Abstract
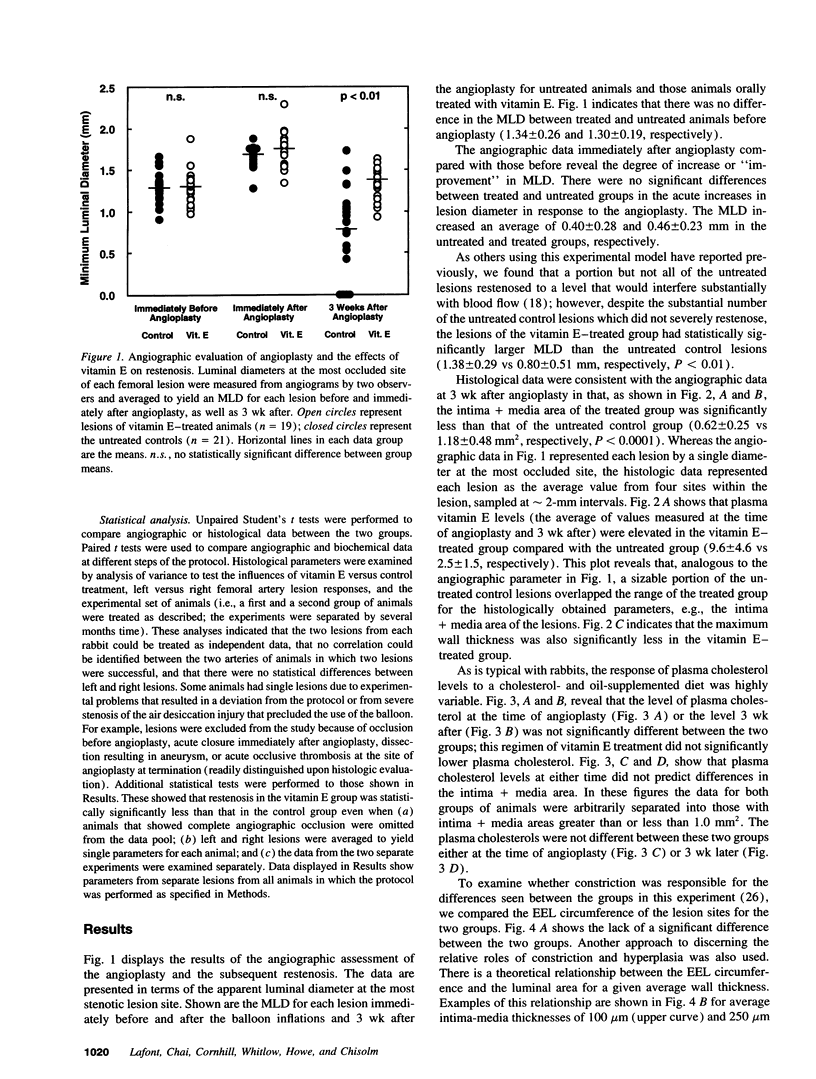
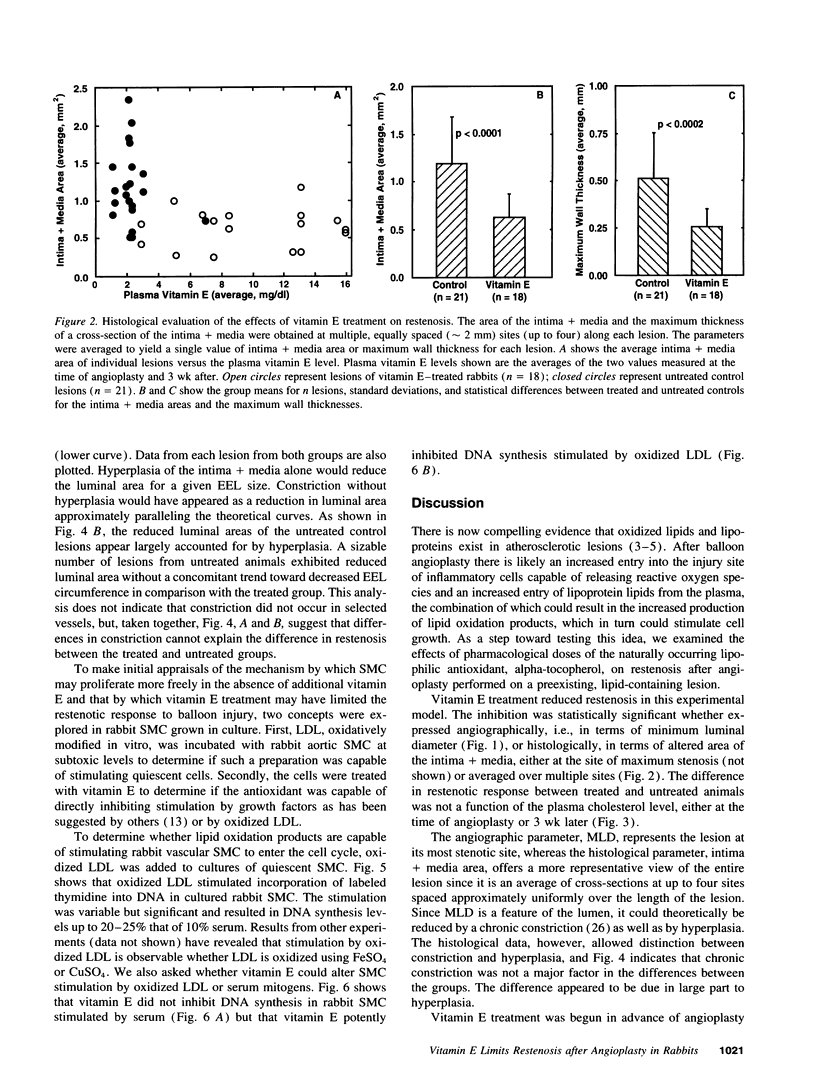
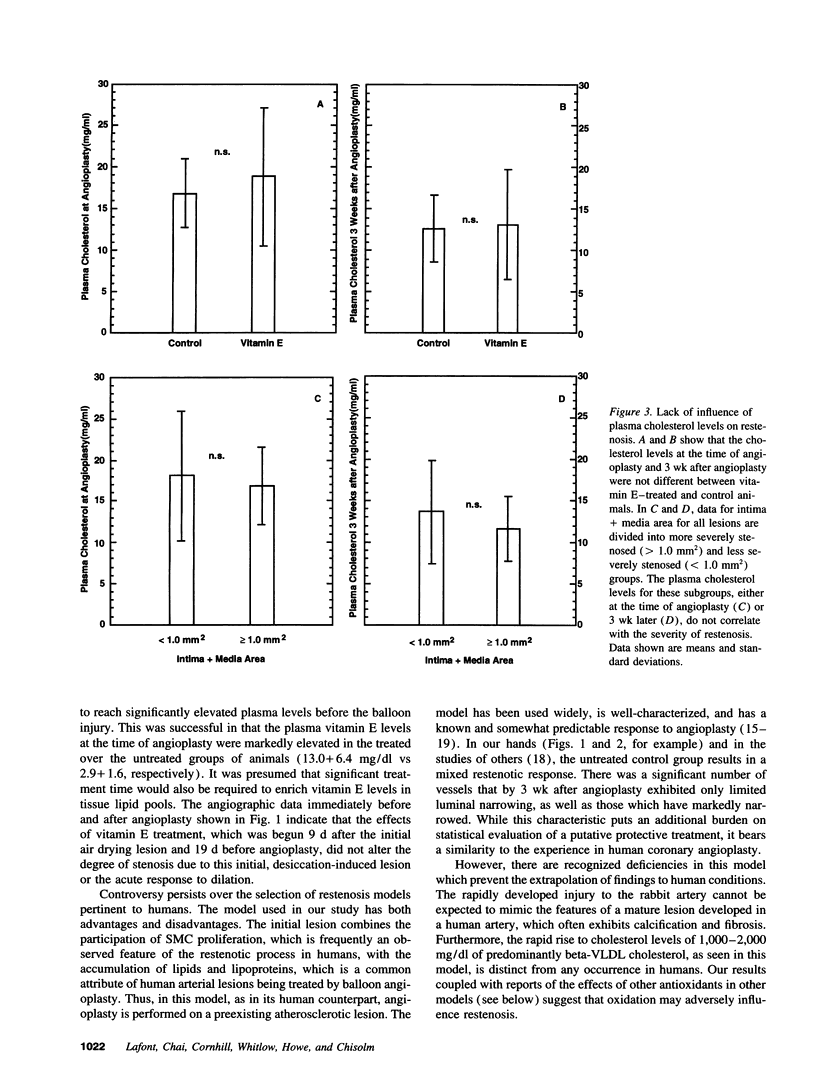
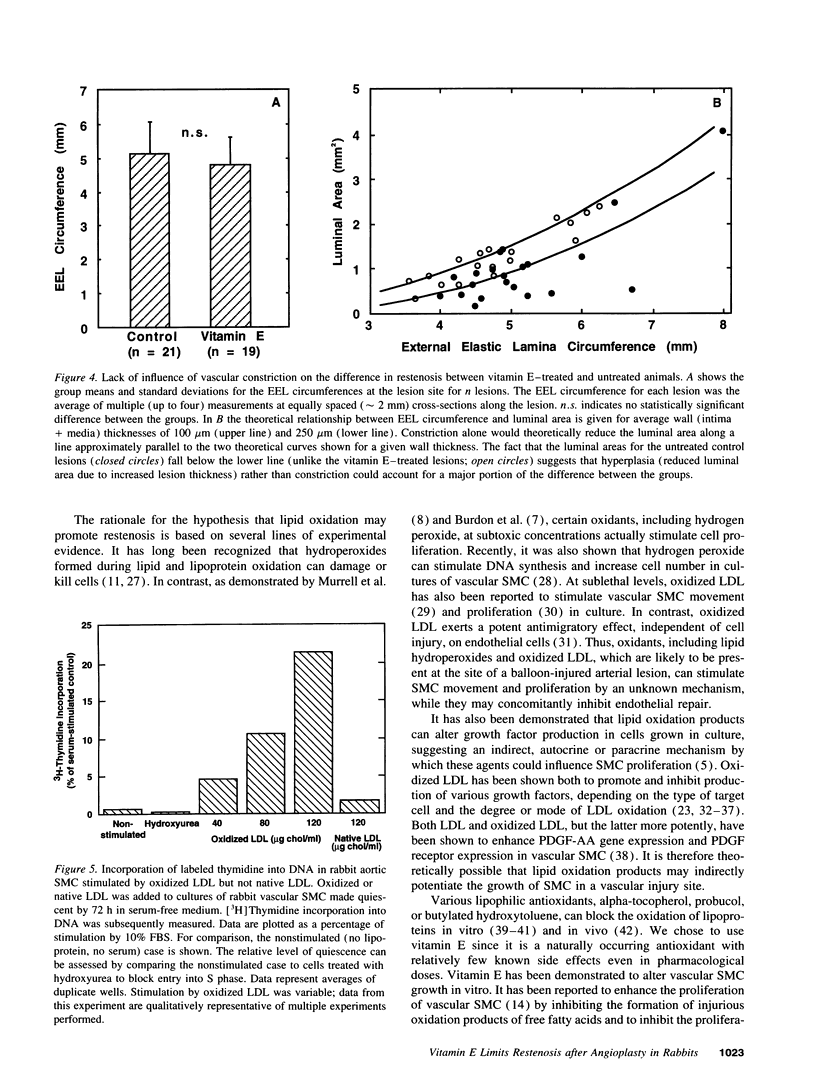
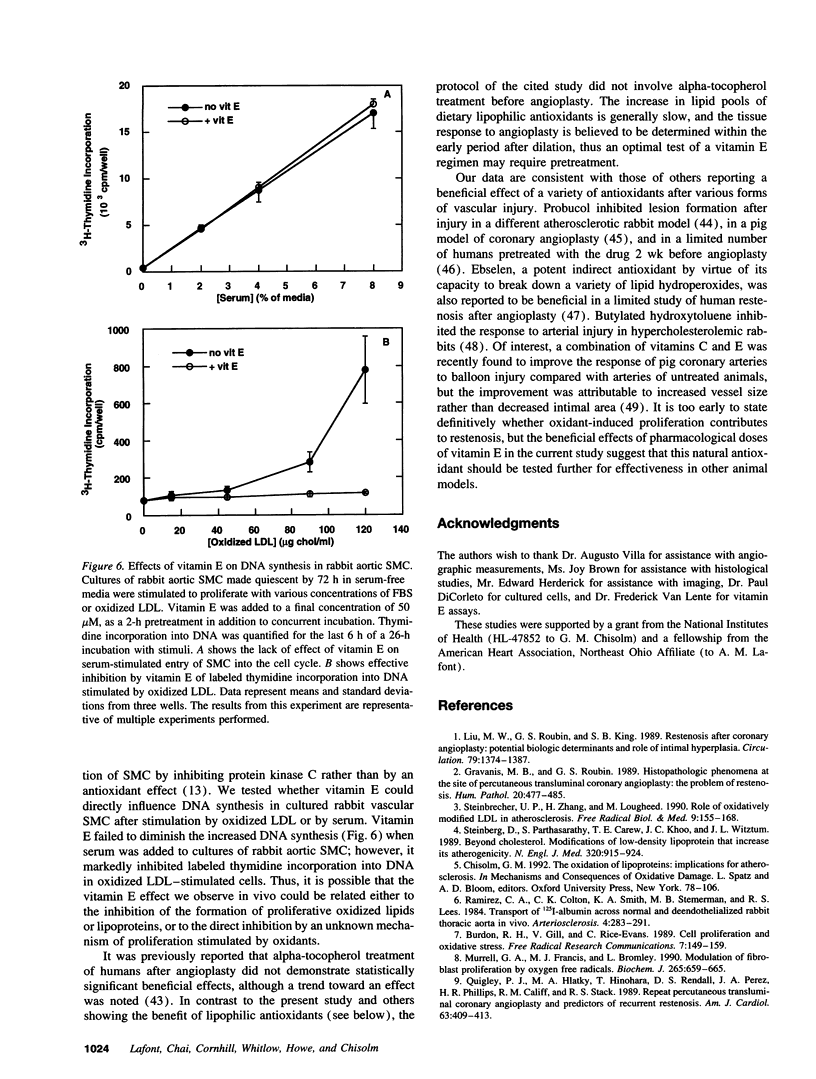
The ability of alpha-tocopherol to reduce restenosis after angioplasty was tested in a rabbit model in which angioplasty was performed on established atherosclerotic lesions. Lesions induced by 4 wk of cholesterol feeding after focal desiccation of femoral arteries were balloon dilated. 3 wk after angioplasty, angiographically determined minimum luminal diameters were less in the untreated group (0.80 +/- 0.51 mm) than in the group treated with oral alpha-tocopherol beginning 19 d before angioplasty (1.38 +/- 0.29 mm; P < 0.01). The cross-sectional area of the intima-media was greater in the untreated group (1.18 +/- 0.48 mm2) than in the alpha-tocopherol group (0.62 +/- 0.25 mm2, P < 0.0001). These differences were not due to vasoconstriction or altered plasma cholesterol. Alpha-tocopherol thus reduced restenosis after angioplasty in this model. In rabbit vascular smooth muscle cells, oxidized low density lipoprotein stimulated DNA synthesis. Alpha-tocopherol treatment inhibited DNA synthesis stimulated by oxidized low density lipoprotein, but not by serum. The findings are consistent with the hypothesis that oxidized lipids can stimulate hyperplasia and that antioxidants may limit hyperplasia by inhibiting either the oxidation or the proliferative effects of oxidants on cells.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Autio I., Jaakkola O., Solakivi T., Nikkari T. Oxidized low-density lipoprotein is chemotactic for arterial smooth muscle cells in culture. FEBS Lett. 1990 Dec 17;277(1-2):247–249. doi: 10.1016/0014-5793(90)80857-f. [DOI] [PubMed] [Google Scholar]
- Bieri J. G., Tolliver T. J., Catignani G. L. Simultaneous determination of alpha-tocopherol and retinol in plasma or red cells by high pressure liquid chromatography. Am J Clin Nutr. 1979 Oct;32(10):2143–2149. doi: 10.1093/ajcn/32.10.2143. [DOI] [PubMed] [Google Scholar]
- Boscoboinik D., Szewczyk A., Hensey C., Azzi A. Inhibition of cell proliferation by alpha-tocopherol. Role of protein kinase C. J Biol Chem. 1991 Apr 5;266(10):6188–6194. [PubMed] [Google Scholar]
- Boulanger C. M., Tanner F. C., Béa M. L., Hahn A. W., Werner A., Lüscher T. F. Oxidized low density lipoproteins induce mRNA expression and release of endothelin from human and porcine endothelium. Circ Res. 1992 Jun;70(6):1191–1197. doi: 10.1161/01.res.70.6.1191. [DOI] [PubMed] [Google Scholar]
- Burdon R. H., Gill V., Rice-Evans C. Cell proliferation and oxidative stress. Free Radic Res Commun. 1989;7(3-6):149–159. doi: 10.3109/10715768909087937. [DOI] [PubMed] [Google Scholar]
- Chatterjee S. Role of oxidized human plasma low density lipoproteins in atherosclerosis: effects on smooth muscle cell proliferation. Mol Cell Biochem. 1992 Apr;111(1-2):143–147. doi: 10.1007/BF00229586. [DOI] [PubMed] [Google Scholar]
- Chisolm G. M., Irwin K. C., Penn M. S. Lipoprotein oxidation and lipoprotein-induced cell injury in diabetes. Diabetes. 1992 Oct;41 (Suppl 2):61–66. doi: 10.2337/diab.41.2.s61. [DOI] [PubMed] [Google Scholar]
- DeMaio S. J., King S. B., 3rd, Lembo N. J., Roubin G. S., Hearn J. A., Bhagavan H. N., Sgoutas D. S. Vitamin E supplementation, plasma lipids and incidence of restenosis after percutaneous transluminal coronary angioplasty (PTCA). J Am Coll Nutr. 1992 Feb;11(1):68–73. doi: 10.1080/07315724.1992.10718198. [DOI] [PubMed] [Google Scholar]
- Farber J. L., Kyle M. E., Coleman J. B. Mechanisms of cell injury by activated oxygen species. Lab Invest. 1990 Jun;62(6):670–679. [PubMed] [Google Scholar]
- Ferns G. A., Forster L., Stewart-Lee A., Konneh M., Nourooz-Zadeh J., Anggård E. E. Probucol inhibits neointimal thickening and macrophage accumulation after balloon injury in the cholesterol-fed rabbit. Proc Natl Acad Sci U S A. 1992 Dec 1;89(23):11312–11316. doi: 10.1073/pnas.89.23.11312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fong L. G., Fong T. A., Cooper A. D. Inhibition of lipopolysaccharide-induced interleukin-1 beta mRNA expression in mouse macrophages by oxidized low density lipoprotein. J Lipid Res. 1991 Dec;32(12):1899–1910. [PubMed] [Google Scholar]
- Fox P. L., Chisolm G. M., DiCorleto P. E. Lipoprotein-mediated inhibition of endothelial cell production of platelet-derived growth factor-like protein depends on free radical lipid peroxidation. J Biol Chem. 1987 May 5;262(13):6046–6054. [PubMed] [Google Scholar]
- Freyschuss A., Stiko-Rahm A., Swedenborg J., Henriksson P., Björkhem I., Berglund L., Nilsson J. Antioxidant treatment inhibits the development of intimal thickening after balloon injury of the aorta in hypercholesterolemic rabbits. J Clin Invest. 1993 Apr;91(4):1282–1288. doi: 10.1172/JCI116326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gravanis M. B., Roubin G. S. Histopathologic phenomena at the site of percutaneous transluminal coronary angioplasty: the problem of restenosis. Hum Pathol. 1989 May;20(5):477–485. doi: 10.1016/0046-8177(89)90014-2. [DOI] [PubMed] [Google Scholar]
- Hamilton T. A., Ma G. P., Chisolm G. M. Oxidized low density lipoprotein suppresses the expression of tumor necrosis factor-alpha mRNA in stimulated murine peritoneal macrophages. J Immunol. 1990 Mar 15;144(6):2343–2350. [PubMed] [Google Scholar]
- Heinecke J. W., Rosen H., Chait A. Iron and copper promote modification of low density lipoprotein by human arterial smooth muscle cells in culture. J Clin Invest. 1984 Nov;74(5):1890–1894. doi: 10.1172/JCI111609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jougasaki M., Kugiyama K., Saito Y., Nakao K., Imura H., Yasue H. Suppression of endothelin-1 secretion by lysophosphatidylcholine in oxidized low density lipoprotein in cultured vascular endothelial cells. Circ Res. 1992 Sep;71(3):614–619. doi: 10.1161/01.res.71.3.614. [DOI] [PubMed] [Google Scholar]
- Kosugi K., Morel D. W., DiCorleto P. E., Chisolm G. M. Toxicity of oxidized low-density lipoprotein to cultured fibroblasts is selective for S phase of the cell cycle. J Cell Physiol. 1987 Mar;130(3):311–320. doi: 10.1002/jcp.1041300302. [DOI] [PubMed] [Google Scholar]
- LeVeen R. F., Wolf G. L., Villanueva T. G. New rabbit atherosclerosis model for the investigation of transluminal angioplasty. Invest Radiol. 1982 Sep-Oct;17(5):470–475. doi: 10.1097/00004424-198209000-00006. [DOI] [PubMed] [Google Scholar]
- Lindsey J. A., Zhang H. F., Kaseki H., Morisaki N., Sato T., Cornwell D. G. Fatty acid metabolism and cell proliferation. VII. Antioxidant effects of tocopherols and their quinones. Lipids. 1985 Mar;20(3):151–157. doi: 10.1007/BF02534247. [DOI] [PubMed] [Google Scholar]
- Liu M. W., Roubin G. S., King S. B., 3rd Restenosis after coronary angioplasty. Potential biologic determinants and role of intimal hyperplasia. Circulation. 1989 Jun;79(6):1374–1387. doi: 10.1161/01.cir.79.6.1374. [DOI] [PubMed] [Google Scholar]
- Malden L. T., Chait A., Raines E. W., Ross R. The influence of oxidatively modified low density lipoproteins on expression of platelet-derived growth factor by human monocyte-derived macrophages. J Biol Chem. 1991 Jul 25;266(21):13901–13907. [PubMed] [Google Scholar]
- McNamara C. A., Sarembock I. J., Gimple L. W., Fenton J. W., 2nd, Coughlin S. R., Owens G. K. Thrombin stimulates proliferation of cultured rat aortic smooth muscle cells by a proteolytically activated receptor. J Clin Invest. 1993 Jan;91(1):94–98. doi: 10.1172/JCI116206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morel D. W., Chisolm G. M. Antioxidant treatment of diabetic rats inhibits lipoprotein oxidation and cytotoxicity. J Lipid Res. 1989 Dec;30(12):1827–1834. [PubMed] [Google Scholar]
- Morel D. W., DiCorleto P. E., Chisolm G. M. Endothelial and smooth muscle cells alter low density lipoprotein in vitro by free radical oxidation. Arteriosclerosis. 1984 Jul-Aug;4(4):357–364. doi: 10.1161/01.atv.4.4.357. [DOI] [PubMed] [Google Scholar]
- Morel D. W., DiCorleto P. E., Chisolm G. M. Modulation of endotoxin-induced endothelial cell toxicity by low density lipoprotein. Lab Invest. 1986 Oct;55(4):419–426. [PubMed] [Google Scholar]
- Morel D. W., Hessler J. R., Chisolm G. M. Low density lipoprotein cytotoxicity induced by free radical peroxidation of lipid. J Lipid Res. 1983 Aug;24(8):1070–1076. [PubMed] [Google Scholar]
- Murrell G. A., Francis M. J., Bromley L. Modulation of fibroblast proliferation by oxygen free radicals. Biochem J. 1990 Feb 1;265(3):659–665. doi: 10.1042/bj2650659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murugesan G., Chisolm G. M., Fox P. L. Oxidized low density lipoprotein inhibits the migration of aortic endothelial cells in vitro. J Cell Biol. 1993 Feb;120(4):1011–1019. doi: 10.1083/jcb.120.4.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quigley P. J., Hlatky M. A., Hinohara T., Rendall D. S., Perez J. A., Phillips H. R., Califf R. M., Stack R. S. Repeat percutaneous transluminal coronary angioplasty and predictors of recurrent restenosis. Am J Cardiol. 1989 Feb 15;63(7):409–413. doi: 10.1016/0002-9149(89)90309-3. [DOI] [PubMed] [Google Scholar]
- Rajavashisth T. B., Andalibi A., Territo M. C., Berliner J. A., Navab M., Fogelman A. M., Lusis A. J. Induction of endothelial cell expression of granulocyte and macrophage colony-stimulating factors by modified low-density lipoproteins. Nature. 1990 Mar 15;344(6263):254–257. doi: 10.1038/344254a0. [DOI] [PubMed] [Google Scholar]
- Ramirez C. A., Colton C. K., Smith K. A., Stemerman M. B., Lees R. S. Transport of 125I-albumin across normal and deendothelialized rabbit thoracic aorta in vivo. Arteriosclerosis. 1984 May-Jun;4(3):283–291. doi: 10.1161/01.atv.4.3.283. [DOI] [PubMed] [Google Scholar]
- Rao G. N., Berk B. C. Active oxygen species stimulate vascular smooth muscle cell growth and proto-oncogene expression. Circ Res. 1992 Mar;70(3):593–599. doi: 10.1161/01.res.70.3.593. [DOI] [PubMed] [Google Scholar]
- Ross R. The smooth muscle cell. II. Growth of smooth muscle in culture and formation of elastic fibers. J Cell Biol. 1971 Jul;50(1):172–186. doi: 10.1083/jcb.50.1.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarembock I. J., Gertz S. D., Gimple L. W., Owen R. M., Powers E. R., Roberts W. C. Effectiveness of recombinant desulphatohirudin in reducing restenosis after balloon angioplasty of atherosclerotic femoral arteries in rabbits. Circulation. 1991 Jul;84(1):232–243. doi: 10.1161/01.cir.84.1.232. [DOI] [PubMed] [Google Scholar]
- Sarembock I. J., LaVeau P. J., Sigal S. L., Timms I., Sussman J., Haudenschild C., Ezekowitz M. D. Influence of inflation pressure and balloon size on the development of intimal hyperplasia after balloon angioplasty. A study in the atherosclerotic rabbit. Circulation. 1989 Oct;80(4):1029–1040. doi: 10.1161/01.cir.80.4.1029. [DOI] [PubMed] [Google Scholar]
- Schneider J. E., Berk B. C., Gravanis M. B., Santoian E. C., Cipolla G. D., Tarazona N., Lassegue B., King S. B., 3rd Probucol decreases neointimal formation in a swine model of coronary artery balloon injury. A possible role for antioxidants in restenosis. Circulation. 1993 Aug;88(2):628–637. doi: 10.1161/01.cir.88.2.628. [DOI] [PubMed] [Google Scholar]
- Schuh J., Fairclough G. F., Jr, Haschemeyer R. H. Oxygen-mediated heterogeneity of apo-low-density lipoprotein. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3173–3177. doi: 10.1073/pnas.75.7.3173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg D., Parthasarathy S., Carew T. E., Khoo J. C., Witztum J. L. Beyond cholesterol. Modifications of low-density lipoprotein that increase its atherogenicity. N Engl J Med. 1989 Apr 6;320(14):915–924. doi: 10.1056/NEJM198904063201407. [DOI] [PubMed] [Google Scholar]
- Steinbrecher U. P., Parthasarathy S., Leake D. S., Witztum J. L., Steinberg D. Modification of low density lipoprotein by endothelial cells involves lipid peroxidation and degradation of low density lipoprotein phospholipids. Proc Natl Acad Sci U S A. 1984 Jun;81(12):3883–3887. doi: 10.1073/pnas.81.12.3883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinbrecher U. P., Zhang H. F., Lougheed M. Role of oxidatively modified LDL in atherosclerosis. Free Radic Biol Med. 1990;9(2):155–168. doi: 10.1016/0891-5849(90)90119-4. [DOI] [PubMed] [Google Scholar]
- Stiko-Rahm A., Hultgårdh-Nilsson A., Regnström J., Hamsten A., Nilsson J. Native and oxidized LDL enhances production of PDGF AA and the surface expression of PDGF receptors in cultured human smooth muscle cells. Arterioscler Thromb. 1992 Sep;12(9):1099–1109. doi: 10.1161/01.atv.12.9.1099. [DOI] [PubMed] [Google Scholar]



