Abstract
Background and Aims
Clinical trials of therapy against chronic hepatitis C virus (HCV) infection including boceprevir (BOC) or telaprevir (TVR) plus pegylated interferon and ribavirin (PR) have reported considerably higher response rates than those achieved with PR alone. This study sought to evaluate the efficacy and safety of triple therapy including BOC or TVR in combination with PR in HIV/HCV-coinfected patients under real-life conditions.
Methods
In a multicentre study conducted in 24 sites throughout five European countries, all HIV/HCV-coinfected patients who initiated a combination of BOC or TVR plus PR and who had at least 60 weeks of follow-up, were analyzed. Sustained virologic response 12 weeks after the scheduled end of therapy date (SVR12) and the rate of discontinuations due to adverse events (AE) were evaluated.
Results
Of the 159 subjects included, 127 (79.9%) were male, 45 (34.4%) were treatment-naïve for PR and 60 (45.4%) showed cirrhosis. SVR12 was observed in 31/46 (67.4%) patients treated with BOC and 69/113 (61.1%) patients treated with TVR. Overall discontinuations due to AE rates were 8.7% for BOC and 8% for TVR. Grade 3 or 4 hematological abnormalities were frequently observed; anemia 7%, thrombocytopenia 17.2% and neutropenia 16.4%.
Conclusion
The efficacy and safety of triple therapy including BOC or TVR plus PR under real-life conditions of use in the HIV/HCV-coinfected population was similar to what is observed in clinical trials. Hematological side effects are frequent but manageable.
Introduction
Treatment strategies aimed to enhance rates of sustained virologic response (SVR) are of highest priority in patients with chronic hepatitis C and HIV coinfection, since SVR dramatically reduces the incidence of hepatic decompensations and mortality in this setting [1,2]. Until 2009, standard-of-care for treating chronic hepatitis C virus (HCV) infection consisted of dual therapy with pegylated interferon (peg-IFN) plus ribavirin (RBV). However, in the setting of HIV/HCV genotype 1-coinfected patients, SVR rates with dual therapy did not exceed 25% in clinical practice [3]. The arrival of direct acting antivirals (DAA) against HCV has ushered in a new era in the management of chronic hepatitis C. Considerable increases of SVR to triple combination of the first generation HCV-protease inhibitors (PI) boceprevir (BOC) or telaprevir (TVR) with peg-IFN and RBV in the HCV-monoinfected population have been reported in pivotal clinical trials in HCV-monoinfected and in HIV/HCV-coinfected subjects [4–11]. As a consequence, in 2012, triple therapy including BOC or TVR in addition to peg-IFN and RBV became the standard therapy for patients coinfected with HIV and HCV genotype 1 in Europe [12]. Although current recommendations include next-generation DAA [13], first generation PIs are still widely used in some countries due to financial constraints.
However, the use of first-generation HCV PIs raises important concerns, such as tolerability, increased cost, drug-drug interactions, and decreased adherence due to a high pill burden and multiple dosing. Furthermore, data on safety and efficacy of DAA-based therapies are derived from clinical trials and the so-called “real-life” studies are actually based on early access programmes or compassionate use programmes [8,9,14–17]. The sparse data derived from studies conducted in real-life settings mainly come from HCV-monoinfected patients [18–22]. Some of these studies report evidence of reduced tolerability and efficacy of BOC and TVR-based treatments as observed in pivotal trials. However, the population characteristics of these studies are diverse and response rates and safety profiles of DAAs may not be comparable between HCV-monoinfected and HIV/HCV-coinfected patients. In this context, the coinfected population has specific issues such as the co-administration of antiretroviral therapy (ART), which increases the pill-burden even further and may cause drug-drug interactions, making modification of ART or TVR dose adjustment necessary in a high number of patients. Still, to date there is no study available to evaluate this issue in large populations of HIV/HCV-coinfected patients and under real-life conditions. Data on the efficacy and tolerability of first-generation DAA-based regimens in the clinical practice are needed.
Therefore, the aim of this study was to determine the efficacy and safety of triple therapy including BOC or TVR plus peg-IFN and RBV in patients with chronic hepatitis C and HIV coinfection in routine clinical practice.
Patients and Methods
Patients and study design
All patients from populations prospectively followed in 24 health care settings from Austria, Germany, Spain, Switzerland and the United Kingdom were included in this observational study if they were i) older than 18 years, ii) coinfected with HIV, iii) were given triple therapy against HCV including BOC or TVR in combination with Peg-IFN plus RBV, according to the standard of care for chronic hepatitis caused by HCV genotype 1 at the time of starting therapy and iv) started therapy before January 2013 in order to have a minimum follow-up of 60 weeks, so that SVR 12 weeks after ending therapy might be evaluated. Key exclusion criteria were participation in a clinical trial or having received prior DAA-based therapy. Visits were scheduled at least at treatment weeks (TW) zero, four, 12, 24, and 48, as well as 12 weeks after the scheduled end of treatment (EOT). Those patients who had a four-week lead-in phase with Peg-IFN/RBV alone were additionally seen at TW8. At each visit, plasma HCV-RNA was quantified and hematological parameters were determined. Management of adverse events (AE), as well as the decision to discontinue therapy due to side effects, was carried out according to the criteria of caring physicians.
Treatment duration, dosing and definition of response
The scheduled treatment duration for all patients was 48 weeks. Patients received a three-drug combination based on Peg-IFN alfa-2a or Peg-IFN alfa-2b at a dose of 180 μg or 1.5 μg/kg once per week, respectively, and oral RBV at daily doses of 800–1200 mg. Treatment duration and futility rules were in accordance with the package insert of BOC and TVR and international guidelines [23–25]. BOC was administered orally at doses of 800 mg every eight hours for 44 weeks. Oral TVR was given at doses of 750 mg every eight hours or 1125 mg twice a day during the first 12 weeks of therapy, or from TW5 to TW16 in patients who underwent a lead-in phase first with Peg-IFN/RBV alone, followed by Peg-IFN/RBV dual therapy until reaching the scheduled EOT. Non-response to the respective DAA was assumed when the stopping rules were met: all treatment was discontinued and non-response to BOC was assumed when plasma HCV RNA was >100 IU/mL at TW12 or when HCV RNA was detectable at W24. TVR-based therapy was discontinued and non-response was assumed if plasma HCV RNA was >1000 IU/mL four or 12 weeks after TVR initiation, or if it was detectable at TW24For both HCV PIs, an increase of HCV RNA ≥1 log10 IU/mL following a decline, as well as detectable HCV RNA following undetectability, were considered viral breakthrough (VB) and, consequently, treatment was stopped. Detectable HCV RNA at the SVR12 evaluation time point after achieving EOT response was considered relapse. SVR12 was defined as undetectable HCV-RNA 12 weeks after scheduled EOT.
Definition of previous response and hepatic fibrosis
Null response to previous interferon-based therapy was considered when patients had not achieved early virological response (EVR; a 2 log10 decline of HCV RNA or HCV RNA undetectable) at treatment week 12, while partial response was defined as having achieved EVR at week 12 without reaching undetectable HCV RNA in week 24. Patients who had achieved undetectable HCV RNA but had detectable HCV RNA at the EOT were defined as having suffered VB while those who had undetectable HCV RNA at the EOT but subsequently showed detectable HCV RNA at week 24 post-treatment were considered previous relapsers. Cirrhosis was determined by liver biopsy and defined as F4 according to the Scheuer Index [26]. If liver biopsy was unavailable, liver stiffness measurement by transient elastometry using a cut-off value of 14·6 kPa was acceptable for a diagnosis of cirrhosis [27].
Determination of HCV RNA
Plasma HCV RNA was determined by a quantitative polymerase chain reaction assay according to the available technique at each center (Cobas AmpliPrep/Cobas TaqMan HCV test v2.0, Roche Diagnostic Corporation, Pleasanton, CA, USA or Roche Diagnostics International AG, Rotkreuz, Switzerland, detection limit: 10 IU/mL; Abbott M2000 Real Time System, Abbott Diagnostic, Chicago, IL, USA; detection limit 12 IU/mL).
Statistical analysis
Descriptive statistics were conducted for the study population. Continuous variables were expressed as median [interquartile range (Q1-Q3)] and categorical variables as number [percentage; 95% confidence interval (CI)]. The primary efficacy and safety endpoints were SVR12 and the percentage of patients who discontinued therapy due to AEs, respectively. The rate of SVR12 was evaluated in i) an intention-to-treat approach, where all patients were considered and missing values were treated as failures and ii) an on-treatment approach, where those patients who discontinued therapy due to AEs, those who voluntarily dropped out and those who were lost to follow-up were not considered. The association of categorical variables with SVR12 or the proportion of patients who discontinued therapy due to AEs were analyzed using the χ2-test or the Fisher’s test, when applicable. Those factors that showed an association in the univariate analysis with a p<0.2, as well as those with a biologically plausible influence, were entered into a multivariate logistic regression model in order to identify independent predictors for SVR12. The adjusted odds ratio (AOR) and the respective 95% CI were calculated. The positive predictive value (PPV) and the negative predictive value (NPV) of SVR12 in patients achieving undetectable HCV RNA at week four on PI-based therapy were calculated. The latter analysis was restricted to the patients treated according the package inserts of BOC and TVR. Data analysis was conducted using the SPSS statistical software package release 21.0 (IBM Corporation, Somers, NY, USA), STATA 9.0 (StataCorp LP, College Station, TX, USA) and Fisterra.com (Elsevier 2012; http://www.fisterra.com/mbe/investiga/pruebas_diagnosticas/pruebas_diagnosticas.asp).
Ethical issues
Both study design and conduct conformed to the Helsinki declaration and was approved by the local Ethics Committees of the Valme University Hospital (Comité de Ética de la Investigación Sevilla Sur, Seville, Spain; Ref: 00C3u00A9). All patients gave their written informed consent to participate in the study.
Results
Study population
A total of 584 individuals coinfected with HCV genotype 1 and HIV have started BOC or TVR-based therapy at the participating institutions. Of these, 159 patients had reached week 60 of follow-up at the time of analysis (April 2014) and, thus, fullfilled the inclusion criteria for this study: 46 patients received triple therapy based on BOC and 113 individuals were treated with TVR and Peg-INF/RBV. TVR was administered as a bid regimen in ten (8.8%) of these 113 patients. The median (Q1-Q3) age was 48 (42.8–51) years, 127 (79.9%) were male and 45 (34.4%) were treatment-naïve. Assessment of liver fibrosis prior to therapy was available in 133 subjects. Of these, 63 (47.4%) were cirrhotic. The main baseline characteristics of the two groups are shown in Table 1. Five (10.9%) patients received BOC without a lead-in phase and three (2.7%) of those treated with TVR had a four-week lead-in phase prior to TVR initiation. ART was administered in all but one patient (99.4%) and, in the majority of cases, consisted of a combination of tenofovir plus emtricitabine [119 individuals (74.8%)] or abacavir plus lamivudine [20 individuals (12.6%)], along with raltegravir [81 patients (50.9%)] or ritonavir-boosted atazanavir [44 individuals, 27.7%)]. Data on HCV RNA at TW8 was available in 84 patients.
Table 1. Baseline characteristics of the patients treated with BOC or TVR.
| Parameter | BOC (n = 46) | TVR (n = 113) |
|---|---|---|
| Age (years)* | 45.4 (41.6–50.9) | 48.3 (43–51.4) |
| Male gender, no. (%) | 35 (76.1) | 92 (81.4) |
| IL28B rs12979860 CT/TT, no. (%) ± | 25 (59.5) | 70 (70) |
| HCV subtype 1a, no. (%) # | 27 (66) | 62 (66.7) |
| Plasma HCV RNA >8*105 IU/mL, no. (%) | 37 (80·4) | 77 (68.1) |
| Cirrhosis, no. (%) ¶ | 21 (51.2) | 42 (45.7) |
| Liver stiffness | 14.8 (6.8–21) | 12,3 (7.9–19.5) |
| Platelets (cells/μL) | 178 (119–228) | 168 (107–220) |
| Albumin (g/dL) | 4 (3.9–4.3) | 4 (3.9–4.2) |
| Low-density lipoprotein cholesterol (mg/dL)* | 103 (77.7–120) | 71 (46–102) |
| Alanine aminotransferase, IU/mL* | 62 (39–96) | 63 (41–97) |
| Previous response to anti-HCV therapy § | ||
| Naive, no. (%) | 19 (41.3) | 26 (23) |
| Null responders, no. (%) | 6 (13) | 36 (31.9) |
| Partial responders, no. (%) | 0 | 11 (9.7) |
| Relapsers, no. (%) | 14 (30.4) | 19 (16.8) |
| Other, no. (%) | 7 (15.2) | 21 (18.6) |
| CD4 cell count (cells/μL)* | 536 (370–670) | 630 (414–802) |
| Undetectable HIV RNA, no. (%) | 37 (80.4) | 96 (85) |
*Median (interquartile range);
±available in 100 patients receiving TVR and 42 subjects receiving BOC;
#available in 93 patients receiving TVR and 41 subjects receiving BOC;
¶available in 92 patients receiving TVR and 41 subjects receiving BOC;
§as referred to dual therapy with peg-IFN plus RBV.
Response to therapy
The median (Q1-Q3) HCV RNA decline was 1.63 (0.68–2.93) log10 IU/mL after lead-in and 5 (11.4%) subjects had an undetectable HCV-RNA at this time point. A total of 51/159 patients discontinued therapy before reaching the scheduled EOT. Among those who received BOC or TVR, respectively, the numbers were: 5 (10.7%) and 17 (12.8%) met futility rules; 4 (8.7%) and 9 (8%) discontinued due to adverse events; 3 (6.5%) and 8 (7.1%) experienced VB; 1 (2.2%) and 4 (3.5%) voluntary dropped out and 2 (4.3%) and 6 (5.3%) relapsed. A total of 100 patients achieved SVR12, accounting for 62.9% (95% CI: 54.9%-70.4%) treatment success in an intention-to-treat approach (missing = failure): 31/46 (67.4%; 95% CI: 52%-80.5%) of the subjects treated with BOC and 69/113 (61.1%; 95% CI: 51.4%-70.1%) of those treated with TVR. The numbers of patients with an undetectable HCV RNA were 60 (56·6%) at TW4, 60 (69%) at TW8, 114 (71·7%) at TW12 and 112 (70.4%) at TW24, respectively, and 108 (67.9%) patients had an undetectable HCV RNA at the scheduled EOT. The proportions of patients with undetectable HCV RNA at the different treatment points according to the treatment regimen are depicted in Fig 1.
Fig 1. Proportion of patients who presented undetectable HCV RNA at the different treatment weeks (TW).
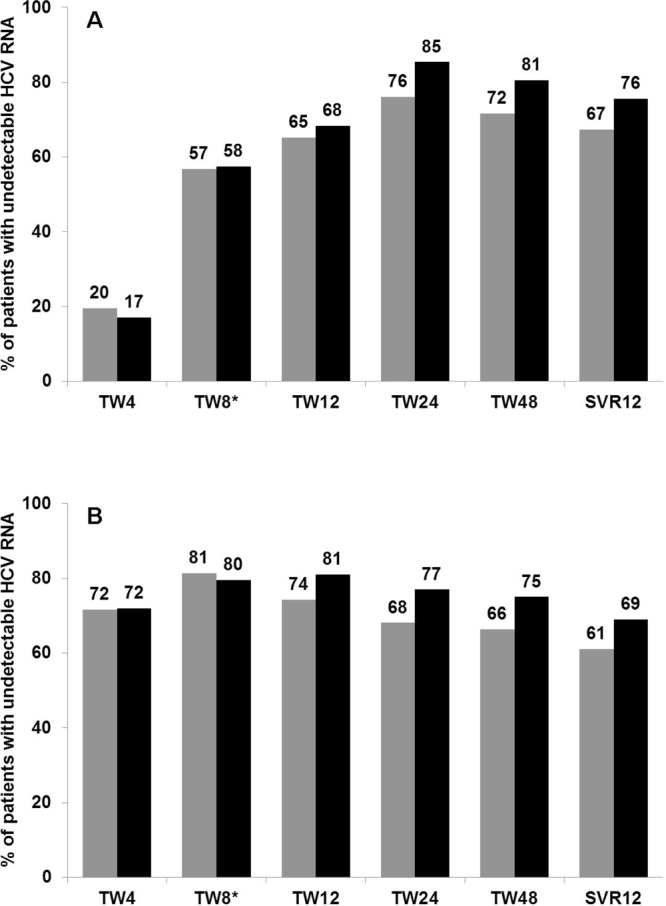
(A) Proportion of patients with undetectable HCV RNA during therapy including peg-IFN plus RBV in combination with BOC. (B) Proportion of patients with undetectable HCV RNA during therapy including peg-IFN plus RBV in combination with TVR. Grey bars: Intention-to-treat analysis; black bars: on-treatment analysis. *TW8 data was available in 44 patients receiving BOC and 43 patients receiving TVR.
Safety analysis
Treatment was discontinued due to AEs in 13/159 (8.2%; 95% CI: 4.4%-13.6%) subjects. Any AE were reported in 38/46 (82.6%) of the patients treated with BOC and 92/113 (81·4%) of those treated with TVR. Grade 3 or 4 anemia was reported in 9 (7%) patients, grade 3 or 4 thrombocytopenia was observed in 22 (17.2%) patients and 21 (16.4%) individuals presented grade 3 or 4 neutropenia. Erythropoietin was administered in 36 (22.6%) individuals and nine (5.7%) received blood transfusion. General dose reductions of Peg-IFN and RBV were reported in 16 (17.4%) and 40 (43.5%) patients of the 92 subjects respectively in whom these data were available. Table 2 summarizes detailed hematological abnormalities observed in the subgroups receiving BOC- or TVR-based therapy. Four (2.5%) individuals developed hepatic decompensation: two (4.3%) individuals treated with BOC and two (1.8%) treated with TVR. One of these patients died due to hepatic failure at TW24 on triple therapy with BOC. Two treatment discontinuations due to AEs to BOC-based therapy were due to severe hematological abnormalities and one due to development of ascites. The AEs leading to early treatment discontinuation in those who received TVR-based therapy were: hematological abnormalities (three patients), depression (three patients), hepatic encephalopathy (one patient), gastrointestinal bleeding and severe skin rash (one patient). Discontinuations due to AEs was observed in 6/73 (8.2%) of those with cirrhosis at baseline versus 7/60 (11.7%) of those without (p = 0.505). Likewise, discontinuations due to AEs was observed in 4 (3.7%) out of 109 patients with a platelet count ≥100 cells/μL versus seven (25%) of the 28 subjects with a platelet count of <100 cells/μL (p<0.001) and in 7/50 (14%) versus 2/6 (33%) of those with baseline albumin values above or below 3.5 g/L (p = 0.223), respectively. Fig 2 depicts the additive effect of baseline platelet count and albumin concentration on discontinuations due to AEs.
Table 2. Hematological abnormalities observed among patients receiving BOC- or TVR-based triple therapy and actions taken.
| Event, n (%) | BOC (n = 46) | TVR (n = 113) |
| Anemia (hemoglobin mg/dL)* | ||
| 9.5–10.9 | 16 (37.2) | 28 (32.9) |
| 8–9.4 | 3 (7) | 11 (12.9) |
| <8 | 5 (11.6) | 4 (4.7) |
| Thrombocytopenia (platelets/μL)* | ||
| 70000–99000 | 8 (18.6) | 18 (21.2) |
| 50000–69000 | 10 (23.3) | 12 (14.1) |
| <50000 | 7 (16.3) | 15 (17.6) |
| Neutropenia (neutrophils/μL)* | ||
| 1000–1499 | 11 (25.6) | 26 (30.6) |
| 750–999 | 7 (16.3) | 3 (3.5) |
| <750 | 7 (16.3) | 14 (16.5) |
| Use of erythropoietin | 22 (47.8) | 14 (12.4) |
| Blood transfusion | 5 (10.9) | 4 (3.5) |
| Dose reduction of ribavirin ± | 28 (66.7) | 63 (67.7) |
*detailed hematological data was available for 85 patients receiving TVR and 43 subjects receiving BOC;
±information available in 93 patients receiving TVR and 42 subjects receiving BOC.
Fig 2. Rate of discontinuations due to adverse events.
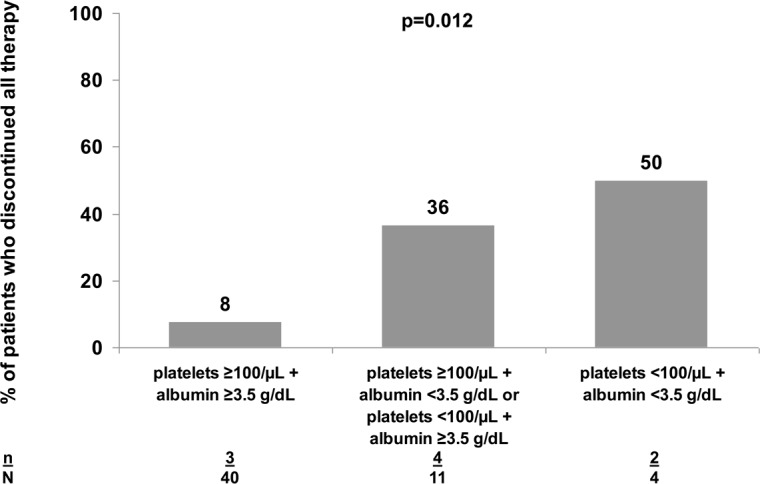
Proportion of patients who permanently discontinued all therapy due to adverse events after stratifying for baseline platelet count and albumin concentration. Data was available in 46 patients.
Predictors of response
The analysis of predictive factors for treatment success was carried out with regard to SVR12 in an on-treatment approach. A total of 24/36 (66.7%) patients who received ritonavir-boosted atazanavir and 53/74 (71.6%) individuals who received raltegravir achieved SVR12 (p = 0.821). The rates of SVR12 (n/N patients) according to HCV subtype 1a and 1b were 71.8% (56/78) versus 70% (28/40) (p = 0.839). Seventy-four (71.8%) out of 103 subjects with a platelet count of ≥100 cells/μL at baseline versus 11 (57.9%) of 19 patients with <100 cells/μL at baseline achieved SVR12 (p = 0.224). All four patients who had plasma albumin levels below 3.5 g/L versus 26/42 (61.9%) of those with baseline albumin values higher than 3.5 g/L achieved SVR12 (p = 0.126). The relationship between other possible predictors and SVR12 is shown in Table 3. In the multivariate analysis, previous response to dual therapy and lower baseline HCV RNA levels were independently associated with SVR12 (Table 3). Among those patients who achieved undetectable HCV RNA four weeks after HCV PI initiation, 97 (86.6%) achieved SVR12 versus 16 (36.4%) of those who had detectable viral load at week four (p<0.001). The PPV to predict SVR12 in patients achieving undetectable HCV RNA at week four was 85·5% (95% CI: 75.2%-92.2%) while the NPV was 49.2% (95% CI: 36.1%-62.4).
Table 3. Univariate and multivariate analysis to identify factors associated with sustained virologic response 12 weeks after scheduled end of therapy (SVR12).
| Parameter | SVR12, | P | AOR | p |
| n (%) | univariate | (95% CI) | multivariate | |
| Age | ||||
| ≤48 years | 50 (68.5) | 0.511 | 0.983 (0.906–1.066) | 0.672 |
| >48 years | 50 (73.5) | 1 | ||
| Gender | ||||
| Male | 82 (70.7) | 0.896 | 0.563 (0.417–2.157) | 0.402 |
| Female | 18 (72) | 1 | ||
| Baseline cirrhosis ± | ||||
| No | 47 (73.4) | 0.279 | 1 | |
| Yes | 34 (64.2) | 0.936 (0.306–2.865) | 0.908 | |
| Baseline HCV RNA | ||||
| <800 kIU/mL | 33 (84.6) | 0.027 | 11.959 (2.145–66.69) | 0.005 |
| ≥800 kIU/mL | 67 (65.7) | 1 | ||
| IL28B rs12979860 | ||||
| CC | 33 (84.6) | 0.025 | 1.741 (0.477–6.361) | 0.401 |
| CT/TT | 58 (65.2) | 1 | ||
| Previous response ¶ | 0.022 | |||
| Naïve | 26 (74.4) | 0.01 | 1 | |
| Relapse | 25 (92.6) | 6.799 (0.698–66.248) | 0.099 | |
| Partial response | 6 (66.7) | 3.165 (0.298–33.576) | 0.339 | |
| Null response | 22 (55) | 0.316 (0.094–1.062) | 0.062 |
AOR: adjusted odds ratio; CI: confidence interval; IL28B: interleukin 28B.
±available in 100 patients receiving TVR and 42 subject receiving BOC;
¶as referred to dual therapy with peg-IFN plus RBV.
Discussion
The results of this study show that the efficacy of triple therapy including BOC or TVR in combination with peg-IFN/RBV plus RBV in patients coinfected with HIV and HCV genotype 1 under real life conditions is comparable to that observed in clinical trials, both in HCV-monoinfected [4–7] and in HIV/HCV-coinfected [8,9] subjects. The described response rates are very well in line with the new guidelines of the European Association for the Study of the Liver (EASL) [13] which no longer separate between HIV/HCV and HCV monoinfected patients with regard to indication and choice of therapy, other than drug-drug interactions, due to similar cure rates in the two populations. In addition, these therapies are tolerable for most patients; indeed, the number of discontinuations due to AEs is slightly lower than that previously reported for dual therapy with Peg-IFN plus RBV [28,29].
A number of studies have evaluated the efficacy of first-generation PIs [16–19], however, data are derived from different populations and are thus not comparable since they differ in the proportion of patients with advanced liver damage, ethiology and social background that may impact on the adherence. The CUPIC study (compassionate use of BOC and TVR in France) reported SVR12 rates of 42.9% and 51.8%, respectively, in a treatment-experienced, cirrhotic population [16]. Furthermore preliminary results obtained from the expanded access study HEP3002 suggest that the efficacy of TVR in patients with advanced fibrosis treated in the clinical practice is comparable to that observed in clinical trials [17]. Importantly, and in contrast to the present study, these data were obtained from HIV (-) patients. Data on the use of HCV PIs in HIV/HCV-coinfected patients in clinical practice are scarce and mainly available for TVR-based triple therapy [30,31]. The present study includes a large number of HIV/HCV-coinfected patients derived from various centers throughout Europe. Most were treated with TVR, of whom 61% achieved SVR12 in an intention-to-treat analysis. This is a somewhat lower response when compared to phase II clinical trials conducted in treatment-naïve HIV/HCV-coinfected patients, with a low frequency of cirrhosis [9,15]. This is not surprising, since this study includes a higher proportion of difficult-to-treat subjects; specifically individuals bearing advanced fibrosis or cirrhosis and previous non-responders to dual therapy. These findings are, however, consistent with interim and SVR12 data reported from studies conducted in HIV/HCV-coinfected patients [30–32]. With regards to those who received BOC-based triple therapy in the present study, a SVR12 rate of 67% was observed. It is likely that the high number of previously naïve patients and relapsers included herein accounts for this comparably high response rate.
The rates of discontinuations due to AEs were 8% in the present study and thus somewhat lower than those reported in clinical trials [8,9,14,15] and even lower than that observed with dual therapy with historical Peg-IFN/RBV in some of the centers participating in the study [28,29]. This probably represents increased tolerability of medication associated side-effects in patients accessing new therapy. In this context, rates of discontinuations due to AEs have improved over time when compared within the same cohorts [28,29]. AEs, predominantly flu-like symptoms, were reported in the majority of the patients. Despite the high proportion of cirrhotic patients, only four individuals developed hepatic decompensations and the rate of discontinuations due to AEs was not influenced by baseline cirrhosis. On the other hand, and in accordance with previously reported data from the CUPIC study [16], baseline platelet count and albumin levels have an impact on the likelihood of discontinuations due to AEs. These observations suggest that although AEs are frequently observed, they are manageable, and that discontinuations rates due to AEs are not higher to what was observed with dual therapy. On the other hand our population was also highly motived as they were waiting for the first generation PI since a long time and hence they were struggling hard not to stop therapy before week 48.
The present analysis shows that treatment response four weeks after initiation of the HCV PI has a high PPV for SVR12 in HIV/HCV-coinfected patients, in real life conditions, including previous non-responders and cirrhotic patients. The role of response in the first weeks of BOC- or TVR- based therapy as predictor of treatment response had been previously described in HCV-monoinfected patients with cirrhosis [33]. In fact, response-guided therapy in HIV/HCV-coinfected patients can be applied in TVR-based therapy in previous relapsers or treatment-naïve patients without cirrhosis [24,25]. Likewise, the role of response-guided therapy in BOC-based regimens is currently being evaluated in HIV/HCV co-infected patients [34,35]. Our results suggests that response-guided shortening of therapy duration should be evaluated not only in naïve or relapsing patients, but might be also appropriate in harder-to-cure subjects.
Previous relapsers showed highest SVR12 rates when compared to treatment-naïve subjects and previous null or partial responders, while the latter show the poorest response to therapy. This is in accordance with data obtained from clinical trials conducted in HCV-monoinfected and HIV/HCV-coinfected patients [5,7], as well as the UNITE trial [10]. On the other hand, a diagnosis of cirrhosis did not significantly influence the achievement of SVR12. The reason for this finding could be that in most patients cirrhosis diagnosis was based on liver stiffness measurement or biopsy, both allowing an earlier diagnosis than clinical or biological data [36]. Late-stage cirrhosis usually affects treatment outcome. However, in this study subjects were treated with ITF-based combinations. Consequently, the vast majority of the cirrhotic patients presented compensated cirrhosis and, in this setting, the impact on SVR could be less obvious. Nevertheless, there was a difference of 9 percent-points between subjects who presented F0-F3 versus those with F4 at baseline. It is therefore likely that a higher sample size would have led to a statistically significant impact of cirrhosis on treatment outcome. However, for patients with biological abnormalities consistent with advanced cirrhosis and portal hypertension, such as hypoalbuminemia or thrombocytopenia, the rate of adverse effects was higher.
The present work has a certain limitations. The clinical impact of this work might diminish since next generation DAAs show improved efficacy over TVR or BOC, show better tolerability, a lower potential of DDIs and are easier to administer [37,38]. However, these regimens may not be widely used in the next years, because of financial constraints even in developed countries. Therefore, triple therapy including BOC or TVR may well remain of importance in the near future. Additionally, this study represents an important historic reference to evaluate the evolution of SVR rates to different regimens over time under real-life condition in the coinfected population.
In conclusion, the efficacy and safety of triple therapy including BOC or TVR in combination with Peg-IFN plus RBV in routine clinical practice in a HIV/HCV-coinfected population was acceptable and comparable to what is observed in clinical trials. Drug-related toxicity can be managed and AEs do not lead to discontinuations in the majority of the patients.
Acknowledgments
The members of the Swiss HIV Cohort Study are: Aubert V, Barth J, Battegay M, Bernasconi E, Böni J, Bucher HC, Burton-Jeangros C, Calmy A, Cavassini M, Egger M, Elzi L, Fehr J, Fellay J, Furrer H (Chairman of the Clinical and Laboratory Committee), Fux CA, Gorgievski M, Günthard H (President of the SHCS), Haerry D (deputy of "Positive Council"), Hasse B, Hirsch HH, Hösli I, Kahlert C, Kaiser L, Keiser O, Klimkait T, Kouyos R, Kovari H, Ledergerber B, Martinetti G, Martinez de Tejada B, Metzner K, Müller N, Nadal D, Pantaleo G, Rauch A (Chairman of the Scientific Board), Regenass S, Rickenbach M (Head of Data Center), Rudin C (Chairman of the Mother & Child Substudy), Schöni-Affolter F, Schmid P, Schultze D, Schüpbach J, Speck R, Staehelin C, Tarr P, Telenti A, Trkola A, Vernazza P, Weber R, and Yerly S. The data from the Swiss patients are gathered by the Five Swiss University Hospitals, two Cantonal Hospitals, 15 affiliated hospitals and 36 private physicians (listed in http://www.shcs.ch/31-health-care-providers. The members of the Vienna HIV and Liver Study Group are, Reiberger T, Schabl P, Payer BA, and Peck-Radosavljevic M.
Data Availability
All relevant data are within the paper.
Funding Statement
This work was supported by the RD12/0017/0012 (RIS-HEP07 Study Group), the Ministerio de Sanidad y Servicios Sociales (grant number EC11-304), the Fundación Progreso y Salud, Consejería de Salud de la Junta de Andalucía (grant number PI-0492-2012) and the framework of the Swiss HIV Cohort Study, supported by the Swiss National Science Foundation (grant number 134277 and SHCS project number 688). K.N. is the recipient of a Miguel Servet research grant from the Instituto de Salud Carlos III (grant number CP13/00187). D.M. is a participant in the European AIDS Clinical Society Exchange Medical Programme. A.R.-J. is the recipient of a post-doctoral extension grant of the Fundación Progreso y Salud of the Junta de Andalucía (grant number RH-0024-2013). J.A.P is the recipient of an intensification grant from the Instituto de Salud Carlos III (grant number Programa-I3SNS).
References
- 1. Mira JA, Rivero-Juárez A, López-Cortés LF, Girón-González JA, Téllez F, de los Santos-Gil I, et al. (2013) Benefits from sustained virologic response to pegylated interferon plus ribavirin in HIV/hepatitis C virus-coinfected patients with compensated cirrhosis. Clin Infect Dis 56:1646–1653. 10.1093/cid/cit103 [DOI] [PubMed] [Google Scholar]
- 2. Limketkai BN, Mehta SH, Sutcliffe CG, Higgins YM, Torbenson MS, Brinkley SC, et al. (2012) Relationship of liver disease stage and antiviral therapy with liver-related events and death in adults coinfected with HIV/HCV. JAMA 308: 370–378. 10.1001/jama.2012.7844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mira JA, Rivero A, de Los Santos-Gil I, López-Cortés LF, Girón-González JA, Márquez M, et al. (2012) Hepatitis C virus genotype 4 responds better to pegylated interferon with ribavirin than genotype 1 in HIV-infected patients. AIDS 26: 1721–1724. [DOI] [PubMed] [Google Scholar]
- 4. Poordad F, McCone J Jr, Bacon BR, Bruno S, Manns MP, Sulkowski MS, et al. (2011) Boceprevir for untreated chronic HCV genotype 1 infection. N Engl J Med 364: 1195–1206. 10.1056/NEJMoa1010494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bacon BR, Gordon SC, Lawitz E, Marcellin P, Vierling JM, Zeuzem S, et al. (2011) Boceprevir for previously treated chronic HCV genotype 1 infection. N Engl J Med 364: 1207–1217. 10.1056/NEJMoa1009482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jacobson IM, McHutchison JG, Dusheiko G, Di Bisceglie AM, Reddy KR, Bzowej NH, et al. (2011) Telaprevir for previously untreated chronic hepatitis C virus infection. N Engl J Med 364: 2405–2416. 10.1056/NEJMoa1012912 [DOI] [PubMed] [Google Scholar]
- 7. Zeuzem S, Andreone P, Pol S, Lawitz E, Diago M, Roberts S, et al. (2011) Telaprevir for retreatment of HCV infection. New Engl J Med 364: 1195–206. 10.1056/NEJMoa1010494 [DOI] [PubMed] [Google Scholar]
- 8. Sulkowski M, Pol S, Mallolas J, Fainboim H, Cooper C, Slim J, et al. (2013) Boceprevir versus placebo with pegylated interferon alfa-2b and ribavirin for treatment of hepatitis C virus genotype 1 in patients with HIV: a randomised, double-blind, controlled phase 2 trial. Lancet Infect Dis 13: 597–605. 10.1016/S1473-3099(13)70149-X [DOI] [PubMed] [Google Scholar]
- 9. Sulkowski MS, Sherman KE, Dieterich DT, Bsharat M, Mahnke L, Rockstroh JK, et al. (2013) Combination therapy with telaprevir for chronic hepatitis C virus genotype 1 infection in patients with HIV: a randomized trial. Ann Intern Med 159: 86–96. 10.7326/0003-4819-159-2-201307160-00654 [DOI] [PubMed] [Google Scholar]
- 10. Hare CB, Sherman KE, Talal A, Teba P, Bshara M, Friedman M, et al. (2013) Simplified Dosing and Response-Guided Therapy Using Telaprevir Combination Treatment for Genotype 1 HCV Treatment-Naïve or-Experienced HIV Co-infected Patients: UNITE Interim Analysis [abstract 64]. In: Global Antiviral Journal, Volume 9, Suppl. 2: HEP DART 2013: Frontiers in Drug Development for Viral Hepatitis; Hawaii, USA. [Google Scholar]
- 11.Montes M, Nelson M, Girard M, Sasadeusz J, Horban A, Grinsztejn B, al. (2013) Telaprevir combination therapy in treatment-naive and-experienced patients co-infected with hepatitis C virus and HIV: Week 12 analysis of INSIGHT [abstract 38]. In: Program and Abstracts of the 64th Annual Meeting of the American Association for the Study of Liver Diseases (Washington DC). Alexandria, VA: American Association for the Study of Liver Disease.
- 12.European AIDS Clinical Society (EACS). Clinical Management and Treatment of Chronic HBV and HCV Co-infection in HIV-positive Persons. Available: http://www.eacsociety.org/Portals/0/Guidelines_Online_131014.pdf. Accessed 30 2014.
- 13.European Association for the Study of the Liver (EASL). EASL Recommendations on Treatment of Hepatitis C 2014. Available: http://files.easl.eu/easl-recommendations-on-treatment-of-hepatitis-C.pdf. Accessed 27 August 2014. [DOI] [PubMed]
- 14.Poizot-Martin I, Bellissant E, Colson P, Renault A, Piroth L, Solas C, et al. (2014) Boceprevir for previously treated HCV-HIV coinfected patients: the ARNS-HC27 BocepreVIH Trial [abstract 659LB]. In: Program and Abstracts of the 21st Conference on Retroviruses and Opportunistic Infections (Boston). Boston, MA: Infectious Diseases Society of America.
- 15. Cotte L, Braun J, Lascoux-Combe C, Vincent C, Valantin MA, Sogni P, et al. (2014) Telaprevir for HIV-HCV coinfected patients failing peginterferon-ribavirin (ANRS HC26 TelapreVIH): an open-label, single-arm, phase 2 trial. Clin Infect Dis 15; 59: 1768–1776. [DOI] [PubMed] [Google Scholar]
- 16. Hezode C, Fontaine H, Dorival C, Zoulim F, Larrey D, Canva V, et al. (2014) Effectiveness of Telaprevir or Boceprevir in Treatment-experienced Patients with HCV Genotype 1 Infection and Cirrhosis. Gastroenterology 147:132–142.e4. 10.1053/j.gastro.2014.03.051 [DOI] [PubMed] [Google Scholar]
- 17. Colombo M, Fernández I, Abdurakhmanov D, Ferreira PA, Strasser SI, Urbanek P, et al. (2013) Safety and on-treatment efficacy of telaprevir: the early access programme for patients with advanced hepatitis C. Gut 63:1150–1158. 10.1136/gutjnl-2013-305667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Akiyama MJ, Piotrowski JI, Roytman MM, Chan SM, Hong LK, Huddleston L, et al. (2013) New triple therapy for chronic hepatitis C: real life clinical experience in a community setting. Hawaii J Med Public Health 72 Suppl 4:6–13. [PMC free article] [PubMed] [Google Scholar]
- 19. Ioannou GN, Beste LA, Green PK. (2013) Similar Effectiveness of Boceprevir and Telaprevir Treatment Regimens for Hepatitis C Virus Infection on the Basis of a Nationwide Study of Veterans. Clin Gastroenterol Hepatol 12:1371–1380. 10.1016/j.cgh.2013.12.011 [DOI] [PubMed] [Google Scholar]
- 20.Di Bisceglie A, Kuo A, Rustgi V, Sulkowski MS, Sterling RK, Stewart T, et al. (2013)Virologic Outcomes and Adherence to Treatment Algorithms in a Longitudinal Study of Patients with Chronic Hepatitis C Treated with Boceprevir (BOC) or Telaprevir (TVR) in the United States (HCV-TARGET) [abstract 41]. In: Program and Abstracts of the 64th Annual Meeting of the American Association for the Study of Liver Diseases (Washington DC). Alexandria, VA: American Association for the Study of Liver Disease.
- 21. Werner CR, Franz C, Egetemeyr DP, Janke-Maier P, Malek NP, Lauer UM, et al. (2014) Efficacy and safety of telaprevir (TVR) triple therapy in a 'real-life' cohort of 102 patients with HCV genotype 1: interim analysis after 24 weeks of treatment. J Viral Hepat 21: 333–340. 10.1111/jvh.12145 [DOI] [PubMed] [Google Scholar]
- 22. Gheorghe L, Iacob S, Simionov I, Caruntu F, Motoc A, Arama V, et al. (2014) A real life boceprevir use in treatment-experienced HCV genotype 1 patients with advanced fibrosis. J Gastrointestin Liver Dis 23: 45–50. [DOI] [PubMed] [Google Scholar]
- 23.European AIDS Clinical Society. Clinical Management and Treatment of Chronic HBV and HCV Co-infection in HIV-positive Persons. Available: http://eacsociety.org/Portals/0/Guidelines_Online_131014.pdf. Accessed 30 November 2014.
- 24.INCIVEK (telaprevir) full prescribing description. Available: http://pi.vrtx.com/files/uspi_telaprevir.pdf. Accessed 30 August 2014.
- 25.VICTRELIS (boceprevir) full prescribing description. Available: http://www.merck.com/product/usa/pi_circulars/v/victrelis/victrelis_pi.pdf. Accessed 3 October 2014.
- 26. Scheuer PJ (1991) Classification of chronic viral hepatitis: a need for reassessment. J Hepatol 13: 372–374. [DOI] [PubMed] [Google Scholar]
- 27. Vergara S, Macías J, Rivero A, Gutiérrez-Valencia A, González-Serrano M, Merino D, et al. (2007) The use of transient elastometry for assessing liver fibrosis in patients with HIV and hepatitis C virus coinfection. Clin Infect Dis 45: 969–974. [DOI] [PubMed] [Google Scholar]
- 28. Pineda JA, Mira JA, Gil IL, Valera-Bestard B, Rivero A, Merino D, et al. (2007) Influence of concomitant antiretroviral therapy on the rate of sustained virological response to pegylated interferon plus ribavirin in hepatitis C virus/HIV-coinfected patients. J Antimicrob Chemother 60: 1347–1354. [DOI] [PubMed] [Google Scholar]
- 29. Zinkernagel AS, von Wyl V, Ledergerber B, Rickenbach M, Furrer H, Battegay M, et al. (2006) Eligibility for and outcome of hepatitis C treatment of HIV-coinfected individuals in clinical practice: the Swiss HIV cohort study. Antivir Ther 11: 131–142. [PubMed] [Google Scholar]
- 30. Lacombe K, Valinb N, Stitoud H, Gozlane J, Thibault V, Boyd A, et al. (2013) Efficacy and tolerance of telaprevir in HIV-hepatitis C virus genotype 1-coinfected patients failing previous anti hepatitis C virus therapy: 24-week results. AIDS 27: 1356–1359. 10.1097/QAD.0b013e32836138d0 [DOI] [PubMed] [Google Scholar]
- 31.Martel-Laferriere V, Brinkley V, Bichoupan K, Posner S, Stivala A, Perumalswami P, et al. (2013) On-treatment responses to telaprevir-based hepatitis C treatment are similar in HIV/Hepatitis C Virus co-infected and Hepatitis C Virus mono-infected patients [abstract 679]. In: Program and Abstracts of the 20th Conference on Retroviruses and Opportunistic Infections (Atlanta). Boston, MA: Infectious Diseases Society of America.
- 32.von Wichmann MA, Moreno A, Ortega E, Mira JA, Montes M, Tellez MJ, et al. (2013) Efficacy and safety of therapy with Telaprevir (DAA) in genotype 1 coinfected patients with advanced fibrosis. Six month follow-up data. [abstract 1874]. In: Program and Abstracts of the 64th Annual Meeting of the American Association for the Study of Liver Diseases (Washington DC). Alexandria, VA: American Association for the Study of Liver Disease.
- 33. Vierling JM, Zeuzem S, Poordad F, Bronowicki JP, Manns MP, Bacon BR, et al. (2014) Safety & Efficacy of Boceprevir/Peginterferon/Ribavirin for HCV G1 Compensated Cirrhotics: Meta-Analysis of 5 Trials. J Hepatol 61: 200–209. 10.1016/j.jhep.2014.03.022 [DOI] [PubMed] [Google Scholar]
- 34.A Study to Evaluate Safety and Efficacy of Boceprevir-response Guided Therapy in Controlled HIV Patients With Chronic Hepatitis C Genotype 1 Infection Who Failed Previously to Peginterferon /Ribavirin. Available: http://clinicaltrials.gov/show/NCT01718301. Accessed 3 October 2014.
- 35. Mandorfer M, Steiner S, Schwabl P, Payer BA, Aichelburg MC, Lang G, et al. (2014) Response-guided Boceprevir-based Triple-Therapy in HIV/HCV-coinfected Patients: The HIVCOBOC-RGT Study. J Infect Dis 2015; 211: 729–735. 10.1093/infdis/jiu516 [DOI] [PubMed] [Google Scholar]
- 36. Pineda JA, Aguilar-Guisado M, Rivero A, Girón-González JA, Ruiz-Morales J, Merino D, et al. (2009) Natural history of compensated hepatitis C virus-related cirrhosis in HIV-infected patients. Clin Infect Dis 49: 1274–1282. 10.1086/605676 [DOI] [PubMed] [Google Scholar]
- 37.U.S. Food and Drug Administration. Olysio (simeprevir) for the treatment of chronic hepatitis C in combination antiviral treatment. Available: http://www.fda.gov/forconsumers/byaudience/forpatientadvocates/ucm377234.htm. Accessed 3 December 2014.
- 38.U.S. Food and Drug Administration. Approval of Sovaldi (sofosbuvir) tablets for the treatment of chronic hepatitis C. Available: http://www.fda.gov/forconsumers/byaudience/forpatientadvocates/ucm377920.htm Accessed 3 December 2014.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.


