = .027 | F(4,148) = 4.27, p < .05, η2 = .104 |
Repeated measures ANOVA
Significant time by condition interactions are highlighted in bold. Results remained essentially unaltered after adjustment for gender and BMI.
PEP= pre-ejection period; RMSSD= root mean square of successive differences
Salivary Cortisol responses
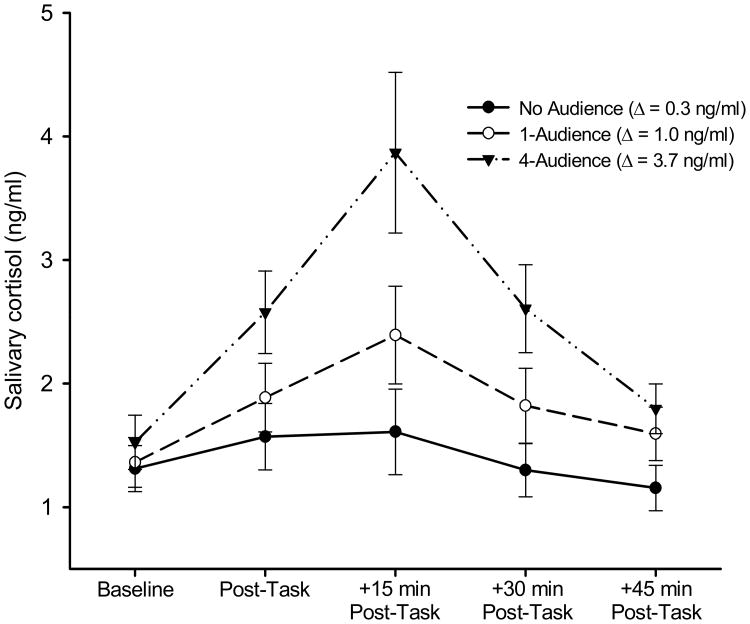
As shown in Figure 3, irrespective of condition, cortisol levels showed the expected peak at +15 min post-task and had largely returned to baseline levels at +45 min post-task (main-effect of time: F(4,212) = 24.03, p < .001, η2 = .312). Separate analyses of each condition showed no significant cortisol change in the ‘no audience’ condition, F(4,64) = 2.57, p = .085, η2 = .139, whereas there were main effects of time for both the 1-audience and 4-audience conditions, F(4,76) = 8.84, p = .001, η2 = .318, and F(4,72) = 15.55, p < .001, η2 = .464, respectively. Importantly, there was also a significant Time × Condition interaction effect (F(8,212) = 5.50, p = .001, η2 = .172). Table 2 presents the outcomes of pair-wise comparisons of the cortisol responses during each condition. There were significantly larger cortisol changes in the 4-audience compared to the control condition and the 1-audience condition, whereas the 1-audience and control conditions were not significantly different (see Table 2). Adjustment for gender, baseline cortisol, and BMI did not alter these outcomes.
Figure 3.
Mean (± SEM) salivary cortisol values in the No Audience (filled circles), 1-Audience (open circles), and 4-Audience (filled triangles) conditions (Δ = 15-min post-task value minus Baseline value)
Mediation analyses
Attention then turned to whether the affective responses or autonomic reactivity predicted, i.e., mediated, the condition-dependent rises in cortisol. For mediation, it is necessary to show that: 1) the independent variable (task condition) affects the dependent variable (cortisol responses); 2) the independent variable predicts the mediator (e.g., affective responses); 3) the selected mediator predicts the dependent variable; and 4) the association between the independent and the dependent variable is substantially attenuated after taking the putative mediator into account. In the preceding sections, we have shown a significant Time × Condition interaction for cortisol, and importantly, that all the putative mediators (affective and autonomic responses) showed Time × Condition interactions, satisfying the first and second condition for mediation.
Associations between candidate mediator variables and the dependent variable were tested using repeated measures ANCOVA. To assess whether affective responses mediate the effect of condition on cortisol responses, changes in anxiety, shame/embarrassment and negative affect were entered as covariates. ANCOVA was also used to test the extent to which mediators accounted for the association between the predictor (condition) and the dependent variable (cortisol response). If affective responses predict or mediate differential cortisol responses across conditions this would result in; 1) a significant mediator by time interaction (i.e., the covariate would be significant), and; 2) an attenuation of the Time × Condition interaction effect. However, ANCOVA revealed that none of these covariates were significantly associated with cortisol responses; anxiety (F(4,208) = 0.12, p = .851, η2 = .002), shame/embarrassment (F(4,196) = 0.16, p = .816, η2 = .003), negative affect (F(4,204) = 1.36, p = .260, η2 = .026). Unsurprisingly, the Time × Condition interaction remained virtually unaltered when these parameters were entered as covariates. Together these results indicated that the condition effects on cortisol profile were unrelated to these affective responses.
To determine whether sympathetic cardiac responses during the tasks predicted subsequent cortisol responses a similar mediation analyses was conducted, using change in PEP as a covariate in the ANCOVA. First, ANCOVA yielded a significant association between change in PEP and cortisol response (F(4,208) = 4.13, p = .024, η2 = .074). Second, adjusting for PEP attenuated the significant cortisol Time × Condition interaction to non-significance in conjunction with a substantial reduction in effect size: The variance in the cortisol response explained by condition fell from 17.2% (shown above) to 5.7% by the adding change in PEP as a covariate (F(8,208) = 1.86, p = .133, η2 = .057). This reduction was statistically significant (F(8,212) = 4.10; p < .01). Replicating these analyses using cortisol area under curve as the dependent variable (as an alternative to using a repeated measures approach) yielded virtually identical results. No evidence for mediation was found for heart rate variability (analyses not shown).
Discussion
Social interaction and a need to belong are intrinsic to human existence, which makes it understandable that situations involving social transgression and threats to social standing are powerful stressors (49). The Social Self Preservation Theory (11, 17) contends that HPA activation is a characteristic of such social stressors. The current results confirmed HPA activation in response to social evaluative threat. However, while cortisol activity clearly differentiated evaluative from non-evaluative task conditions, so too did heart rate, sympathetic cardiac activation (PEP), and vagal tone (RMSSD). In fact, based on effect sizes, autonomic and cardiac reactions appeared more sensitive than cortisol responses in differentiating SET from non-SET.
The current study manipulated levels of evaluative threat by increasing audience size (33). This enabled further examination of whether social evaluation determines the pattern, rather than the magnitude, of physiological reactivity. In keeping with previous findings (17), it was anticipated that the magnitude of cortisol reactivity, but not cardiac reactivity, would correlate positively with increasing audience size. The data did not support this prediction; cortisol, heart rate, and PEP all displayed comparable response gradients. For example, heart rate reactivity increased in a linear fashion from 9 bpm (no audience), to 14 bpm (1-audience), to 20 bpm (4-audience). RMSSD was the minor exception to this pattern; although vagal responses clearly differentiated evaluative from non-evaluative conditions, the difference in vagal withdrawal between the 1- and 4-audience conditions did not reach statistical significance. Taken together, however, it seems reasonable to conclude from the present data that social evaluation potentiates a general, rather than a HPA-specific, physiological reactivity.
Further support for a general reactivity-enhancing effect of SET was provided by mediation analyses, which indicated that the effects on HPA activation could not be separated from the effects on sympathetic activation. The finding that autonomic responses during the tasks predicted the subsequent elevation in cortisol replicates previous research (24, 50-54), and is also consistent with evidence of extensive interaction between the two response systems (9, 55-57). The present observations contrast, however, with the findings of Gruenewald et al. (17), which showed a relative insensitivity of cardiovascular measures to evaluative threat together with a selective cortisol reactivity. This discrepancy could have been due to confounding by movement and posture changes, which we aimed to minimise in the current study. A clear demonstration of the effects of such confounding is provided by Rohleder et al. (32). Their study showed that a control condition, imposing the mere physical activities of the TSST, elicited nearly similar changes in heart rate and heart rate variability as the full TSST protocol with the speech and mental arithmetic stressor (32). Thus, movement artefacts appear capable of partly masking the effects of the TSST stressors on the cardiovascular system.
The social self preservation theory (SSPT) is a rebuttal to assumptions based on Selye's generality theory which posits that all stressors will activate the HPA-axis. It partly rests on the outcome of a meta-analysis showing a clear difference in HPA activation between ‘regular’ performance tasks (e.g., metal arithmetic) and performance tasks that include the additional element of social evaluation or uncontrollability (11). However, Selye's concept of stress, based on experiments that applied severe physical threats to animals, may not generalize to the comparatively sedate human performance tasks for more fundamental physiological reasons: the activation of different physiological systems may require different intensities of provocation. That is, while mildly engaging problem-solving tasks readily perturb cardiac and autonomic activity, HPA activation appears to require more provocative manipulations, such as evaluated speech tasks. Such tasks elicit higher levels of physiological activation in general2 (19, 23). A comparable elevated threshold for HPA activation is also observed during physical stressors like exercise (26). These observations lead us to tentatively propose an alternative ‘threshold activation’ model of HPA reactivity during performance stressors. This threshold activation model postulates that some stressors, such as those without elements of social evaluation or uncontrollability, fail to induce a cortisol response simply because they are less likely to induce a level of activation sufficient to engage the HPA axis.
An implication of this threshold activation model is that provocative elements like social evaluation or uncontrollability engage the HPA axis not because of a unique psychological-physiological response association, but by intensifying an otherwise moderately arousing task. Accordingly, evaluation can create a perception of response specificity when it is response intensity that is actually manipulated. An illustration of this phenomenon is provided by a study of Cacioppo and co-workers (53). In line with the extant literature (11, 23, 24), they observed that a simple performance task (metal arithmetic) does not create a significant average increase in cortisol. However, consistent with a threshold-activation model, further analyses of individual differences showed that the task did elicit cortisol release, but only in individuals exhibiting a strong cardiac autonomic activation. This finding is consistent with the idea that it is primarily the magnitude of physiological (e.g., autonomic) activation during a performance task that predicts whether the HPA axis becomes engaged.
Although the primary aim of our study was to assess the specificity of cortisol responses to social evaluative threat, our findings warrant a brief comment on the specificity of self-conscious emotions during such situations. It seems counter-intuitive that a context designed to elicit evaluation apprehension and which increases embarrassment and shame (emotions that reflect social threat) would not also increase apprehension and anxiety (49). However, such was the finding reported by Gruenewald et al. (17). In contrast, the current data showed that anxiety and shame/embarrassment increased in a parallel fashion. A possible explanation for this discrepancy could be that in the Gruenewald study participants in the social evaluation condition already reported elevated anxiety at baseline (compared to the non-evaluative condition). These baseline differences might have masked a differential effect of condition on anxiety. Another notable finding of the present study was that self-report measures revealed no differences between the 1- and 4-audience conditions, even though physiological responses clearly differentiated the conditions. This observation resembles that of other studies which manipulated social context (e.g., (58, 59). Thus, it is possible that audience size drives physiological responses independent of affective mechanisms that were assessed here.
Several limitations should be noted. Like most research in this area, the present study was performed with university students, and replication of our findings in other populations is an important next step. Also, the group sizes were relatively small and the resulting lack in power requires a cautious interpretation of two null-findings: i.e., the lack of association between vagal reactivity and increasing audience size, and the absence of a significant correlation between shame/embarrassment and cortisol responses. A further limitation, shared with the study of Gruenewald et al. (17), is that the Shame/Embarrassment mood-scale does not differentiate between these two distinct emotions, which may have different physiological correlates (60, 61).
In summary, our findings were consistent with the general observation from social facilitation and social anxiety research that performance involving social evaluation elicits heightened physiological reactivity (19, 20). We proposed a threshold activation model as an alternative explanation for the observation that social evaluative stressors characteristically elicit a cortisol response. This model is based on the observation that cortisol is less readily perturbed during psychological, and physical, stressors than cardiovascular and autonomic parameters. An illusion of response specificity may thus occur when a provocative element (e.g., lack of control, social evaluation) is incorporated into a less provoking challenge (e.g., performing arithmetic). We may add that this model does not imply that physiological responses to evaluative and non-evaluative stressors can simply be differentiated on a single dimension of activation or arousal (62). Our contention is merely that a threatening evaluative context appears to broadly enhance reactivity of multiple physiological systems. It may be that this ability to elicit a robust generalised response explains why social stressors have consistently been associated with a range of health outcomes.
Acknowledgments
We thank Prof. John T. Cacioppo (University of Chicago) and Prof. Gary G. Berntson (Ohio State University) for comments on the rationale of the study and its interpretation. We also very grateful for the help of Claire Sawyer, Gareth Tantram, Sarah Alderton, and Lewis Moore, who assisted with participant recruitment and experimental procedures. (19, 63, 64)
This study has been made possible through funding awarded to Dr Jos A. Bosch by the 'Birmingham Interdisciplinary Bridging in Engineering, Medicine & Science' (BIBEM) and the NIDCR (NIH RO3-DE-16726).
Abbreviations
- ECG
electrocardiograph
- HPA
hypothalamic-pituitary-adrenal
- ICG
impedance cardiograph
- PEP
pre-ejection period
- RMSSD
root mean square of successive differences
- SAM
sympathetic-adrenal-medullary
- SET
social evaluative threat
- TSST
Trier Social Stress Test
Footnotes
This subscale has been developed as a measure of “shame” for the study by Gruenewald et al. (17). Because the scale contains both embarrassment and shame items it was denoted as “shame/embarrassment” in this paper.
References
- 1.Cacioppo JT, Tassinary LG. Inferring psychological significance from physiological signals. The American psychologist. 1990;45:16–28. doi: 10.1037//0003-066x.45.1.16. [DOI] [PubMed] [Google Scholar]
- 2.Stern RM, Sison CEE. Response patterning. In: Cacioppo JT, Tassinary LG, editors. Principles of Psychophysiology: Physical, social, and inferential elements. Cambridge: Cambridge University Press; 1990. pp. 193–216. [Google Scholar]
- 3.Kemeny ME. Psychobiological responses to social threat: Evolution of a psychological model in psychoneuroimmunology. Brain Behav Immun. 2008 doi: 10.1016/j.bbi.2008.08.008. [DOI] [PubMed] [Google Scholar]
- 4.Schneiderman N, Ironson G, Siegel SD. Stress and health: psychological, behavioral, and biological determinants. Annu Rev Clin Psychol. 2005;1:607–28. doi: 10.1146/annurev.clinpsy.1.102803.144141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Taylor SE, Klein LC, Lewis BP, Gruenewald TL, Gurung RA, Updegraff JA. Biobehavioral responses to stress in females: tend-and-befriend, not fight-or-flight. Psychological review. 2000;107:411–29. doi: 10.1037/0033-295x.107.3.411. [DOI] [PubMed] [Google Scholar]
- 6.Weiner H. Perturbing the Organism: The Biology of Stressful Experience. Chicago: University of Chicago Press; 1992. [Google Scholar]
- 7.Dienstbier RA. Arousal and physiological toughness: implications for mental and physical health. Psychological review. 1989;96:84–100. doi: 10.1037/0033-295x.96.1.84. [DOI] [PubMed] [Google Scholar]
- 8.Uchino BN, Cacioppo JT, Kiecolt-Glaser JK. The relationship between social support and physiological processes: a review with emphasis on underlying mechanisms and implications for health. Psychological bulletin. 1996;119:488–531. doi: 10.1037/0033-2909.119.3.488. [DOI] [PubMed] [Google Scholar]
- 9.Sapolsky RM, Romero LM, Munck AU. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr Rev. 2000;21:55–89. doi: 10.1210/edrv.21.1.0389. [DOI] [PubMed] [Google Scholar]
- 10.McEwen BS. Physiology and neurobiology of stress and adaptation: central role of the brain. Physiological reviews. 2007;87:873–904. doi: 10.1152/physrev.00041.2006. [DOI] [PubMed] [Google Scholar]
- 11.Dickerson SS, Kemeny ME. Acute stressors and cortisol responses: a theoretical integration and synthesis of laboratory research. Psychological bulletin. 2004;130:355–91. doi: 10.1037/0033-2909.130.3.355. [DOI] [PubMed] [Google Scholar]
- 12.Miller GE, Chen E, Zhou ES. If it goes up, must it come down? Chronic stress and the hypothalamicpituitary-adrenocortical axis in humans. Psychological bulletin. 2007;133:25–45. doi: 10.1037/0033-2909.133.1.25. [DOI] [PubMed] [Google Scholar]
- 13.Frankenhaeuser M. Challenge-control interaction as reflected in sympathetic-adrenal and pituitary-adrenal activity: comparison between the sexes. Scand J Psychol. 1982;(Suppl 1):158–64. doi: 10.1111/j.1467-9450.1982.tb00466.x. [DOI] [PubMed] [Google Scholar]
- 14.Henry JP. Neuroendocrine patterns of emotional response. In: Plutchick R, Kellerman H, editors. Emotion: Theory, Research and Experiences. San Diego: Academic Press; 1986. [Google Scholar]
- 15.Schommer NC, Hellhammer DH, Kirschbaum C. Dissociation between reactivity of the hypothalamus-pituitary-adrenal axis and the sympathetic-adrenal-medullary system to repeated psychosocial stress. Psychosomatic medicine. 2003;65:450–60. doi: 10.1097/01.psy.0000035721.12441.17. [DOI] [PubMed] [Google Scholar]
- 16.Dickerson SS, Gruenewald TL, Kemeny ME. When the social self is threatened: shame, physiology, and health. Journal of personality. 2004;72:1191–216. doi: 10.1111/j.1467-6494.2004.00295.x. [DOI] [PubMed] [Google Scholar]
- 17.Gruenewald TL, Kemeny ME, Aziz N, Fahey JL. Acute threat to the social self: shame, social self-esteem, and cortisol activity. Psychosomatic medicine. 2004;66:915–24. doi: 10.1097/01.psy.0000143639.61693.ef. [DOI] [PubMed] [Google Scholar]
- 18.Kirschbaum C, Pirke KM, Hellhammer DH. The ‘Trier Social Stress Test’--a tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology. 1993;28:76–81. doi: 10.1159/000119004. [DOI] [PubMed] [Google Scholar]
- 19.Cacioppo JT, Rourke PA, Marshall-Goodell BS, Tassinary LG, Baron RS. Rudimentary physiological effects of mere observation. Psychophysiology. 1990;27:177–86. doi: 10.1111/j.1469-8986.1990.tb00368.x. [DOI] [PubMed] [Google Scholar]
- 20.Wright RA, Tunstall AM, Williams BJ, Goodwin JS, Harmon-Jones E. Social evaluation and cardiovascular response: an active coping approach. Journal of personality and social psychology. 1995;69:530–43. doi: 10.1037//0022-3514.69.3.530. [DOI] [PubMed] [Google Scholar]
- 21.Smith TW, Nealey JB, Kircher JC, Limon JP. Social determinants of cardiovascular reactivity: effects of incentive to exert influence and evaluative threat. Psychophysiology. 1997;34:65–73. doi: 10.1111/j.1469-8986.1997.tb02417.x. [DOI] [PubMed] [Google Scholar]
- 22.Kamarck TW, Annunziato B, Amateau LM. Affiliation moderates the effects of social threat on stress-related cardiovascular responses: boundary conditions for a laboratory model of social support. Psychosomatic medicine. 1995;57:183–94. doi: 10.1097/00006842-199503000-00011. [DOI] [PubMed] [Google Scholar]
- 23.Linden W, Rutledge T, Con A. A case for the usefulness of laboratory social stressors. Ann Behav Med. 1998;20:310–6. doi: 10.1007/BF02886380. [DOI] [PubMed] [Google Scholar]
- 24.Al'Absi M, Bongard S, Buchanan T, Pincomb GA, Licinio J, Lovallo WR. Cardiovascular and neuroendocrine adjustment to public speaking and mental arithmetic stressors. Psychophysiology. 1997;34:266–75. doi: 10.1111/j.1469-8986.1997.tb02397.x. [DOI] [PubMed] [Google Scholar]
- 25.Ewart CK, Kolodner KB. Social competence interview for assessing physiological reactivity in adolescents. Psychosomatic medicine. 1991;53:289–304. doi: 10.1097/00006842-199105000-00003. [DOI] [PubMed] [Google Scholar]
- 26.Luger A, Deuster PA, Gold PW, Loriaux DL, Chrousos GP. Hormonal responses to the stress of exercise. Adv Exp Med Biol. 1988;245:273–80. doi: 10.1007/978-1-4899-2064-5_22. [DOI] [PubMed] [Google Scholar]
- 27.Berntson GG, Uchino BN, Cacioppo JT. Origins of baseline variance and the Law of Initial Values. Psychophysiology. 1994;31:204–10. doi: 10.1111/j.1469-8986.1994.tb01042.x. [DOI] [PubMed] [Google Scholar]
- 28.Veldhuijzen van Zanten JJ, Ring C, Burns VE, Edwards KM, Drayson M, Carroll D. Mental stress-induced hemoconcentration: Sex differences and mechanisms. Psychophysiology. 2004;41:541–51. doi: 10.1111/j.1469-8986.2004.00190.x. [DOI] [PubMed] [Google Scholar]
- 29.Tulen JH, Boomsma F, Man in 't Veld AJ. Cardiovascular control and plasma catecholamines during rest and mental stress: effects of posture. Clin Sci (Lond) 1999;96:567–76. [PubMed] [Google Scholar]
- 30.Obrist PA. Cardiovascular psychophysiology: a perspective. New York: Plenum Press; 1981. [Google Scholar]
- 31.Houtveen JH, Groot PF, Geus EJ. Effects of variation in posture and respiration on RSA and pre-ejection period. Psychophysiology. 2005;42:713–9. doi: 10.1111/j.1469-8986.2005.00363.x. [DOI] [PubMed] [Google Scholar]
- 32.Rohleder N, Wolf JM, Maldonado EF, Kirschbaum C. The psychosocial stress-induced increase in salivary alpha-amylase is independent of saliva flow rate. Psychophysiology. 2006;43:645–52. doi: 10.1111/j.1469-8986.2006.00457.x. [DOI] [PubMed] [Google Scholar]
- 33.Knowles ES. Social physics and the effects of others: tests of the effects of audience size and distance on social judgements and behavior. Journal of personality and social psychology. 1983;45:1263–79. [Google Scholar]
- 34.Kirschbaum C, Kudielka BM, Gaab J, Schommer NC, Hellhammer DH. Impact of gender, menstrual cycle phase, and oral contraceptives on the activity of the hypothalamus-pituitary-adrenal axis. Psychosomatic medicine. 1999;61:154–62. doi: 10.1097/00006842-199903000-00006. [DOI] [PubMed] [Google Scholar]
- 35.Bosch JA, de Geus EJ, Veerman EC, Hoogstraten J, Nieuw Amerongen AV. Innate secretory immunity in response to laboratory stressors that evoke distinct patterns of cardiac autonomic activity. Psychosomatic medicine. 2003;65:245–58. doi: 10.1097/01.psy.0000058376.50240.2d. [DOI] [PubMed] [Google Scholar]
- 36.Bosch JA, Berntson GG, Cacioppo JT, Marucha PT. Differential mobilization of functionally distinct natural killer subsets during acute psychologic stress. Psychosomatic medicine. 2005;67:366–75. doi: 10.1097/01.psy.0000160469.00312.8e. [DOI] [PubMed] [Google Scholar]
- 37.Saab PG, Matthews KA, Stoney CM, McDonald RH. Premenopausal and postmenopausal women differ in their cardiovascular and neuroendocrine responses to behavioral stressors. Psychophysiology. 1989;26:270–80. doi: 10.1111/j.1469-8986.1989.tb01917.x. [DOI] [PubMed] [Google Scholar]
- 38.van Eck MM, Nicolson NA, Berkhof H, Sulon J. Individual differences in cortisol responses to a laboratory speech task and their relationship to responses to stressful daily events. Biological psychology. 1996;43:69–84. doi: 10.1016/0301-0511(95)05159-7. [DOI] [PubMed] [Google Scholar]
- 39.Sarason IG, Stoops R. Test anxiety and the passage of time. J Consult Clin Psychol. 1978;46:102–9. doi: 10.1037//0022-006x.46.1.102. [DOI] [PubMed] [Google Scholar]
- 40.Leary MR. Social anxiousness: the construct and its measurement. J Pers Assess. 1983;47:66–75. doi: 10.1207/s15327752jpa4701_8. [DOI] [PubMed] [Google Scholar]
- 41.Heatherton TF, Polivy J, Herman CP. Restraint, weight loss, and variability of body weight. J Abnorm Psychol. 1991;100:78–83. doi: 10.1037//0021-843x.100.1.78. [DOI] [PubMed] [Google Scholar]
- 42.Derogatis LR, Yevzeroff H, Wittelsberger B. Social class, psychological disorder, and the nature of the psychopathologic indicator. J Consult Clin Psychol. 1975;43:183–91. doi: 10.1037/h0076514. [DOI] [PubMed] [Google Scholar]
- 43.Berntson GG, Cacioppo JT, Quigley KS. Cardiac psychophysiology and autonomic space in humans: empirical perspectives and conceptual implications. Psychological bulletin. 1993;114:296–322. doi: 10.1037/0033-2909.114.2.296. [DOI] [PubMed] [Google Scholar]
- 44.Bosch JA, de Geus EJ, Kelder A, Veerman EC, Hoogstraten J, Amerongen AV. Differential effects of active versus passive coping on secretory immunity. Psychophysiology. 2001;38:836–46. [PubMed] [Google Scholar]
- 45.Willemsen GH, De Geus EJ, Klaver CH, Van Doornen LJ, Carroll D. Ambulatory monitoring of the impedance cardiogram. Psychophysiology. 1996;33:184–93. doi: 10.1111/j.1469-8986.1996.tb02122.x. [DOI] [PubMed] [Google Scholar]
- 46.Bosch JA, Berntson GG, Cacioppo JT, Dhabhar FS, Marucha PT. Acute stress evokes selective mobilization of T cells that differ in chemokine receptor expression: a potential pathway linking immunologic reactivity to cardiovascular disease. Brain Behav Immun. 2003;17:251–9. doi: 10.1016/s0889-1591(03)00054-0. [DOI] [PubMed] [Google Scholar]
- 47.Berntson GG, Cacioppo JT, Binkley PF, Uchino BN, Quigley KS, Fieldstone A. Autonomic cardiac control. III. Psychological stress and cardiac response in autonomic space as revealed by pharmacological blockades. Psychophysiology. 1994;31:599–608. doi: 10.1111/j.1469-8986.1994.tb02352.x. [DOI] [PubMed] [Google Scholar]
- 48.Cacioppo JT, Berntson GG, Binkley PF, Quigley KS, Uchino BN, Fieldstone A. Autonomic cardiac control. II. Noninvasive indices and basal response as revealed by autonomic blockades. Psychophysiology. 1994;31:586–98. doi: 10.1111/j.1469-8986.1994.tb02351.x. [DOI] [PubMed] [Google Scholar]
- 49.Baumeister RF, Leary MR. The need to belong: desire for interpersonal attachments as a fundamental human motivation. Psychological bulletin. 1995;117:497–529. [PubMed] [Google Scholar]
- 50.Uchino BN, Cacioppo JT, Malarkey W, Glaser R. Individual differences in cardiac sympathetic control predict endocrine and immune responses to acute psychological stress. Journal of personality and social psychology. 1995;69:736–43. doi: 10.1037//0022-3514.69.4.736. [DOI] [PubMed] [Google Scholar]
- 51.Sgoutas-Emch SA, Cacioppo JT, Uchino BN, Malarkey W, Pearl D, Kiecolt-Glaser JK, Glaser R. The effects of an acute psychological stressor on cardiovascular, endocrine, and cellular immune response: a prospective study of individuals high and low in heart rate reactivity. Psychophysiology. 1994;31:264–71. doi: 10.1111/j.1469-8986.1994.tb02215.x. [DOI] [PubMed] [Google Scholar]
- 52.Sgoifo A, Braglia F, Costoli T, Musso E, Meerlo P, Ceresini G, Troisi A. Cardiac autonomic reactivity and salivary cortisol in men and women exposed to social stressors: relationship with individual ethological profile. Neuroscience and biobehavioral reviews. 2003;27:179–88. doi: 10.1016/s0149-7634(03)00019-8. [DOI] [PubMed] [Google Scholar]
- 53.Cacioppo JT, Malarkey WB, Kiecolt-Glaser JK, Uchino BN, Sgoutas-Emch SA, Sheridan JF, Berntson GG, Glaser R. Heterogeneity in neuroendocrine and immune responses to brief psychological stressors as a function of autonomic cardiac activation. Psychosomatic medicine. 1995;57:154–64. doi: 10.1097/00006842-199503000-00008. [DOI] [PubMed] [Google Scholar]
- 54.Doussard-Roosevelt JA, Montgomery LA, Porges SW. Short-term stability of physiological measures in kindergarten children: respiratory sinus arrhythmia, heart period, and cortisol. Developmental psychobiology. 2003;43:230–42. doi: 10.1002/dev.10136. [DOI] [PubMed] [Google Scholar]
- 55.Habib KE, Gold PW, Chrousos GP. Neuroendocrinology of stress. Endocrinol Metab Clin North Am. 2001;30:695–728. vii–viii. doi: 10.1016/s0889-8529(05)70208-5. [DOI] [PubMed] [Google Scholar]
- 56.Cacioppo JT. Social neuroscience: autonomic, neuroendocrine, and immune responses to stress. Psychophysiology. 1994;31:113–28. doi: 10.1111/j.1469-8986.1994.tb01032.x. [DOI] [PubMed] [Google Scholar]
- 57.Wang J, Rao H, Wetmore GS, Furlan PM, Korczykowski M, Dinges DF, Detre JA. Perfusion functional MRI reveals cerebral blood flow pattern under psychological stress. Proceedings of the National Academy of Sciences of the United States of America; 2005; pp. 17804–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gerin W, Pieper C, Levy R, Pickering TG. Social support in social interaction: a moderator of cardiovascular reactivity. Psychosomatic medicine. 1992;54:324–36. doi: 10.1097/00006842-199205000-00008. [DOI] [PubMed] [Google Scholar]
- 59.Kamarck TW, Manuck SB, Jennings JR. Social support reduces cardiovascular reactivity to psychological challenge: a laboratory model. Psychosomatic medicine. 1990;52:42–58. doi: 10.1097/00006842-199001000-00004. [DOI] [PubMed] [Google Scholar]
- 60.Leary MR. Motivational and emotional aspects of the self. Annual review of psychology. 2007;58:317–44. doi: 10.1146/annurev.psych.58.110405.085658. [DOI] [PubMed] [Google Scholar]
- 61.Drummond PD, Back K, Harrison J, Helgadottir FD, Lange B, Lee C, Leavy K, Novatscou C, Orner A, Pham H, Prance J, Radford D, Wheatley L. Blushing during social interactions in people with a fear of blushing. Behav Res Ther. 2007;45:1601–8. doi: 10.1016/j.brat.2006.06.012. [DOI] [PubMed] [Google Scholar]
- 62.Berntson GG. Reasoning about brains. In: Cacioppo JT, Visser PS, Pickett CL, editors. Social Neuroscience: people thinking about people. Cambridge, MA: The MIT press; 2006. pp. 1–11. [Google Scholar]
- 63.Baron RS. Distraction-conflict theory: Progress and problems. Adv Exp Soc Psychol. 1986;19:1–40. [Google Scholar]
- 64.Wright RA, Killebrew K, Pimpalapure D. Cardiovascular incentive effects where a challenge is unfixed: demonstrations involving social evaluation, evaluator status, and monetary reward. Psychophysiology. 2002;39:188–97. doi: 10.1017/S0048577202011137. [DOI] [PubMed] [Google Scholar]