Abstract
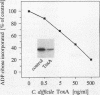
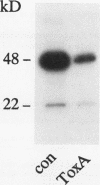
Enterotoxin A is one of the major virulence factors of Clostridium difficile, and the causative agent of antibiotic-associated pseudomembranous colitis. In cell culture (NIH-3T3, rat basophilic leukemia cells) toxin A inhibits Clostridium botulinum ADP-ribosyltransferase C3 (C3)-catalyzed ADP-ribosylation of the low molecular mass GTP-binding Rho proteins. Rho participates in the regulation of the microfilament cytoskeleton. Decrease in ADP-ribosylation of Rho occurs in a time- and concentration-dependent manner and precedes the toxin A-induced destruction of the actin cytoskeleton. Action of toxin A is not due to proteolytical degradation of Rho or to an inherent ADP-ribosyltransferase activity of toxin A. Toxin A-induced decrease in ADP-ribosylation is observed also in cell lysates and with recombinant RhoA protein. A heat stable low molecular mass cytosolic factor is essential for the toxin effect on Rho. Thus, the enterotoxin (toxin A) resembles the effects of the C. difficile cytotoxin (toxin B) on Rho proteins (Just, I., G. Fritz, K. Aktories, M. Giry, M. R. Popoff, P. Boquet, S. Hegenbath, and C. Von Eichel-Streiber. 1994. J. Biol. Chem. 269:10706-10712). The data indicate that despite different in vivo effects, toxin A and toxin B act on the same cellular target protein Rho to elicit their toxic effects.
Full text
PDF
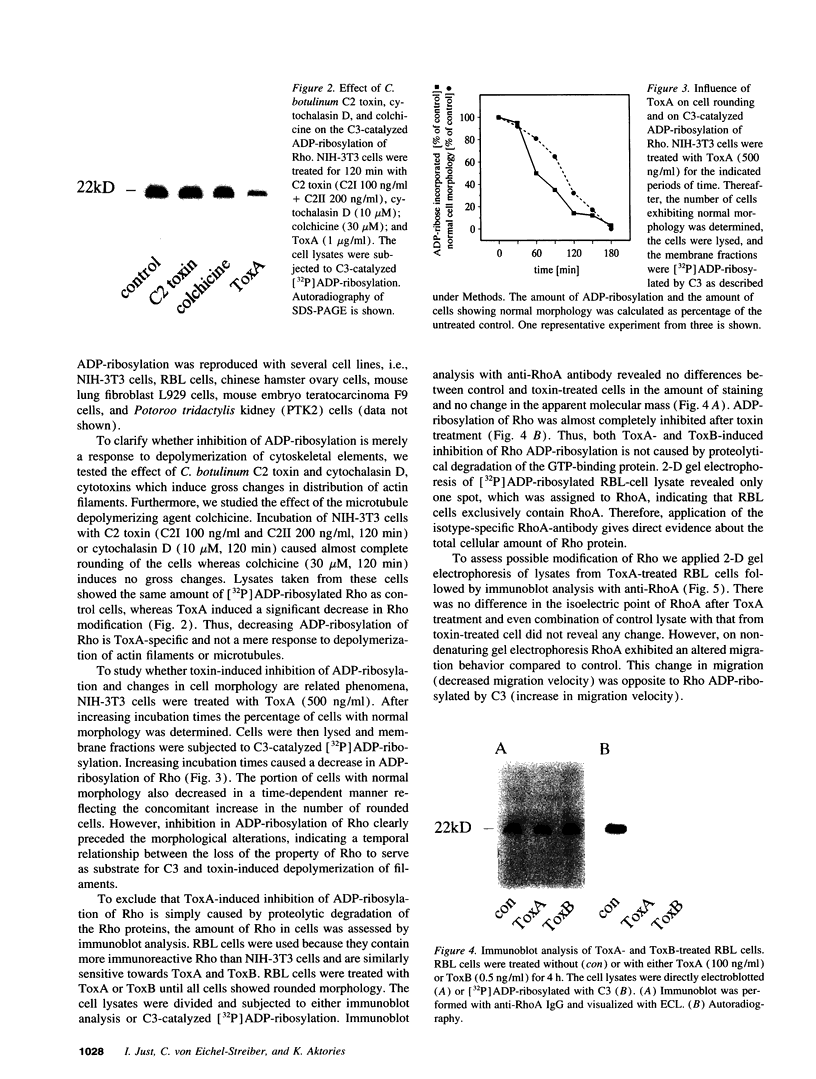
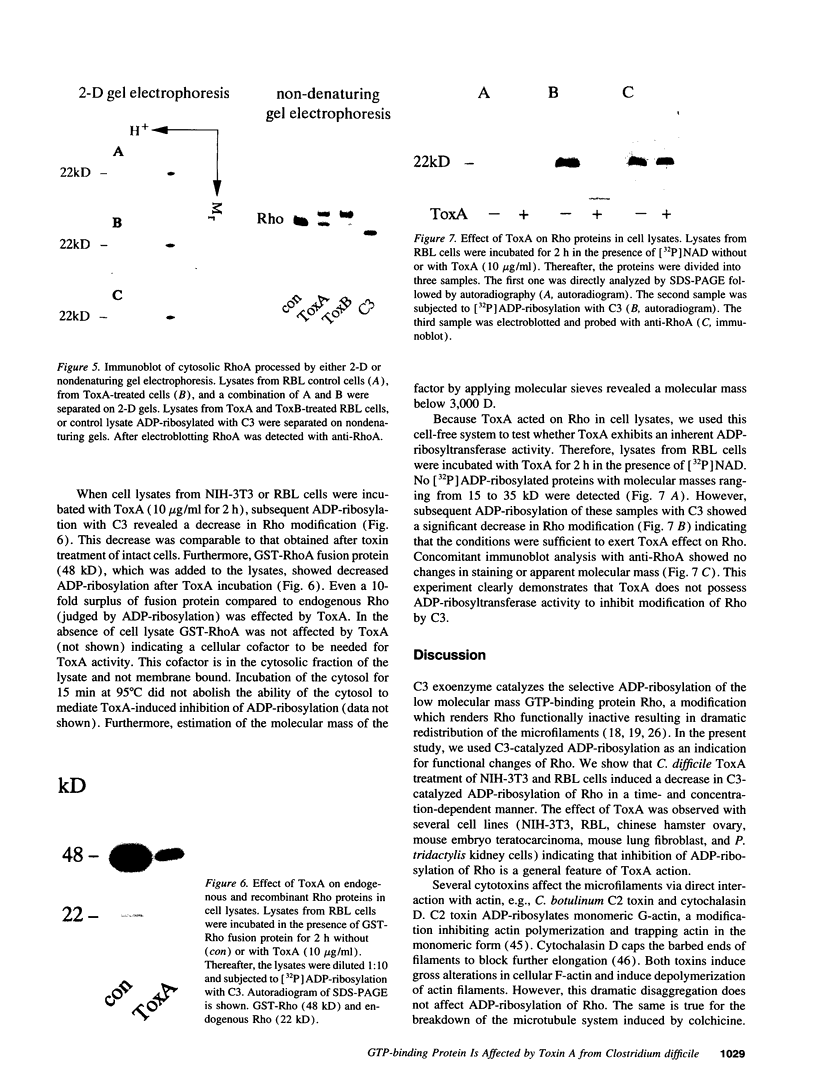


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aktories K., Mohr C., Koch G. Clostridium botulinum C3 ADP-ribosyltransferase. Curr Top Microbiol Immunol. 1992;175:115–131. doi: 10.1007/978-3-642-76966-5_6. [DOI] [PubMed] [Google Scholar]
- Aktories K., Rösener S., Blaschke U., Chhatwal G. S. Botulinum ADP-ribosyltransferase C3. Purification of the enzyme and characterization of the ADP-ribosylation reaction in platelet membranes. Eur J Biochem. 1988 Mar 1;172(2):445–450. doi: 10.1111/j.1432-1033.1988.tb13908.x. [DOI] [PubMed] [Google Scholar]
- Arnon S. S., Mills D. C., Day P. A., Henrickson R. V., Sullivan N. M., Wilkins T. D. Rapid death of infant rhesus monkeys injected with Clostridium difficile toxins A and B: physiologic and pathologic basis. J Pediatr. 1984 Jan;104(1):34–40. doi: 10.1016/s0022-3476(84)80585-5. [DOI] [PubMed] [Google Scholar]
- Barroso L. A., Wang S. Z., Phelps C. J., Johnson J. L., Wilkins T. D. Nucleotide sequence of Clostridium difficile toxin B gene. Nucleic Acids Res. 1990 Jul 11;18(13):4004–4004. doi: 10.1093/nar/18.13.4004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourmeyster N., Stasia M. J., Garin J., Gagnon J., Boquet P., Vignais P. V. Copurification of rho protein and the rho-GDP dissociation inhibitor from bovine neutrophil cytosol. Effect of phosphoinositides on rho ADP-ribosylation by the C3 exoenzyme of Clostridium botulinum. Biochemistry. 1992 Dec 29;31(51):12863–12869. doi: 10.1021/bi00166a022. [DOI] [PubMed] [Google Scholar]
- Chardin P., Boquet P., Madaule P., Popoff M. R., Rubin E. J., Gill D. M. The mammalian G protein rhoC is ADP-ribosylated by Clostridium botulinum exoenzyme C3 and affects actin microfilaments in Vero cells. EMBO J. 1989 Apr;8(4):1087–1092. doi: 10.1002/j.1460-2075.1989.tb03477.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper J. A. Effects of cytochalasin and phalloidin on actin. J Cell Biol. 1987 Oct;105(4):1473–1478. doi: 10.1083/jcb.105.4.1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorentini C., Arancia G., Paradisi S., Donelli G., Giuliano M., Piemonte F., Mastrantonio P. Effects of Clostridium difficile toxins A and B on cytoskeleton organization in HEp-2 cells: a comparative morphological study. Toxicon. 1989;27(11):1209–1218. doi: 10.1016/0041-0101(89)90029-9. [DOI] [PubMed] [Google Scholar]
- Fiorentini C., Thelestam M. Clostridium difficile toxin A and its effects on cells. Toxicon. 1991;29(6):543–567. doi: 10.1016/0041-0101(91)90050-2. [DOI] [PubMed] [Google Scholar]
- Florin I., Thelestam M. Lysosomal involvement in cellular intoxication with Clostridium difficile toxin B. Microb Pathog. 1986 Aug;1(4):373–385. doi: 10.1016/0882-4010(86)90069-0. [DOI] [PubMed] [Google Scholar]
- Fukumoto Y., Kaibuchi K., Hori Y., Fujioka H., Araki S., Ueda T., Kikuchi A., Takai Y. Molecular cloning and characterization of a novel type of regulatory protein (GDI) for the rho proteins, ras p21-like small GTP-binding proteins. Oncogene. 1990 Sep;5(9):1321–1328. [PubMed] [Google Scholar]
- Henriques B., Florin I., Thelestam M. Cellular internalisation of Clostridium difficile toxin A. Microb Pathog. 1987 Jun;2(6):455–463. doi: 10.1016/0882-4010(87)90052-0. [DOI] [PubMed] [Google Scholar]
- Hirata K., Kikuchi A., Sasaki T., Kuroda S., Kaibuchi K., Matsuura Y., Seki H., Saida K., Takai Y. Involvement of rho p21 in the GTP-enhanced calcium ion sensitivity of smooth muscle contraction. J Biol Chem. 1992 May 5;267(13):8719–8722. [PubMed] [Google Scholar]
- Isomura M., Kaibuchi K., Yamamoto T., Kawamura S., Katayama M., Takai Y. Partial purification and characterization of GDP dissociation stimulator (GDS) for the rho proteins from bovine brain cytosol. Biochem Biophys Res Commun. 1990 Jun 15;169(2):652–659. doi: 10.1016/0006-291x(90)90380-6. [DOI] [PubMed] [Google Scholar]
- Just I., Fritz G., Aktories K., Giry M., Popoff M. R., Boquet P., Hegenbarth S., von Eichel-Streiber C. Clostridium difficile toxin B acts on the GTP-binding protein Rho. J Biol Chem. 1994 Apr 8;269(14):10706–10712. [PubMed] [Google Scholar]
- Just I., Mohr C., Schallehn G., Menard L., Didsbury J. R., Vandekerckhove J., van Damme J., Aktories K. Purification and characterization of an ADP-ribosyltransferase produced by Clostridium limosum. J Biol Chem. 1992 May 25;267(15):10274–10280. [PubMed] [Google Scholar]
- Just I., Schallehn G., Aktories K. ADP-ribosylation of small GTP-binding proteins by Bacillus cereus. Biochem Biophys Res Commun. 1992 Mar 31;183(3):931–936. doi: 10.1016/s0006-291x(05)80279-7. [DOI] [PubMed] [Google Scholar]
- Kelly C. P., Pothoulakis C., LaMont J. T. Clostridium difficile colitis. N Engl J Med. 1994 Jan 27;330(4):257–262. doi: 10.1056/NEJM199401273300406. [DOI] [PubMed] [Google Scholar]
- Krautwurst D., Hescheler J., Arndts D., Lösel W., Hammer R., Schultz G. Novel potent inhibitor of receptor-activated nonselective cation currents in HL-60 cells. Mol Pharmacol. 1993 May;43(5):655–659. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lyerly D. M., Krivan H. C., Wilkins T. D. Clostridium difficile: its disease and toxins. Clin Microbiol Rev. 1988 Jan;1(1):1–18. doi: 10.1128/cmr.1.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyerly D. M., Lockwood D. E., Richardson S. H., Wilkins T. D. Biological activities of toxins A and B of Clostridium difficile. Infect Immun. 1982 Mar;35(3):1147–1150. doi: 10.1128/iai.35.3.1147-1150.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyerly D. M., Saum K. E., MacDonald D. K., Wilkins T. D. Effects of Clostridium difficile toxins given intragastrically to animals. Infect Immun. 1985 Feb;47(2):349–352. doi: 10.1128/iai.47.2.349-352.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell M. J., Laughon B. E., Lin S. Biochemical studies on the effect of Clostridium difficile toxin B on actin in vivo and in vitro. Infect Immun. 1987 Jul;55(7):1610–1615. doi: 10.1128/iai.55.7.1610-1615.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morii N., Kawano K., Sekine A., Yamada T., Narumiya S. Purification of GTPase-activating protein specific for the rho gene products. J Biol Chem. 1991 Apr 25;266(12):7646–7650. [PubMed] [Google Scholar]
- Narumiya S., Morii N. rho gene products, botulinum C3 exoenzyme and cell adhesion. Cell Signal. 1993 Jan;5(1):9–19. doi: 10.1016/0898-6568(93)90003-5. [DOI] [PubMed] [Google Scholar]
- Nemoto Y., Namba T., Kozaki S., Narumiya S. Clostridium botulinum C3 ADP-ribosyltransferase gene. Cloning, sequencing, and expression of a functional protein in Escherichia coli. J Biol Chem. 1991 Oct 15;266(29):19312–19319. [PubMed] [Google Scholar]
- Nitta A. T., Iseman M. D., Newell J. D., Madsen L. A., Goble M. Ten-year experience with artificial pneumoperitoneum for end-stage, drug-resistant pulmonary tuberculosis. Clin Infect Dis. 1993 Feb;16(2):219–222. doi: 10.1093/clind/16.2.219. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Ohishi I., Iwasaki M., Sakaguchi G. Purification and characterization of two components of botulinum C2 toxin. Infect Immun. 1980 Dec;30(3):668–673. doi: 10.1128/iai.30.3.668-673.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottlinger M. E., Lin S. Clostridium difficile toxin B induces reorganization of actin, vinculin, and talin in cultured cells. Exp Cell Res. 1988 Jan;174(1):215–229. doi: 10.1016/0014-4827(88)90156-5. [DOI] [PubMed] [Google Scholar]
- Paterson H. F., Self A. J., Garrett M. D., Just I., Aktories K., Hall A. Microinjection of recombinant p21rho induces rapid changes in cell morphology. J Cell Biol. 1990 Sep;111(3):1001–1007. doi: 10.1083/jcb.111.3.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popoff M. R., Rubin E. J., Gill D. M., Boquet P. Actin-specific ADP-ribosyltransferase produced by a Clostridium difficile strain. Infect Immun. 1988 Sep;56(9):2299–2306. doi: 10.1128/iai.56.9.2299-2306.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridley A. J., Hall A. The small GTP-binding protein rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell. 1992 Aug 7;70(3):389–399. doi: 10.1016/0092-8674(92)90163-7. [DOI] [PubMed] [Google Scholar]
- Rubin E. J., Gill D. M., Boquet P., Popoff M. R. Functional modification of a 21-kilodalton G protein when ADP-ribosylated by exoenzyme C3 of Clostridium botulinum. Mol Cell Biol. 1988 Jan;8(1):418–426. doi: 10.1128/mcb.8.1.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safer D. An electrophoretic procedure for detecting proteins that bind actin monomers. Anal Biochem. 1989 Apr;178(1):32–37. doi: 10.1016/0003-2697(89)90351-5. [DOI] [PubMed] [Google Scholar]
- Schiavo G., Benfenati F., Poulain B., Rossetto O., Polverino de Laureto P., DasGupta B. R., Montecucco C. Tetanus and botulinum-B neurotoxins block neurotransmitter release by proteolytic cleavage of synaptobrevin. Nature. 1992 Oct 29;359(6398):832–835. doi: 10.1038/359832a0. [DOI] [PubMed] [Google Scholar]
- Sekine A., Fujiwara M., Narumiya S. Asparagine residue in the rho gene product is the modification site for botulinum ADP-ribosyltransferase. J Biol Chem. 1989 May 25;264(15):8602–8605. [PubMed] [Google Scholar]
- Stasia M. J., Jouan A., Bourmeyster N., Boquet P., Vignais P. V. ADP-ribosylation of a small size GTP-binding protein in bovine neutrophils by the C3 exoenzyme of Clostridium botulinum and effect on the cell motility. Biochem Biophys Res Commun. 1991 Oct 31;180(2):615–622. doi: 10.1016/s0006-291x(05)81110-6. [DOI] [PubMed] [Google Scholar]
- Sugai M., Hashimoto K., Kikuchi A., Inoue S., Okumura H., Matsumoto K., Goto Y., Ohgai H., Moriishi K., Syuto B. Epidermal cell differentiation inhibitor ADP-ribosylates small GTP-binding proteins and induces hyperplasia of epidermis. J Biol Chem. 1992 Feb 5;267(4):2600–2604. [PubMed] [Google Scholar]
- Takai Y., Kaibuchi K., Kikuchi A., Kawata M. Small GTP-binding proteins. Int Rev Cytol. 1992;133:187–230. doi: 10.1016/s0074-7696(08)61861-6. [DOI] [PubMed] [Google Scholar]
- Takaishi K., Kikuchi A., Kuroda S., Kotani K., Sasaki T., Takai Y. Involvement of rho p21 and its inhibitory GDP/GTP exchange protein (rho GDI) in cell motility. Mol Cell Biol. 1993 Jan;13(1):72–79. doi: 10.1128/mcb.13.1.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triadafilopoulos G., Pothoulakis C., O'Brien M. J., LaMont J. T. Differential effects of Clostridium difficile toxins A and B on rabbit ileum. Gastroenterology. 1987 Aug;93(2):273–279. doi: 10.1016/0016-5085(87)91014-6. [DOI] [PubMed] [Google Scholar]
- Ueda T., Kikuchi A., Ohga N., Yamamoto J., Takai Y. Purification and characterization from bovine brain cytosol of a novel regulatory protein inhibiting the dissociation of GDP from and the subsequent binding of GTP to rhoB p20, a ras p21-like GTP-binding protein. J Biol Chem. 1990 Jun 5;265(16):9373–9380. [PubMed] [Google Scholar]
- Wiegers W., Just I., Müller H., Hellwig A., Traub P., Aktories K. Alteration of the cytoskeleton of mammalian cells cultured in vitro by Clostridium botulinum C2 toxin and C3 ADP-ribosyltransferase. Eur J Cell Biol. 1991 Apr;54(2):237–245. [PubMed] [Google Scholar]
- Yamamoto J., Kikuchi A., Ueda T., Ohga N., Takai Y. A GTPase-activating protein for rhoB p20, a ras p21-like GTP-binding protein--partial purification, characterization and subcellular distribution in rat brain. Brain Res Mol Brain Res. 1990 Jul;8(2):105–111. doi: 10.1016/0169-328x(90)90054-h. [DOI] [PubMed] [Google Scholar]
- von Eichel-Streiber C., Harperath U., Bosse D., Hadding U. Purification of two high molecular weight toxins of Clostridium difficile which are antigenically related. Microb Pathog. 1987 May;2(5):307–318. doi: 10.1016/0882-4010(87)90073-8. [DOI] [PubMed] [Google Scholar]
- von Eichel-Streiber C., Sauerborn M. Clostridium difficile toxin A carries a C-terminal repetitive structure homologous to the carbohydrate binding region of streptococcal glycosyltransferases. Gene. 1990 Nov 30;96(1):107–113. doi: 10.1016/0378-1119(90)90348-u. [DOI] [PubMed] [Google Scholar]