Abstract
Background
We hypothesized that the addition of toceranib to metronomic cyclophosphamide/piroxicam therapy would significantly improve disease-free interval (DFI) and overall survival (OS) in dogs with appendicular osteosarcoma (OSA) following amputation and carboplatin chemotherapy.
Methods and Findings
This was a randomized, prospective clinical trial in which dogs with OSA free of gross metastatic disease (n = 126) received carboplatin chemotherapy (4 doses) following amputation. On study entry, dogs were randomized to receive piroxicam/cyclophosphamide with or without toceranib (n = 63 each) after completing chemotherapy. Patient demographics were not significantly different between both groups. During or immediately following carboplatin chemotherapy, 32 dogs (n = 13 toceranib; n = 19 control) developed metastatic disease, and 13 dogs left the study due to other medical conditions or owner preference. Following carboplatin chemotherapy, 81 dogs (n = 46 toceranib; n = 35 control) received the metronomic treatment; 35 dogs (n = 20 toceranib; n = 15 control) developed metastatic disease during the maintenance therapy, and 26 dogs left the study due to other medical conditions or owner preference. Nine toceranib-treated and 11 control dogs completed the study without evidence of metastatic disease 1-year following amputation. Toceranib-treated dogs experienced more episodes of diarrhea, neutropenia and weight loss than control dogs, although these toxicities were low-grade and typically resolved with supportive care. More toceranib-treated dogs (n = 8) were removed from the study for therapy-associated adverse events compared to control dogs (n = 1). The median DFI for control and toceranib treated dogs was 215 and 233 days, respectively (p = 0.274); the median OS for control and toceranib treated dogs was 242 and 318 days, respectively (p = 0.08). The one year survival rate for control dogs was 35% compared to 38% for dogs receiving toceranib.
Conclusions
The addition of toceranib to metronomic piroxicam/cyclophosphamide therapy following amputation and carboplatin chemotherapy did not improve median DFI, OS or the 1-year survival rate in dogs with OSA.
Introduction
Osteosarcoma (OSA) is the most common primary bone tumor in dogs, comprising up to 85% of all reported bone neoplasia [1]. Median survival times approach 4–5 months with amputation alone, and adjuvant chemotherapy improves median survival times to 8–12 months[2]. Despite the use of various chemotherapy protocols and novel treatment approaches, clinically meaningful improvements in survival have not been achieved and 90% of dogs die of metastatic disease within 2 years after treatment [3–8].
Given that most dogs with OSA possess microscopic metastatic disease at the time of presentation, a goal of therapeutic development has been to influence factors in the tumor microenvironment that are critical for outgrowth of the metastatic tumor cells. In particular, efforts have been directed at modulating immune responses and altering tumor access to critical elements, such as blood supply as an alternative to the standard cytotoxic approaches to treatment. In veterinary medicine, metronomic chemotherapy typically consists of piroxicam and low-dose cyclophosphamide and several publications have demonstrated modulation of both the tumor microenvironment and immune response with this approach [9–11]. In both veterinary and human medicine, metronomic cyclophosphamide has been shown to downregulate CD4+ Tregs [11,12]. Metronomic cyclophosphamide administered at 15 mg/m2/day has also been associated with decreased tumor microvessel density in dogs with soft tissue sarcoma [11]. In addition, metronomic cyclophosphamide has been shown to decrease mobilization and viability of circulating endothelial precursors (CEPs) [13,14].
While NSAIDs are not included in most human metronomic chemotherapy protocols, piroxicam has been frequently used in veterinary medicine as it has been hypothesized that COX-2 expression in CEPs is important for their survival. As such COX-2 inhibition may decrease the ability of CEPs to survive and proliferate in the tumor microenvironment [15]. The combination of metronomic cyclophosphamide with piroxicam in mice with canine melanoma xenografts resulted in decreased tumor microvessel density, decreased VEGF secretion and increased TSP-1 secretion, supporting the concurrent use of metronomic chemotherapy and NSAIDs [16].
With respect to tumor angiogenesis, vascular endothelial growth factor (VEGF) and its receptor (VEGFR) are known to play critical roles in this process. In endothelial cells activation of the VEGFR stimulates multiple signaling pathways that promote endothelial cell survival, increased vascular permeability and mobilization of CEPs. Tumor cells can drive the migration of VEGFR2 expressing CEPs from the bone marrow to the tumor microenvironment through the production of VEGF and anti-VEGF/VEGFR therapy has been shown to decrease survival signaling and mobilization of CEPs to the site of tumor growth [17–20]. VEGF is detectable in both human and canine OSA, and has been associated with increased malignant potential and poor prognosis [21–25].
In human cancer therapy there are several approved inhibitors of the VEGF/VEGFR signaling axis, including the small molecules sunitinib, sorafenib, and pazopanib and the monoclonal antibody bevacizumab. While these drugs exhibit significant activity in mouse models of disease, their effects on angiogenesis and tumor progression in humans have been questionable [26–29]. With respect to OSA, several multi-targeted VEGF inhibitors have been evaluated in murine xenograft models of OSA and have demonstrated antitumor activity [30,31]. In addition, cediranib combined with gefitinib demonstrated antitumor activity and changes in VEGF and VEGFR2 levels in patients with solid tumors, including one with OSA[32]. Toceranib phosphate (toceranib; Palladia) is a multi-targeted small molecule inhibitor of several receptor tyrosine kinases (RTKs) including VEGFR, PDGFR, KIT, and FLT-3 that was developed to be an orally bioavailable anti-angiogenic agent [33–35]. Although toceranib was approved for the treatment of canine mast cell tumors by virtue of its inhibition of KIT, activity against a wide variety of tumors has been demonstrated in dogs, likely due to dysregulation of other RTKs inhibited by toceranib [33,36]. Previously published data demonstrate that doses of 2.4–2.75 mg/kg of toceranib given every other day result in statistically significant increases in plasma VEGF, a surrogate biomarker of VEGFR2 inhibition [37]. As such, the doses of toceranib currently used to treat dogs for a variety of tumors would be expected to have a similar effect.
In the setting of metastatic OSA, a retrospective study showed that approximately 48% of dogs experienced clinical benefit (primarily consisting of stable disease) following toceranib therapy [38]. In this study, many of the dogs were also treated with metronomic cyclophosphamide and/or an NSAID in conjunction with toceranib. More recently, toceranib has been shown to downregulate levels of circulating regulatory T cells in dogs with cancer, suggesting that some of its effects on tumors may be exerted through immunomodulation [39].
Given the potential complementary effects of metronomic chemotherapy and VEGF/VEGFR inhibitors, they have been combined in human medicine with variable outcomes [26,40–42]. However, in women with advanced breast cancer clinical benefit was documented in a substantial number of patients (63.6–68%) using combinations of bevacizumb and cyclophosphamide with capecitabine or methotrexate [26,42]. These data suggest that administration of continuous low-dose metronomic chemotherapy with anti-angiogenic targeted therapies may represent a valid strategy to address microscopic metastatic disease. Therefore, the purpose of this clinical trial was to evaluate the impact of toceranib phosphate combined with metronomic cyclophosphamide and piroxicam on disease free interval (DFI) and overall survival (OS) in dogs with appendicular OSA following amputation and carboplatin chemotherapy.
Materials and Methods
Eligibility and Ethics Statement
The Clinical Research and Advising Committee at the College of Veterinary Medicine at The Ohio State University and the Institutional Animal Care and Use Committee (IACUC) at The Ohio State University approved this study. IACUC approval was also obtained at the University of Wisconsin-Madison, North Carolina State University, Texas A&M University, University of Minnesota, University of Missouri, University of Georgia, Colorado State University. The private specialty practices that participated in this clinical trial do not require IACUC approval; participation in the clinical trial was at the discretion of the Veterinary Medical Oncologist designated as principle investigator at these study sites (Hope Veterinary Specialists, Malvern, PA; Veterinary Cancer Group, Tustin, CA,; New England Veterinary Oncology Group, Waltham, MA; Oradell Animal Hospital, Paramus, NJ; Southeast Veterinary Oncology and Medicine, Orange Park, FL; Sage Centers for Veterinary Specialty and Emergency Care, Concord, CA; VCA Katonah Bedford Veterinary Center, Bedford Hill, NY; Southwest Veterinary Oncology, Tucson, AZ; Veterinary Specialty Hospital of the Carolinas, Cary, NC; Veterinary Specialty Hospital, San Diego, CA; The Veterinary Cancer Center, Norwalk, CT). Informed consent was obtained from all owners prior to study entry.
Dogs free of gross metastatic disease with histologically confirmed appendicular osteosarcoma that had undergone amputation were considered for enrollment. Only appendicular sites involving long bones were considered for enrollment. Prior to enrollment, dogs underwent diagnostic tests including thoracic radiographs, complete blood count (CBC), serum biochemistry profile and urinalysis. Dogs were excluded if they received prior treatment with radiation therapy or chemotherapy, or had received non-steroidal anti-inflammatory (NSAID) drugs within 72 hours of beginning carboplatin chemotherapy.
Study Design
A total of 126 dogs with histologically confirmed appendicular osteosarcoma without evidence of gross metastatic disease were enrolled in this study following amputation. A computer generated randomization table was established prior to study initiation and study group assignment followed this table as patients were enrolled. Upon enrollment, dogs were randomized to receive piroxicam/cyclophosphamide with or without toceranib (provided by Zoetis, Florham Park, NJ) after completing carboplatin chemotherapy. All dogs began treatment with 4 cycles of single agent carboplatin (300 mg/m2 IV) every three weeks within 14 days of amputation. Prior to the fourth carboplatin treatment dogs were re-evaluated with thoracic radiographs. Dogs without evidence of pulmonary metastatic disease began oral maintenance therapy with piroxicam at 0.3 mg/kg PO EOD and cyclophosphamide at 10 mg/m2 PO EOD (alternating day of dosing with toceranib) with or without toceranib at 2.75 mg/kg PO every other day (EOD); oral therapy was initiated 3 weeks after completion of the fourth carboplatin treatment. All dogs received 0.5 mg/kg PO famotidine every 12 hours at the start of oral maintenance therapy. Chlorambucil was administered at 5 mg/m2 PO EOD in place of cyclophosphamide in the event of sterile hemorrhagic cystitis. Dogs were evaluated 2 weeks after starting oral maintenance therapy, then once every 4 weeks thereafter for the next 8 months or until progressive disease was noted. CBC and biochemistry profile were performed every 4 weeks; urinalysis was obtained every 16 weeks after starting oral maintenance therapy. Restaging with thoracic radiographs was performed every 8 weeks.
Drug Products and Concomitant Medications
Cyclophosphamide and piroxicam were compounded by the Apothecary Shoppe (now Avella Specialty Pharmacy, Columbus, OH) and mailed directly to owners. The Apothecary Shoppe is certified by the Pharmacy Compounding Accreditation Board (PCAB), and was used to decrease the likelihood of inaccurate dosing. Chlorambucil was compounded for the 8 dogs that developed cystitis/gastroenteritis on the cyclophosphamide by Diamondback Drugs (Phoenix Arizona). Toceranib was provided by Pfizer Animal Heath (now Zoetis) in 10 mg, 15 mg and 50 mg size tablets.
Adverse events were recorded and graded using the VCOG-CTCAE [43]. Concomitant medications to prevent and/or treat drug related toxicities were used at the discretion of the attending clinician and included the following: antibiotics (ciprofloxacin, cefazolin, cephalexin, amoxicillin clavulanic acid, trimethoprim-sulfamethoxazole, enrofloxacin, marbofloxacin), anti-emetics (ondansetron, maropitant, metoclopramide), gastrointestinal protectants (omeprazole, famotidine, sucralfate, pantoprazole, misoprostol, ranitidine), anti-diarrheal (loperamide, metronidazole, Pepto-Bismol, probiotics), and pain (tramadol, gabapentin, buprenorphine, fentanyl), proteinuria/hypertension (benazepril, enalapril), elevated liver transaminases (Denamarin, SAMe).
Statistical Analysis
Historical control groups were taken from two previous studies of 48 and 155 dogs with osteosarcoma treated with amputation and carboplatin chemotherapy to perform sample size calculations for the study. The reported 1-year survival rate in these two populations was 35% [3,7]. Assuming 80% power and 95% confidence, 47 dogs in each group were required to demonstrate a 50% increase in the 1-year survival rate reported in the historical control population. Due to the high attrition rate, 32 additional dogs (63 dogs total per group) were enrolled for a total of 126 dogs. Patient characteristics were summarized by treatment group (mean ± standard deviation (SD) for continuous variables and frequency (%) for categorical variables). The period between the date of amputation and development of detectable metastatic disease was defined as the disease free interval (DFI). Dogs were censored from the DFI analysis if they did not have documented metastatic disease at the time of death or last follow-up. Necropsy examination was not required, however dogs with radiographic, cytologic/histopathologic evidence of disease progression were included in the DFI analysis. Overall survival (OS) was defined as the time from amputation to the date of death or euthanasia. Dogs were censored from the survival analysis if they were alive at the time of last follow-up or were lost to follow-up. Kaplan-Meier curves were used to describe DFI and OS for each group and Cox regression was used to test for a difference in DFI or OS adjusting for characteristics found to differ by group at baseline. OS was chosen instead of disease-specific survival to avoid under-reporting of disease-specific deaths. Plots of Schoenfeld Residuals [44,45] and tests of an interaction with time (log-transformed) were used to determine if the proportional hazards (PH) assumption of Cox regression was violated. If PH was violated, the interaction with time was retained and hazard ratios were estimated at four time points: 100 days (roughly the time at which maintenance therapy began), time at which the hazards of the two groups converged, one time point halfway between 100 days and time of convergence, and a final time point halfway between the time of convergence and the maximum follow-up time. Two types of analyses were performed for DFI: an intent-to-treat analysis and an adherence analysis. Dogs in both analyses were censored if there was no evidence of metastatic disease. In the intent-to-treat analysis, dogs free of metastatic disease were censored at the last known time alive without metastatic disease. For the adherence analysis, dogs that left the study prior to the development of metastatic disease due to owner preference or another medical condition were censored at the time they withdrew from the study. The remaining dogs in the adherence analysis were censored at the last known time without metastatic disease. An adherence analysis was not performed for OS since many patients withdrew due to medical issues that put them at a greater risk of death than patients who remained in the study; had these patients been included in the analysis, the independent censoring assumption of the Kaplan-Meier estimator and Cox regression would have been violated. Survival outcomes were also compared by tumor location (proximal humerus versus all other locations) to assess whether location impacted outcome between treatment groups. For adverse events that were experienced by at least five patients and at least two per treatment group, event rates were compared across treatment groups using Poisson regression with robust standard errors estimated using Generalized Estimating Equations (GEE). Adverse events occurring in only one treatment group were rare, and only those common enough to be compared statistically were included in this analysis. Survival analyses were performed using Intercooled Stata 11 (StataCorp, College Station, TX). Poisson regression was performed using PROC GENMOD in SAS version 9.2 (SAS Inc., Cary, NC).
Results
Patient Demographics
This was a multi-institutional study, with a total of 126 dogs (63 toceranib-treated, 63 control) enrolled from September 2010 through August 2012. Patients in the control group were heavier and more likely to be male (Table 1). Thus, adjustments were made for patient weight and gender (male, female ignoring castrated/spayed status) in the Cox regression analyses.
Table 1. Distribution of patient characteristics.
| Variable | Toceranib (n = 63) | Control (n = 63) | Total (n = 126) | |
|---|---|---|---|---|
| Age (yrs) | 8.4 ± 2.7 | 8.0 ± 2.6 | 8.2 ± 2.6 | |
| Weight (kg) | 35.9 ± 10.4 | 40.8 ± 11.8 | 38.3 ± 11.3 | |
| Gender | Female | 0 (0%) | 1 (1.6%) | 1 (0.8%) |
| Female Spayed | 36 (57.1%) | 29 (46.0%) | 65 (51.6%) | |
| Male | 1 (1.6%) | 1 (1.6%) | 2 (1.6%) | |
| Male Castrated | 26 (41.3%) | 32 (50.8%) | 58 (46.0%) | |
| Breed | Pure Bred | 13 (20.6%) | 11 (17.5%) | 24 (19.1%) |
| Mixed Breed | 50 (79.4%) | 52 (82.5%) | 102 (80.9%) | |
| Tumor Location | Proximal Humerus | 16 (25.4%) | 15 (23.8%) | 31 (24.6%) |
| Other | 47 (74.6%) | 48 (76.2%) | 95 (75.4%) | |
Dosage and Drug Modifications
The starting dose of carboplatin for all dogs was 300 mg/m2. The median dose of carboplatin administered during the study was 300 mg/m2. Nineteen dogs had dose adjustments (range: 250–290 mg/m2). More than one dose adjustment occurred in 4 dogs. Carboplatin dose-delays due to adverse events occurred in 33 dogs. The median dose of toceranib administered was 2.73 mg/kg EOD. Twenty-seven dogs had toceranib dose-reductions. In addition, temporary toceranib discontinuation occurred in 10 dogs due to adverse events, prior to re-instituting therapy. Drug discontinuation generally lasted 1 week. Cyclophosphamide-induced cystitis resulted in 7 dogs switching from cyclophosphamide to chlorambucil at 5 mg/m2 EOD. Chlorambucil was substituted for cyclophosphamide in 1 dog due to gastrointestinal adverse events.
Patient Outcome
Thirty-two dogs (25%) developed progressive disease prior to beginning oral therapy at week 14, of which 12 dogs developed metastatic lesions outside of the pulmonary parenchyma. This is consistent with previous reports of carboplatin chemotherapy in dogs with appendicular OSA. Thirteen dogs withdrew prior to starting oral maintenance therapy due to owner non-compliance or other unrelated medical conditions. Of the 126 dogs entered into the study, 81 dogs (64%) received the metronomic treatment. Of the 46 dogs that received oral therapy with toceranib, 9 dogs completed the study protocol, 20 dogs withdrew due to the development of metastatic disease, and 17 withdrew due to owner non-compliance or other unrelated medical conditions. Of the 35 dogs in the control group that received oral therapy without toceranib, 11 dogs completed the study protocol, 15 dogs withdrew due to the development of metastatic disease, and 9 withdrew due to owner non-compliance or other unrelated medical conditions.
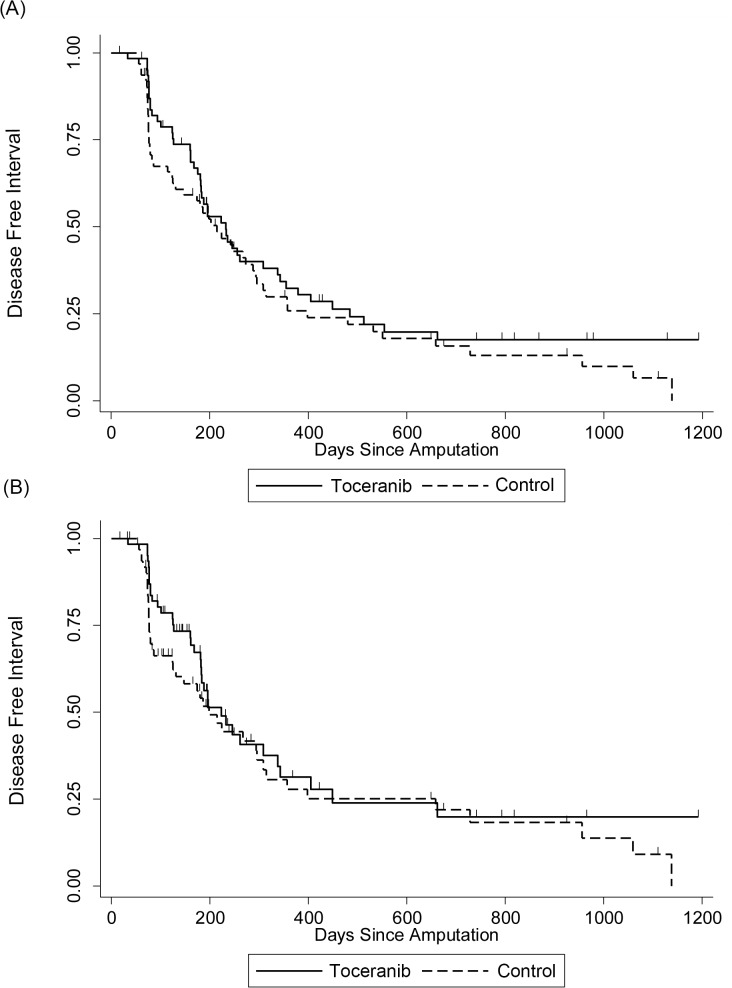
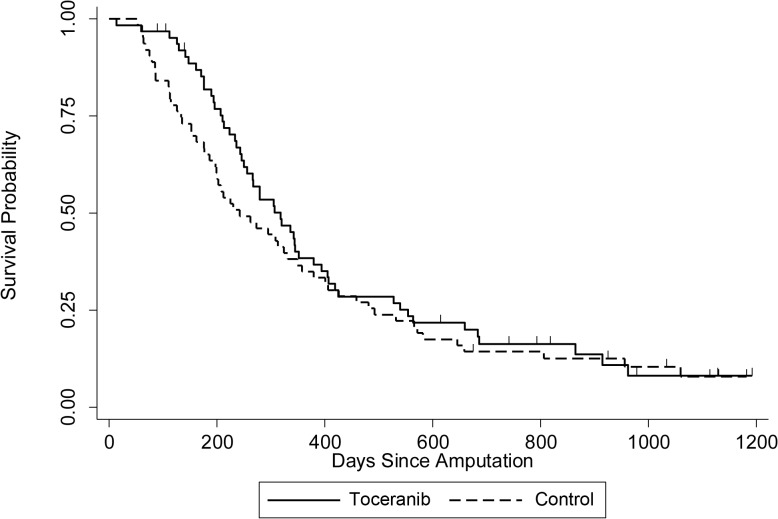
The median DFI reported in the intent-to-treat analysis was 233 days for toceranib-treated dogs and 215 days for control dogs (p = 0.274, Fig 1A, Tables 2 and 3). The median DFI reported in the adherence analysis was 223 days for toceranib-treated dogs and 198 days for control dogs (p = 0.3, Fig 1B, Tables 2 and 3). The median OS was 318 days and 242 days for toceranib-treated and control dogs, respectively (p = 0.08) (Fig 2, Tables 2 and 3). DFI was similar across treatment groups, however there was a time-dependent difference in short-term mortality risk (Fig 2 and Table 3). Initially, the mortality hazard was greater among control patients, but at approximately 400 days the risks converged and were not significantly different at one year (p = 0.961) and two years (p = 0.316). The 1- and 2-year survival rates of toceranib treated group were not significantly different from those of the control group (p = 0.963 and 0.325, respectively) (Table 2). Median DFI (182 days, p = 0.281) and OS (243 days, p = 0.28) were shorter among patients whose cancer was located in the proximal humerus, but this difference was not significant (Table 4). In addition, proximal humeral location did not affect the differences between treatment groups (p > 0.28 for each survival outcome).
Fig 1. Disease Free Interval.
Kaplan-Meier disease-free interval (DFI) curves comparing dogs treated with toceranib to control dogs. Hash marks denote censored observations; n = 63 toceranib-treated dogs; n = 63 control dogs. (A) Intent-to-treat analysis (p = 0.274). (B) Adherence analysis (p = 0.3).
Table 2. Median Disease Free Interval (DFI), Overall Survival (OS) and survival proportions by treatment group.
| Outcome | Survival Proportions | ||||
|---|---|---|---|---|---|
| Analysis | Group | Median (days) | 1 year | 2 years | 3 years |
| DFI ITT | Toceranib | 233 | 0.323 | 0.176 | 0.176 |
| Toceranib | (181, 338) | (0.206, 0.447) | (0.086, 0.292) | (0.086, 0.292) | |
| Control | 215 | 0.259 | 0.131 | 0.065 | |
| Control | (125, 288) | (0.152, 0.378) | (0.055, 0.241) | (0.014, 0.175) | |
| DFI A | Toceranib | 223 | 0.313 | 0.199 | 0.199 |
| Toceranib | (182, 338) | (0.177, 0.459) | (0.083, 0.351) | (0.083, 0.351) | |
| Control | 198 | 0.278 | 0.183 | 0.091 | |
| Control | (125, 296) | (0.153, 0.418) | (0.078, 0.323) | (0.019, 0.235) | |
| OS ITT | Toceranib | 318 | 0.384 | 0.163 | 0.082 |
| Toceranib | (250, 379) | (0.263, 0.505) | (0.082, 0.268) | (0.024, 0.184) | |
| Control | 242 | 0.349 | 0.143 | 0.078 | |
| Control | (197, 331) | (0.235, 0.466) | (0.070, 0.240) | (0.025, 0.171) | |
Intervals reported are 95% confidence intervals.
ITT = Intent to treat; A = Adherence
(N = 63 toceranib-treated dogs, 63 control dogs)
Table 3. Hazard Ratios (HRs) and confidence intervals from Cox regression analysis.
| Unadjusted | Adjusted a | ||||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | p-value | HR | 95% CI | p-value | ||
| DFI | ITT | 0.80 | (0.54, 1.19) | 0.274 | 0.80 | (0.53, 1.20) | 0.461 |
| Adherence | 0.79 | (0.50, 1.23) | 0.300 | 0.76 | (0.48, 1.21) | 0.252 | |
| OS b | 1 year | 1.01 | (0.66, 1.54) | 0.963 | 1.01 | (0.64, 1.59) | 0.961 |
| 2 years | 1.40 | (0.72, 2.75) | 0.325 | 1.45 | (0.70, 3.00) | 0.316 | |
aAdjusted for weight and gender (male, female).
bProportional hazards violated (p = 0.08 for OS ITT analysis, p<0.05 for all others).
ITT = Intent to treat
Fig 2. Overall Survival.
Kaplain-Meier overall survival (OS) curves comparing toceranib-treated dogs with control dogs. Hash marks denote censored observations; n = 63 toceranib-treated dogs; n = 63 control dogs (p = 0.08).
Table 4. Median disease free interval, median survival (in days) and Cox regression analysis of cancer location.
| Analysis | Location | Median (days) | 95% CI | HR | 95% CI | p-value |
|---|---|---|---|---|---|---|
| DFI ITT | Proximal Humerus | 182 | (127, 261) | 1.28 | (0.82, 2.01) | 0.281 |
| Other | 233 | (184, 296) | - | - | - | |
| DFI A | Proximal Humerus | 182 | (124, 338) | 1.17 | (0.70, 1.94) | 0.557 |
| Other | 224 | (183, 309) | - | - | - | |
| OS ITT | Proximal Humerus | 243 | (176, 309) | 1.27 | (0.82, 1.97) | 0.280 |
| Other | 318 | (242, 352) | - | - | - |
(N = 31 proximal humerus, 95 other locations)
ITT = Intent to treat; A = Adherence
Adverse Events
Adverse events were similar to those previously reported for carboplatin and toceranib. Grade 1 (n = 77) and 2 (n = 18) neutropenia and grade 1 thrombocytopenia (n = 26) were the most common hematologic adverse events observed during carboplatin chemotherapy. Grade 1 diarrhea (n = 31), lethargy (n = 22) and vomiting (n = 18) were also commonly reported during carboplatin administration. The primary toxicities observed during oral therapy were gastrointestinal and hematologic. S1 Table shows adverse event rates for all events common enough to be modeled using Poisson regression. Patients randomized to toceranib experienced more episodes of diarrhea, neutropenia, and weight loss than patients randomized to control, although these toxicities were low-grade and typically resolved with supportive care and dose modifications. Temporary drug discontinuation due to adverse events was usually 1 week in duration. Any dog requiring drug discontinuation longer than 2 weeks was removed from the study. More toceranib-treated dogs (n = 8) were removed from the study for therapy-associated adverse events compared to control dogs (n = 1). Seven dogs developed cyclophosphamide-induced cystitis (n = 2 control; n = 5 toceranib-treated), resulting in replacement of cyclophosphamide with chlorambucil. Gastrointestinal adverse events in one toceranib-treated dog resulted in a switch from cyclophosphamide to chlorambucil.
Gastrointestinal
Thirteen dogs experienced grade 1 vomiting while on toceranib, two of which experienced more than one episode. One toceranib-treated dog developed grade 4 vomiting and diarrhea in conjunction with pancreatitis. The vomiting and diarrhea resolved with supportive care and temporary drug discontinuation. Ten control dogs experienced grade 1 vomiting, with 3 dogs experiencing more than one episode. Seventeen toceranib-treated dogs developed grade 1 diarrhea, with 12 dogs experiencing more than 1 episode. Sixteen dogs developed grade 2 diarrhea while on toceranib, with 6 dogs experiencing more than 1 episode. Two toceranib-treated dogs developed grade 3 diarrhea, with one dog experiencing 3 episodes of diarrhea. Both dogs with grade 3 diarrhea were removed from the study due to gastrointestinal adverse events. Seven dogs not receiving toceranib developed grade 1 diarrhea, with one dog experiencing 2 episodes of diarrhea. Anorexia was generally mild and limited to grade 1 and 2 adverse events in both arms of the study.
Hematologic
Grade 1 neutropenia was the most common hematologic adverse event in toceranib-treated dogs, with 14 dogs developing transient neutropenia. No dose adjustments or temporary drug discontinuation were necessary due to transient low-grade hematologic toxicity. Six dogs receiving toceranib, and 8 control dogs experienced grade 1 thrombocytopenia. One dog experienced a grade 5 thrombocytopenia which occurred in the setting of widespread metastatic disease.
Biochemical
The majority of elevations in liver transaminases were grade 1 and 2. Two dogs receiving toceranib experienced grade 3 ALT elevations that resolved with a temporary toceranib discontinuation and supportive care. One control dog experienced a grade 3 and 4 ALT and ALP elevation, respectively, which resolved with temporary discontinuation of oral therapy.
Neuromuscular
Grade 1 and 2 weakness was seen in 4 toceranib-treated dogs and 3 control dogs. Two dogs receiving toceranib developed grade 3 weakness, with three separate episodes occurring in 1 dog. In both dogs, each episode resolved with a temporary toceranib discontinuation. Low-grade musculoskeletal pain/lameness was noted in the absence of progressive disease in 8 toceranib-treated dogs and 5 control dogs.
Other
Other adverse events observed in dogs enrolled in this study included: elevated creatinine kinase; otitis media; fever; urinary tract infection; epistaxis; stranguria; hyperglycemia; hypoglycemia; indolent ocular ulcer; seizure; shaking/panting; skin erythema; pyoderma; hyperkalemia; hypokalemia; hypoalbuminemia; hypercalcemia; ataxia; motor neuropathy; sensory neuropathy. These adverse events were believed to be unrelated to toceranib/oral therapy, and instead likely represent progression of disease or other co-morbid conditions.
Discussion
The benefits of amputation and adjuvant chemotherapy for dogs with OSA are well established in veterinary medicine, however most dogs will succumb to metastatic disease within 1 year of amputation and adjuvant chemotherapy, with 2 year survival rates reported in most studies of only 10–15% [8,46]. Despite numerous attempts to improve outcome, little progress has been made in extending survival for affected dogs. The purpose of this clinical trial was to determine whether the addition of metronomic chemotherapy using piroxicam and cyclophosphamide alone or in combination with toceranib would impact both disease free interval and overall survival in dogs with appendicular OSA following amputation and carboplatin chemotherapy. Results from this study demonstrate that piroxicam and cyclophosphamide metronomic therapy with toceranib did not improve median DFI, median OS or the 1-year survival rate in dogs with appendicular OSA over piroxicam and cyclophosphamide alone.
The median DFI and survival times reported here are similar to those reported for dogs that receive carboplatin chemotherapy after amputation [3,7]. Furthermore, the 1-year survival rates of 38.4% and 34.9% for toceranib-treated dogs and control dogs, respectively, are comparable to the 35.4% 1-year survival rate reported for carboplatin alone. Analogous results have been obtained with doxorubicin alone or in combination with carboplatin, cisplatin alone, and carboplatin given concurrently with gemcitabine [5,8,47–49]. A recent retrospective study compared multiple chemotherapy protocols used for canine appendicular OSA post-amputation. Differences in dose intensity and the chemotherapy protocol chosen did not significantly influence the reported median DFI and OS of 291 days and 284 days, respectively [8]. These data support the notion that alteration of therapeutic approaches to treat microscopic metastatic disease using currently available cytotoxic chemotherapeutics is unlikely to result in significant benefit for dogs with OSA.
Therapeutic approaches that target both the tumor itself as well as the tumor microenvironment are increasingly recognized as important components in regulating the metastatic process and several studies have investigated this approach. For example, studies in veterinary medicine have reported on safety and efficacy of metronomic chemotherapy protocols in the treatment of various tumors [9,10,50,51]. The combination of metronomic cyclophosphamide (10 mg/m2) and piroxicam (0.3 mg/kg) was shown to be well-tolerated and suggests that metronomic chemotherapy prolongs DFI for incompletely resected canine soft tissue sarcomas [9]. The use of metronomic cyclophosphamide has been also shown to decrease the number of circulating regulatory T-cells (Treg) and tumor microvessel density in tumor-bearing dogs when cyclophosphamide was administered at 15 mg/m2/day [11]. Unfortunately, the doses and regimens used in metronomic chemotherapy protocols are largely anecdotal, and predictive biomarkers of clinical benefit in dogs with OSA have not been thoroughly evaluated. In the present study cyclophosphamide and piroxicam were dosed at 10 mg/m2 and 0.3 mg/kg EOD, respectively. This therapeutic regimen was established based on the available literature at the beginning of the study, prior to demonstration of the immunomodulatory and antiangiogenic effects of higher doses of cyclophosphamide. Given the reported immunomodulatory and anti-angiogenic effects of cyclophosphamide used at higher doses, it is possible that the lower dose of cyclophosphamide in the present study and the use of EOD dosing resulted in a treatment regimen insufficient to significantly affect the tumor microenvironment, the number of circulating Tregs, or both.
While biologic activity of toceranib in metastatic canine appendicular osteosarcoma has been reported [37], the lack of improvement in DFI and OS in the present study suggests there may be different molecular drivers at different stages of disease in canine OSA. It is possible that while effects on tumor growth are observed in dogs with macroscopic metastatic pulmonary lesions following toceranib therapy, microscopic lesions may become resistant to therapy with in a short period of time, thereby negating any potential therapeutic value. Data generated from several murine models have suggested that VEGF inhibitors modulate the tumor microenvironment in a manner that can actually accelerate metastatic tumor growth [28,29,52]. There has therefore been concern that the use of VEGFR inhibitors in the setting of microscopic disease could actually promote a more aggressive phenotype. Importantly, this study demonstrated that the use of toceranib in dogs with microscopic metastatic OSA did not result in shortened survival times or aberrant patterns of metastasis that would indicate the induction of a more aggressive disease phenotype following treatment.
Recently, administration of toceranib at 2.75 mg/kg EOD to cancer-bearing dogs was associated with a significant decrease in circulating Tregs, possibly through indirect immunomodulatory mechanisms [39]. Doses of toceranib below the label dose (ranging from 2.4–2.9 mg/kg EOD) still result in target inhibition, supporting the use of 2.75 mg/kg in the present study [37]. Therefore, the lack of improvement in DFI and OS reported here is unlikely secondary to the use of a lower dose of toceranib than the current label dose. Although VEGFR inhibition was not specifically assessed in this study, upregulation of plasma VEGF concentrations following toceranib treatment has been previously demonstrated in dogs receiving 2.4–2.9 mg/kg EOD, consistent with effective VEGFR2 inhibition [37].
Adverse events necessitating administration of chlorambucil in place of cyclophosphamide occurred in 8 dogs, with all but one of these due to sterile cystitis. The overall incidence of cyclophosphamide-induced cystitis is low in the veterinary literature, and increased risk has been associated with a higher cumulative cyclophosphamide dose and higher dose intensity [53]. As there were a small number of dogs in each group that developed cystitis (n = 2 control, n = 5 toceranib), and it is likely coincidence that a higher number of toceranib-treated dogs developed cystitis. The use of metronomic chlorambucil has been associated with some antitumor activity in a variety of spontaneous canine tumors, and decreases in numbers of circulating Tregs compared to baseline has been documented in dogs receiving chlorambucil [51]. While the immunomodulatory effects of metronomic cyclophosphamide and chlorambucil have been reported, the impact of chlorambucil administration in this study is not known. However, given the small number of dogs that went on to receive this drug in place of cyclophosphamide, it was unlikely to significantly impact outcome in either group.
Multiple investigations have reported on the prognostic significance of OSA occurring in the proximal humerus [3,7,54–57]. While decreased survival times were noted in dogs with tumors located in the proximal humerus in the current study, the difference in OS was not significant compared to other tumor locations. This may have been due to the small number of dogs that enrolled in the study with proximal humerus disease (n = 31). Alternatively, it is possible that the metronomic therapy had an effect on outcome in this population to improve survival. Future controlled studies would be necessary to determine the benefits of metronomic chemotherapy in this subset of patients.
Toceranib-treated dogs had a higher adverse event profile compared to control dogs, although the adverse events reported were consistent with those expected based on prior published studies. It is important to note that in the current study, dogs experiencing adverse events while on toceranib were effectively managed with drug holidays, concomitant medications and/or dose reductions. The frequency of adverse events was lower than that reported in a recent clinical trial of dogs with various solid tumors receiving toceranib at doses of 2.4–2.9 mg/kg [37]. In this study, the adverse event profile was far superior to that associated with the label dose of toceranib (3.25 mg/kg) but both adequate drug exposure and biologic activity were maintained [37]. Despite the inherent difficulties in comparing findings from various studies, the dose of toceranib utilized in the present study (2.75 mg/kg) was better tolerated compared to the label dose, allowing improved adherence to the treatment protocol [36,37].
While necropsy was not required in the current study and the detection of metastatic disease was largely dependent on clinical findings and thoracic radiographs, it is unlikely that gross metastatic disease in other locations was missed in many dogs. The incidence of gross OSA metastasis detectable with abdominal ultrasound has been previously reported, with abdominal metastasis identified in 0–1.7% of dogs that do not have evidence of pulmonary metastatic disease [58,59]. Additionally, the reported rate for detecting unsuspected osseous metastasis with nuclear scintigraphy in dogs with appendicular OSA at the time of initial presentation (prior to amputation) is 7.8% [60]. Thus, while the lack of necropsy may have contributed to an under-reporting of the true incidence of metastasis, thoracic radiography is considered a reliable diagnostic procedure to detect metastatic disease for most dogs following amputation and chemotherapy, with extrathoracic metastases usually identified by virtue of clinical signs (i.e., lameness for bone metastasis).
It is possible that the use of compounded therapeutics could have impacted our results. The Apothecary Shoppe (now Avella Specialty Pharmacy) was chosen as the supplier of compounded cyclophosphamide and piroxicam as this pharmacy is certified by the Pharmacy Compounding Accreditation Board. However it is recognized that compounding may have resulted in inadvertent overdosing or under dosing, resulting in increased toxicity or sub-therapeutic drug concentrations in some patients, respectively.
A potential weakness of the current study is that the metronomic treatment regimen began after completion of carboplatin chemotherapy. This may have given the remaining microscopic metastatic tumor cells time to develop resistance to multiple therapies, thus negating any impact of the subsequent treatment. While the use of metronomic cyclophosphamide and piroxicam administered concurrently with carboplatin for dogs with appendicular osteosarcoma was reported to be well-tolerated in another study [50], no improvement in survival time was noted with concurrent administration. Nevertheless, it may be warranted to determine if concurrent use of toceranib in combination with metronomic chemotherapy and carboplatin significantly impacts DFI and OS.
Conclusions
The incorporation of toceranib into a commonly employed metronomic chemotherapy protocol failed to improve the DFI, OS and 1-year survival rates in dogs with appendicular OSA after amputation and carboplatin chemotherapy compared to metronomic therapy alone. Furthermore, the DFI, OS and 1-year survival rates in this study were similar to those previously found when dogs with OSA undergo amputation and carboplatin chemotherapy treatment alone. This study underscores the need for thorough interrogation of the molecular mechanisms that drive tumor progression at various stages of disease so that novel approaches aimed at optimizing activity against the tumor microenvironment can be successfully employed.
Supporting Information
Table only includes events that were experienced by at least five patients and at least two in each treatment group.
(DOCX)
Acknowledgments
The Clinical Trials Office (CTO) at the Ohio State University College of Veterinary Medicine (Nicole Stingle, Tamra Mathie, Ashley Smith) coordinated all aspects of this study including generation of case report forms, collection of samples, collation of data, quality assurance, coordination of other clinical sites and final quality control on all data from all study sites. This project was supported by the following grants: UL1TR001070 from the National Center for Advancing Translational Sciences and P30CA016058 from the National Cancer Institute to The Ohio State University. Funding for this clinical trial was provided by Zoetis. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Advancing Translational Sciences, National Cancer Institute or the National Institutes of Health or Zoetis.
Data Availability
All relevant data are within the paper and its Supporting Information file.
Funding Statement
This project was supported by the following grants: UL1TR001070 from the National Center for Advancing Translational Sciences and P30CA016058 from the National Cancer Institute to The Ohio State University. Funding for this clinical trial and the toceranib for treated dogs was provided by Zoetis. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Ehrhart NP, Ryan SD, Fan TM. Tumors of the Skeletal System In: Withrow SJ, MacEwen EG, Page RL, editors. Small Animal Clinical Oncology. 5 ed. St. Louis: W B Saunders Co; 2013. pp. 463–503. [Google Scholar]
- 2. Spodnick GJ, Berg J, Rand WM, Schelling SH, Couto G, Harvey J, et al. Prognosis for dogs with appendicular osteosarcoma treated by amputation alone: 162 cases (1978–1988). JAVMA. 1992; 200: 995–999. [PubMed] [Google Scholar]
- 3. Bergman PJ, MacEwen EG, Kurzman ID, Henry CJ, Hammer AS, Knapp DW, et al. Amputation and carboplatin for treatment of dogs with osteosarcoma: 48 cases (1991 to 1993). J Vet Intern Med. 1996. March;10(2):76–81. [DOI] [PubMed] [Google Scholar]
- 4. Chun R, Kurzman ID, Couto CG, Klausner J, Henry C, MacEwen EG. Cisplatin and doxorubicin combination chemotherapy for the treatment of canine osteosarcoma: a pilot study. J Vet Intern Med. 2000. September;14(5):495–8. [DOI] [PubMed] [Google Scholar]
- 5. Kent MS, Strom A, London CA, Seguin B. Alternating carboplatin and doxorubicin as adjunctive chemotherapy to amputation or limb-sparing surgery in the treatment of appendicular osteosarcoma in dogs. J Vet Intern Med. 2004. July;18(4):540–4. [DOI] [PubMed] [Google Scholar]
- 6. Moore AS, Dernell WS, Ogilvie GK, Kristal O, Elmslie R, Kitchell B, et al. Doxorubicin and BAY 12–9566 for the treatment of osteosarcoma in dogs: a randomized, double-blind, placebo-controlled study. J Vet Intern Med. 2007. July;21(4):783–90. [DOI] [PubMed] [Google Scholar]
- 7. Phillips B, Powers BE, Dernell WS, Straw RC, Khanna C, Hogge GS, et al. Use of single-agent carboplatin as adjuvant or neoadjuvant therapy in conjunction with amputation for appendicular osteosarcoma in dogs. Journal of the American Animal Hospital Association. 2009. January;45(1):33–8. [DOI] [PubMed] [Google Scholar]
- 8. Selmic LE, Burton JH, Thamm DH, Withrow SJ, Lana SE. Comparison of carboplatin and doxorubicin-based chemotherapy protocols in 470 dogs after amputation for treatment of appendicular osteosarcoma. J Vet Intern Med. 2014. March;28(2):554–63. 10.1111/jvim.12313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Elmslie RE, Glawe P, Dow SW. Metronomic therapy with cyclophosphamide and piroxicam effectively delays tumor recurrence in dogs with incompletely resected soft tissue sarcomas. J Vet Intern Med. 2008. November;22(6):1373–9. 10.1111/j.1939-1676.2008.0179.x [DOI] [PubMed] [Google Scholar]
- 10. Lana S, U'ren L, Plaza S, Elmslie R, Gustafson D, Morley P, et al. Continuous low-dose oral chemotherapy for adjuvant therapy of splenic hemangiosarcoma in dogs. J Vet Intern Med. 2007. July;21(4):764–9. [DOI] [PubMed] [Google Scholar]
- 11. Burton JH, Mitchell L, Thamm DH, Dow SW, Biller BJ. Low-dose cyclophosphamide selectively decreases regulatory T cells and inhibits angiogenesis in dogs with soft tissue sarcoma. J Vet Intern Med. 2011. July;25(4):920–6. 10.1111/j.1939-1676.2011.0753.x [DOI] [PubMed] [Google Scholar]
- 12. Ghiringhelli F, Menard C, Puig PE, Ladoire S, Roux S, Martin F, et al. Metronomic cyclophosphamide regimen selectively depletes CD4+CD25+ regulatory T cells and restores T and NK effector functions in end stage cancer patients. Cancer Immunol Immunother. 2007. May;56(5):641–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bertolini F, Paul S, Mancuso P, Monestiroli S, Gobbi A, Shaked Y, et al. Maximum tolerable dose and low-dose metronomic chemotherapy have opposite effects on the mobilization and viability of circulating endothelial progenitor cells. Cancer Research. 2003. August 1;63(15):4342–6. [PubMed] [Google Scholar]
- 14. Browder T, Butterfield CE, Kräling BM, Shi B, Marshall B, O'Reilly MS, et al. Antiangiogenic scheduling of chemotherapy improves efficacy against experimental drug-resistant cancer. Cancer Research. 2000. April 1;60(7):1878–86. [PubMed] [Google Scholar]
- 15. Colleselli D, Bijuklic K, Mosheimer BA, Kähler CM. Inhibition of cyclooxygenase (COX)-2 affects endothelial progenitor cell proliferation. Exp Cell Res. 2006. September 10;312(15):2933–41. [DOI] [PubMed] [Google Scholar]
- 16.Choisunirachon N, Jaroensong T, Yoshida K, Saeki K, Mochizuki M, Nishimura R, et al. Effects of low-dose cyclophosphamide with piroxicam on tumour neovascularization in a canine oral malignant melanoma-xenografted mouse model. Veterinary and Comparative Oncology. 2013 Jul 26; InPress. [DOI] [PubMed]
- 17. Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997. February 14;275(5302):964–7. [DOI] [PubMed] [Google Scholar]
- 18. Lyden D, Hattori K, Dias S, Costa C, Blaikie P, Butros L, et al. Impaired recruitment of bone-marrow-derived endothelial and hematopoietic precursor cells blocks tumor angiogenesis and growth. Nat Med. 2001. November;7(11):1194–201. [DOI] [PubMed] [Google Scholar]
- 19. Stoelting S, Trefzer T, Kisro J, Steinke A, Wagner T, Peters SO. Low-dose oral metronomic chemotherapy prevents mobilization of endothelial progenitor cells into the blood of cancer patients. in vivo. 2008. November;22(6):831–6. [PubMed] [Google Scholar]
- 20. Willett CG, Boucher Y, di Tomaso E, Duda DG, Munn LL, Tong RT, et al. Direct evidence that the VEGF-specific antibody bevacizumab has antivascular effects in human rectal cancer. Nat Med. 2004. February;10(2):145–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kaya M, Wada T, Akatsuka T, Kawaguchi S, Nagoya S, Shindoh M, et al. Vascular endothelial growth factor expression in untreated osteosarcoma is predictive of pulmonary metastasis and poor prognosis. Clin Cancer Res. 2000. February;6(2):572–7. [PubMed] [Google Scholar]
- 22. Ohba T, Cates JMM, Cole HA, Slosky DA, Haro H, Ando T, et al. Autocrine VEGF/VEGFR1 signaling in a subpopulation of cells associates with aggressive osteosarcoma. Mol Cancer Res. 2014. August;12(8):1100–11. 10.1158/1541-7786.MCR-14-0037 [DOI] [PubMed] [Google Scholar]
- 23. Yang J, Yang D, Sun Y, Sun B, Wang G, Trent JC, et al. Genetic amplification of the vascular endothelial growth factor (VEGF) pathway genes, including VEGFA, in human osteosarcoma. Cancer. 2011. November 1;117(21):4925–38. 10.1002/cncr.26116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Thamm DH, O'Brien MG, Vail DM. Serum vascular endothelial growth factor concentrations and postsurgical outcome in dogs with osteosarcoma. Veterinary and Comparative Oncology. 2008. June;6(2):126–32. 10.1111/j.1476-5829.2007.00153.x [DOI] [PubMed] [Google Scholar]
- 25. Wergin MC, Kaser-Hotz B. Plasma vascular endothelial growth factor (VEGF) measured in seventy dogs with spontaneously occurring tumours. in vivo. 2004. January;18(1):15–9. [PubMed] [Google Scholar]
- 26. García-Sáenz JA, Martín M, Calles A, Bueno C, Rodríguez L, Bobokova J, et al. Bevacizumab in combination with metronomic chemotherapy in patients with anthracycline- and taxane-refractory breast cancer. J Chemother. 2008. October;20(5):632–9. [DOI] [PubMed] [Google Scholar]
- 27. Klement G, Baruchel S, Rak J, Man S, Clark K, Hicklin DJ, et al. Continuous low-dose therapy with vinblastine and VEGF receptor-2 antibody induces sustained tumor regression without overt toxicity. J Clin Invest. 2000. April;105(8):R15–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pàez-Ribes M, Allen E, Hudock J, Takeda T, Okuyama H, Viñals F, et al. Antiangiogenic therapy elicits malignant progression of tumors to increased local invasion and distant metastasis. Cancer Cell. 2009. March 3;15(3):220–31. 10.1016/j.ccr.2009.01.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ebos JML, Lee CR, Cruz-Munoz W, Bjarnason GA, Christensen JG, Kerbel RS. Accelerated metastasis after short-term treatment with a potent inhibitor of tumor angiogenesis. Cancer Cell. 2009. March 3;15(3):232–9. 10.1016/j.ccr.2009.01.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Maris JM, Courtright J, Houghton PJ, Morton CL, Gorlick R, Kolb EA, et al. Initial testing of the VEGFR inhibitor AZD2171 by the pediatric preclinical testing program. Pediatr Blood Cancer. 2008. March;50(3):581–7. [DOI] [PubMed] [Google Scholar]
- 31. Pignochino Y, Grignani G, Cavalloni G, Motta M, Tapparo M, Bruno S, et al. Sorafenib blocks tumour growth, angiogenesis and metastatic potential in preclinical models of osteosarcoma through a mechanism potentially involving the inhibition of ERK1/2, MCL-1 and ezrin pathways. Mol Cancer. 2009;8(1):118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. van Cruijsen H, Voest EE, Punt CJA, Hoekman K, Witteveen PO, Meijerink MR, et al. Phase I evaluation of cediranib, a selective VEGFR signalling inhibitor, in combination with gefitinib in patients with advanced tumours. Eur J Cancer. 2010. March;46(5):901–11. 10.1016/j.ejca.2009.12.023 [DOI] [PubMed] [Google Scholar]
- 33. London CA, Hannah AL, Zadovoskaya R, Chien MB, Kollias-Baker C, Rosenberg M, et al. Phase I dose-escalating study of SU11654, a small molecule receptor tyrosine kinase inhibitor, in dogs with spontaneous malignancies. Clin Cancer Res. 2003. July;9(7):2755–68. [PubMed] [Google Scholar]
- 34. Mendel DB, Laird AD, Xin X, Louie SG, Christensen JG, Li G, et al. In vivo antitumor activity of SU11248, a novel tyrosine kinase inhibitor targeting vascular endothelial growth factor and platelet-derived growth factor receptors: determination of a pharmacokinetic/pharmacodynamic relationship. 2003. January;9(1):327–37. [PubMed] [Google Scholar]
- 35. Pryer NK, Lee LB, Zadovaskaya R, Yu X, Sukbuntherng J, Cherrington JM, et al. Proof of target for SU11654: inhibition of KIT phosphorylation in canine mast cell tumors. Clin Cancer Res. 2003. November 15;9(15):5729–34. [PubMed] [Google Scholar]
- 36. London CA, Malpas PB, Wood-Follis SL, Boucher JF, Rusk AW, Rosenberg MP, et al. Multi-center, placebo-controlled, double-blind, randomized study of oral toceranib phosphate (SU11654), a receptor tyrosine kinase inhibitor, for the treatment of dogs with recurrent (either local or distant) mast cell tumor following surgical excision. Clin Cancer Res. 2009. June 1;15(11):3856–65. 10.1158/1078-0432.CCR-08-1860 [DOI] [PubMed] [Google Scholar]
- 37. Bernabe LF, Portela R, Nguyen S, Kisseberth WC, Pennell M, Yancey MF, et al. Evaluation of the adverse event profile and pharmacodynamics of toceranib phosphate administered to dogs with solid tumors at doses below the maximum tolerated dose. BMC Vet Res. 2013;9(1):190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. London C, Mathie T, Stingle N, Clifford C, Haney S, Klein MK, et al. Preliminary evidence for biologic activity of toceranib phosphate (Palladia) in solid tumours. Veterinary and Comparative Oncology. 2012. September;10(3):194–205. 10.1111/j.1476-5829.2011.00275.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Mitchell L, Thamm DH, Biller BJ. Clinical and immunomodulatory effects of toceranib combined with low-dose cyclophosphamide in dogs with cancer. J Vet Intern Med. 2012. March;26(2):355–62. 10.1111/j.1939-1676.2011.00883.x [DOI] [PubMed] [Google Scholar]
- 40. Krzyzanowska MK, Tannock IF, Lockwood G, Knox J, Moore M, Bjarnason GA. A phase II trial of continuous low-dose oral cyclophosphamide and celecoxib in patients with renal cell carcinoma. Cancer Chemotherapy and Pharmacology. 2007. June;60(1):135–41. [DOI] [PubMed] [Google Scholar]
- 41. Stempak D, Gammon J, Halton J, Moghrabi A, Koren G, Baruchel S. A pilot pharmacokinetic and antiangiogenic biomarker study of celecoxib and low-dose metronomic vinblastine or cyclophosphamide in pediatric recurrent solid tumors. J Pediatr Hematol Oncol. 2006. November;28(11):720–8. [DOI] [PubMed] [Google Scholar]
- 42. Dellapasqua S, Bertolini F, Bagnardi V, Campagnoli E, Scarano E, Torrisi R, et al. Metronomic cyclophosphamide and capecitabine combined with bevacizumab in advanced breast cancer. Journal of Clinical Oncology. 2008. October 20;26(30):4899–905. 10.1200/JCO.2008.17.4789 [DOI] [PubMed] [Google Scholar]
- 43. Veterinary Co-operative Oncology Group (VCOG). Veterinary Co-operative Oncology Group—Common Terminology Criteria for Adverse Events (VCOG-CTCAE) following chemotherapy or biological antineoplastic therapy in dogs and cats v1.0. Veterinary and Comparative Oncology. 2004. December;2(4):195–213. 10.1111/j.1476-5810.2004.0053b.x [DOI] [PubMed] [Google Scholar]
- 44. Schoenfeld D. Partial residuals for the proportional hazards regression model. Biometrika. 1982;69:239–241. [Google Scholar]
- 45. Grambsch PM, Therneau TM. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika. 1994;81:515–526. [Google Scholar]
- 46. Fenger JM, London CA, Kisseberth WC. Canine osteosarcoma: a naturally occurring disease to inform pediatric oncology. ILAR J. Oxford University Press; 2014;55(1):69–85. 10.1093/ilar/ilu009 [DOI] [PubMed] [Google Scholar]
- 47. Bailey D, Erb H, Williams L, Ruslander D, Hauck M. Carboplatin and doxorubicin combination chemotherapy for the treatment of appendicular osteosarcoma in the dog. J Vet Intern Med. 2003. March;17(2):199–205. [DOI] [PubMed] [Google Scholar]
- 48. Chun R, Garrett LD, Henry C, Wall M, Smith A, Azene NM. Toxicity and efficacy of cisplatin and doxorubicin combination chemotherapy for the treatment of canine osteosarcoma. Journal of the American Animal Hospital Association. 2005. November;41(6):382–7. [DOI] [PubMed] [Google Scholar]
- 49. McMahon M, Mathie T, Stingle N, Romansik E, Vail D, London C. Adjuvant carboplatin and gemcitabine combination chemotherapy postamputation in canine appendicular osteosarcoma. J Vet Intern Med. 2011. May;25(3):511–7. 10.1111/j.1939-1676.2011.0697.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Bracha S, Walshaw R, Danton T, Holland S, Ruaux C, Obradovich J. Evaluation of toxicities from combined metronomic and maximal-tolerated dose chemotherapy in dogs with osteosarcoma. J Small Animal Practice. 2014. Jul;55(7):369–74. 10.1111/jsap.12228 [DOI] [PubMed] [Google Scholar]
- 51. Leach TN, Childress MO, Greene SN, Mohamed AS, Moore GE, Schrempp DR, et al. Prospective trial of metronomic chlorambucil chemotherapy in dogs with naturally occurring cancer. Veterinary and Comparative Oncology. 2012. June;10(2):102–12. 10.1111/j.1476-5829.2011.00280.x [DOI] [PubMed] [Google Scholar]
- 52. Singh M, Couto SS, Forrest WF, Lima A, Cheng JH, Molina R, et al. Anti-VEGF antibody therapy does not promote metastasis in genetically engineered mouse tumour models. The Journal of Pathology. 2012. August;227(4):417–30. 10.1002/path.4053 [DOI] [PubMed] [Google Scholar]
- 53. Gaeta R, Brown D, Cohen R, Sorenmo K. Risk factors for development of sterile haemorrhagic cystitis in canine lymphoma patients receiving oral cyclophosphamide: a case-control study. Veterinary and Comparative Oncology. 2014. December;12(4):277–86. 10.1111/vco.12009 [DOI] [PubMed] [Google Scholar]
- 54. Saam DE, LIPTAK JM, Stalker MJ, Chun R. Predictors of outcome in dogs treated with adjuvant carboplatin for appendicular osteosarcoma: 65 cases (1996–2006). J Am Vet Med Assoc. 2011. January 15;238(2):195–206. 10.2460/javma.238.2.195 [DOI] [PubMed] [Google Scholar]
- 55. Schmidt AF, Nielen M, Klungel OH, Hoes AW, de Boer A, Groenwold RHH, et al. Prognostic factors of early metastasis and mortality in dogs with appendicular osteosarcoma after receiving surgery: an individual patient data meta-analysis. Preventive Veterinary Medicine. 2013. November 1;112(3–4):414–22. 10.1016/j.prevetmed.2013.09.010 [DOI] [PubMed] [Google Scholar]
- 56. Kuntz CA, Asselin TL, Dernell WS. Limb salvage surgery for osteosarcoma of the proximal humerus: outcome in 17 dogs. Veterinary Surgery. 1998;27:417–422. [DOI] [PubMed] [Google Scholar]
- 57. Boerman I, Selvarajah GT, Nielen M, Kirpensteijn J. Prognostic factors in canine appendicular osteosarcoma—a meta-analysis. BMC Vet Res. 2012;8(1):56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Sacornrattana O, Dervisis NG, McNiel EA. Abdominal ultrasonographic findings at diagnosis of osteosarcoma in dogs and association with treatment outcome. 2013. Sep;11(3):199–207. [DOI] [PubMed] [Google Scholar]
- 59. Wallace M, Selmic L, Withrow SJ. Diagnostic utility of abdominal ultrasonography for routine staging at diagnosis of skeletal OSA in dogs. Journal of the American Animal Hospital Association. American Animal Hospital Association; 2013. July;49(4):243–5. 10.5326/JAAHA-MS-5862 [DOI] [PubMed] [Google Scholar]
- 60. Jankowski MK, Steyn PF, Lana SE, Dernell WS, Blom CM, Uhrig JL, et al. Nuclear scanning with 99mTc-HDP for the initial evaluation of osseous metastasis in canine osteosarcoma. Veterinary and Comparative Oncology. 2003. September;1(3):152–8. 10.1111/j.1476-5829.2003.00021.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table only includes events that were experienced by at least five patients and at least two in each treatment group.
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information file.