Abstract
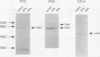
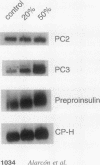
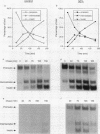
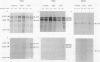
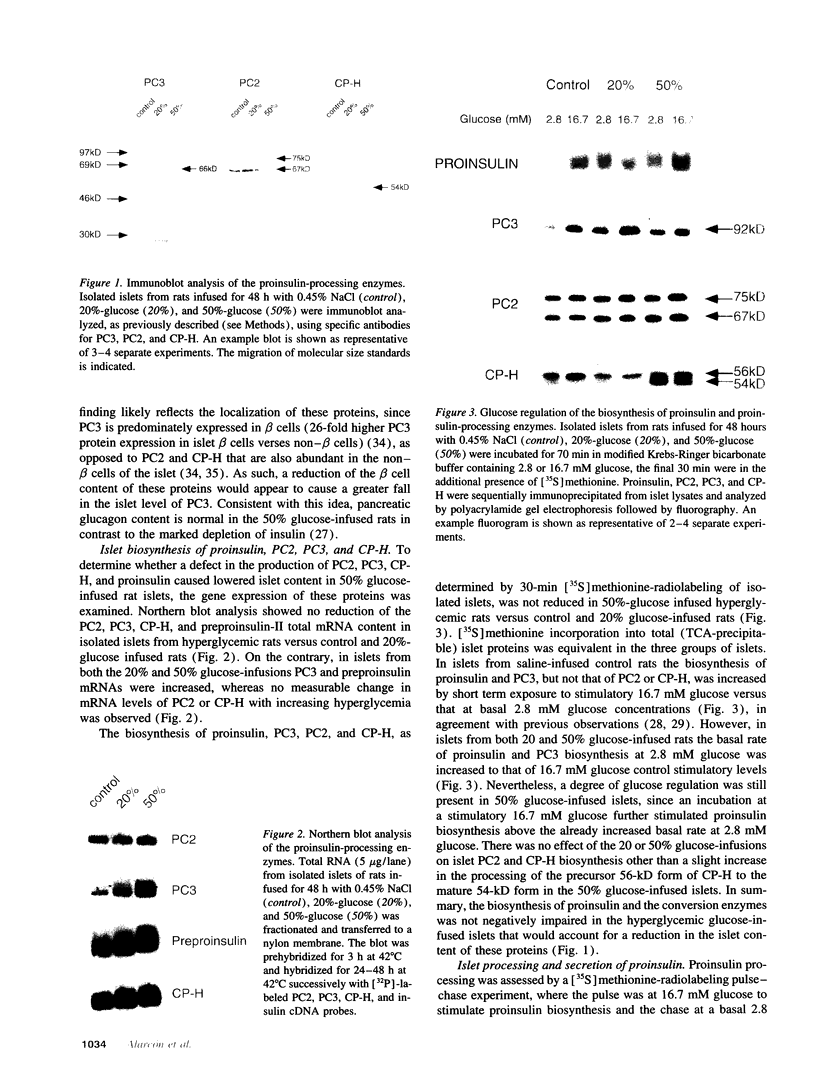
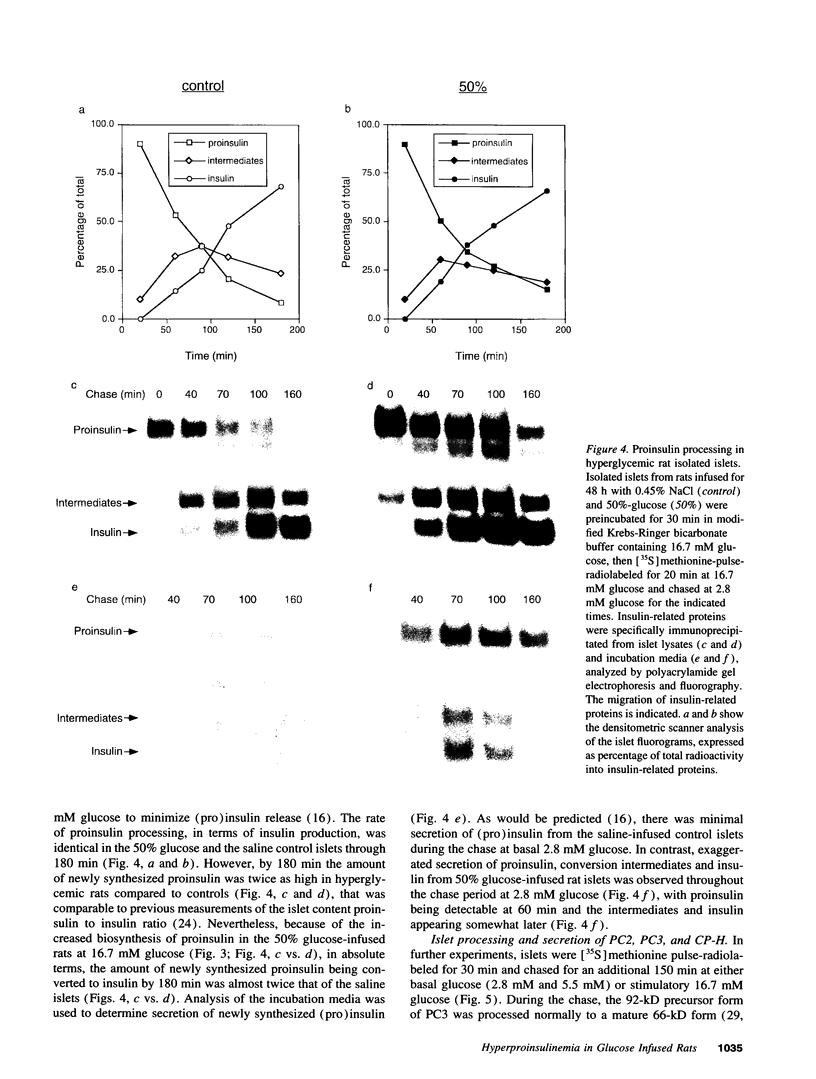
Hyperproinsulinemia in non-insulin-dependent diabetes mellitus (NIDDM) is due to an increased release of proinsulin from pancreatic beta cells. This could reside in increased secretory demand placed on the beta cell by hyperglycemia or in the proinsulin conversion mechanism. In this study, biosynthesis of the proinsulin conversion enzymes (PC2, PC3, and carboxypeptidase-H [CP-H]) and proinsulin, were examined in islets isolated from 48-h infused rats with 50% (wt/vol) glucose (hyperglycemic, hyperinsulinemic, and increased pancreatic proinsulin to insulin ratio), 20% (wt/vol) glucose (normoglycemic but hyperinsulinemic), and 0.45% (wt/vol) saline (controls). A decrease in the islet content of PC2, PC3, and CP-H from hyperglycemic rats was observed. This reduction did not correlate with any deficiency in mRNA levels or biosynthesis of PC2, PC3, CP-H, or proinsulin. Furthermore, proinsulin conversion rate was comparable in islets from hyperglycemic and control rats. However, in islets from hyperglycemic rats an abnormal increased proportion of proinsulin was secreted, that was accompanied by an augmented release of PC2, PC3 and CP-H. Stimulation of the beta cell's secretory pathway by hyperglycemia, resulted in proinsulin being prematurely secreted from islets before its conversion could be completed. Thus, hyperproinsulinemia induced by chronic hyperglycemia likely results from increased beta cell secretory demand, rather than a defect in the proinsulin processing enzymes per se.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alarcón C., Lincoln B., Rhodes C. J. The biosynthesis of the subtilisin-related proprotein convertase PC3, but no that of the PC2 convertase, is regulated by glucose in parallel to proinsulin biosynthesis in rat pancreatic islets. J Biol Chem. 1993 Feb 25;268(6):4276–4280. [PubMed] [Google Scholar]
- Ashcroft S. J. Glucoreceptor mechanisms and the control of insulin release and biosynthesis. Diabetologia. 1980 Jan;18(1):5–15. doi: 10.1007/BF01228295. [DOI] [PubMed] [Google Scholar]
- Bailyes E. M., Bennett D. L., Hutton J. C. Proprotein-processing endopeptidases of the insulin secretory granule. Enzyme. 1991;45(5-6):301–313. doi: 10.1159/000468903. [DOI] [PubMed] [Google Scholar]
- Bailyes E. M., Shennan K. I., Seal A. J., Smeekens S. P., Steiner D. F., Hutton J. C., Docherty K. A member of the eukaryotic subtilisin family (PC3) has the enzymic properties of the type 1 proinsulin-converting endopeptidase. Biochem J. 1992 Jul 15;285(Pt 2):391–394. doi: 10.1042/bj2850391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett D. L., Bailyes E. M., Nielsen E., Guest P. C., Rutherford N. G., Arden S. D., Hutton J. C. Identification of the type 2 proinsulin processing endopeptidase as PC2, a member of the eukaryote subtilisin family. J Biol Chem. 1992 Jul 25;267(21):15229–15236. [PubMed] [Google Scholar]
- Burgess T. L., Kelly R. B. Constitutive and regulated secretion of proteins. Annu Rev Cell Biol. 1987;3:243–293. doi: 10.1146/annurev.cb.03.110187.001331. [DOI] [PubMed] [Google Scholar]
- Davidson H. W., Hutton J. C. The insulin-secretory-granule carboxypeptidase H. Purification and demonstration of involvement in proinsulin processing. Biochem J. 1987 Jul 15;245(2):575–582. doi: 10.1042/bj2450575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fricker L. D., Adelman J. P., Douglass J., Thompson R. C., von Strandmann R. P., Hutton J. Isolation and sequence analysis of cDNA for rat carboxypeptidase E [EC 3.4.17.10], a neuropeptide processing enzyme. Mol Endocrinol. 1989 Apr;3(4):666–673. doi: 10.1210/mend-3-4-666. [DOI] [PubMed] [Google Scholar]
- Fricker L. D., Devi L. Posttranslational processing of carboxypeptidase E, a neuropeptide-processing enzyme, in AtT-20 cells and bovine pituitary secretory granules. J Neurochem. 1993 Oct;61(4):1404–1415. doi: 10.1111/j.1471-4159.1993.tb13634.x. [DOI] [PubMed] [Google Scholar]
- Giddings S. J., Chirgwin J., Permutt M. A. Effects of glucose on proinsulin messenger RNA in rats in vivo. Diabetes. 1982 Jul;31(7):624–629. doi: 10.2337/diab.31.7.624. [DOI] [PubMed] [Google Scholar]
- Gross D. J., Villa-Komaroff L., Kahn C. R., Weir G. C., Halban P. A. Deletion of a highly conserved tetrapeptide sequence of the proinsulin connecting peptide (C-peptide) inhibits proinsulin to insulin conversion by transfected pituitary corticotroph (AtT20) cells. J Biol Chem. 1989 Dec 25;264(36):21486–21490. [PubMed] [Google Scholar]
- Guest P. C., Arden S. D., Bennett D. L., Clark A., Rutherford N. G., Hutton J. C. The post-translational processing and intracellular sorting of PC2 in the islets of Langerhans. J Biol Chem. 1992 Nov 5;267(31):22401–22406. [PubMed] [Google Scholar]
- Guest P. C., Pipeleers D., Rossier J., Rhodes C. J., Hutton J. C. Co-secretion of carboxypeptidase H and insulin from isolated rat islets of Langerhans. Biochem J. 1989 Dec 1;264(2):503–508. doi: 10.1042/bj2640503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guest P. C., Rhodes C. J., Hutton J. C. Regulation of the biosynthesis of insulin-secretory-granule proteins. Co-ordinate translational control is exerted on some, but not all, granule matrix constituents. Biochem J. 1989 Jan 15;257(2):431–437. doi: 10.1042/bj2570431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halban P. A. Differential rates of release of newly synthesized and of stored insulin from pancreatic islets. Endocrinology. 1982 Apr;110(4):1183–1188. doi: 10.1210/endo-110-4-1183. [DOI] [PubMed] [Google Scholar]
- Hutton J. C. Subtilisin-like proteinases involved in the activation of proproteins of the eukaryotic secretory pathway. Curr Opin Cell Biol. 1990 Dec;2(6):1131–1142. doi: 10.1016/0955-0674(90)90167-d. [DOI] [PubMed] [Google Scholar]
- Kaelin D., Renold A. E., Sharp G. W. Glucose stimulated proinsulin biosynthesis rates of turn off after cessation of the stimulus. Diabetologia. 1978 May;14(5):329–335. doi: 10.1007/BF01223025. [DOI] [PubMed] [Google Scholar]
- Kuliawat R., Arvan P. Protein targeting via the "constitutive-like" secretory pathway in isolated pancreatic islets: passive sorting in the immature granule compartment. J Cell Biol. 1992 Aug;118(3):521–529. doi: 10.1083/jcb.118.3.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leahy J. L., Bonner-Weir S., Weir G. C. Beta-cell dysfunction induced by chronic hyperglycemia. Current ideas on mechanism of impaired glucose-induced insulin secretion. Diabetes Care. 1992 Mar;15(3):442–455. doi: 10.2337/diacare.15.3.442. [DOI] [PubMed] [Google Scholar]
- Leahy J. L., Cooper H. E., Deal D. A., Weir G. C. Chronic hyperglycemia is associated with impaired glucose influence on insulin secretion. A study in normal rats using chronic in vivo glucose infusions. J Clin Invest. 1986 Mar;77(3):908–915. doi: 10.1172/JCI112389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leahy J. L., Halban P. A., Weir G. C. Relative hypersecretion of proinsulin in rat model of NIDDM. Diabetes. 1991 Aug;40(8):985–989. doi: 10.2337/diab.40.8.985. [DOI] [PubMed] [Google Scholar]
- Leahy J. L. Increased proinsulin/insulin ratio in pancreas extracts of hyperglycemic rats. Diabetes. 1993 Jan;42(1):22–27. doi: 10.2337/diab.42.1.22. [DOI] [PubMed] [Google Scholar]
- Leahy J. L. Natural history of beta-cell dysfunction in NIDDM. Diabetes Care. 1990 Sep;13(9):992–1010. doi: 10.2337/diacare.13.9.992. [DOI] [PubMed] [Google Scholar]
- Leahy J. L., Weir G. C. Evolution of abnormal insulin secretory responses during 48-h in vivo hyperglycemia. Diabetes. 1988 Feb;37(2):217–222. doi: 10.2337/diab.37.2.217. [DOI] [PubMed] [Google Scholar]
- Neerman-Arbez M., Cirulli V., Halban P. A. Levels of the conversion endoproteases PC1 (PC3) and PC2 distinguish between insulin-producing pancreatic islet beta cells and non-beta cells. Biochem J. 1994 May 15;300(Pt 1):57–61. doi: 10.1042/bj3000057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orci L., Ravazzola M., Storch M. J., Anderson R. G., Vassalli J. D., Perrelet A. Proteolytic maturation of insulin is a post-Golgi event which occurs in acidifying clathrin-coated secretory vesicles. Cell. 1987 Jun 19;49(6):865–868. doi: 10.1016/0092-8674(87)90624-6. [DOI] [PubMed] [Google Scholar]
- Orci L. The insulin factory: a tour of the plant surroundings and a visit to the assembly line. The Minkowski lecture 1973 revisited. Diabetologia. 1985 Aug;28(8):528–546. doi: 10.1007/BF00281987. [DOI] [PubMed] [Google Scholar]
- Pipeleers D. G. Heterogeneity in pancreatic beta-cell population. Diabetes. 1992 Jul;41(7):777–781. doi: 10.2337/diab.41.7.777. [DOI] [PubMed] [Google Scholar]
- Porte D., Jr, Kahn S. E. Hyperproinsulinemia and amyloid in NIDDM. Clues to etiology of islet beta-cell dysfunction? Diabetes. 1989 Nov;38(11):1333–1336. doi: 10.2337/diab.38.11.1333. [DOI] [PubMed] [Google Scholar]
- Rhodes C. J., Alarcón C. What beta-cell defect could lead to hyperproinsulinemia in NIDDM? Some clues from recent advances made in understanding the proinsulin-processing mechanism. Diabetes. 1994 Apr;43(4):511–517. doi: 10.2337/diab.43.4.511. [DOI] [PubMed] [Google Scholar]
- Rhodes C. J., Halban P. A. Newly synthesized proinsulin/insulin and stored insulin are released from pancreatic B cells predominantly via a regulated, rather than a constitutive, pathway. J Cell Biol. 1987 Jul;105(1):145–153. doi: 10.1083/jcb.105.1.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes C. J., Lincoln B., Shoelson S. E. Preferential cleavage of des-31,32-proinsulin over intact proinsulin by the insulin secretory granule type II endopeptidase. Implication of a favored route for prohormone processing. J Biol Chem. 1992 Nov 15;267(32):22719–22727. [PubMed] [Google Scholar]
- Rhodes C. J., Lucas C. A., Mutkoski R. L., Orci L., Halban P. A. Stimulation by ATP of proinsulin to insulin conversion in isolated rat pancreatic islet secretory granules. Association with the ATP-dependent proton pump. J Biol Chem. 1987 Aug 5;262(22):10712–10717. [PubMed] [Google Scholar]
- Seidah N. G., Marcinkiewicz M., Benjannet S., Gaspar L., Beaubien G., Mattei M. G., Lazure C., Mbikay M., Chrétien M. Cloning and primary sequence of a mouse candidate prohormone convertase PC1 homologous to PC2, Furin, and Kex2: distinct chromosomal localization and messenger RNA distribution in brain and pituitary compared to PC2. Mol Endocrinol. 1991 Jan;5(1):111–122. doi: 10.1210/mend-5-1-111. [DOI] [PubMed] [Google Scholar]
- Sizonenko S., Irminger J. C., Buhler L., Deng S., Morel P., Halban P. A. Kinetics of proinsulin conversion in human islets. Diabetes. 1993 Jun;42(6):933–936. doi: 10.2337/diab.42.6.933. [DOI] [PubMed] [Google Scholar]
- Smeekens S. P., Avruch A. S., LaMendola J., Chan S. J., Steiner D. F. Identification of a cDNA encoding a second putative prohormone convertase related to PC2 in AtT20 cells and islets of Langerhans. Proc Natl Acad Sci U S A. 1991 Jan 15;88(2):340–344. doi: 10.1073/pnas.88.2.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smeekens S. P., Montag A. G., Thomas G., Albiges-Rizo C., Carroll R., Benig M., Phillips L. A., Martin S., Ohagi S., Gardner P. Proinsulin processing by the subtilisin-related proprotein convertases furin, PC2, and PC3. Proc Natl Acad Sci U S A. 1992 Sep 15;89(18):8822–8826. doi: 10.1073/pnas.89.18.8822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smeekens S. P., Steiner D. F. Identification of a human insulinoma cDNA encoding a novel mammalian protein structurally related to the yeast dibasic processing protease Kex2. J Biol Chem. 1990 Feb 25;265(6):2997–3000. [PubMed] [Google Scholar]
- Steiner D. F., Smeekens S. P., Ohagi S., Chan S. J. The new enzymology of precursor processing endoproteases. J Biol Chem. 1992 Nov 25;267(33):23435–23438. [PubMed] [Google Scholar]
- Temple R. C., Carrington C. A., Luzio S. D., Owens D. R., Schneider A. E., Sobey W. J., Hales C. N. Insulin deficiency in non-insulin-dependent diabetes. Lancet. 1989 Feb 11;1(8633):293–295. doi: 10.1016/s0140-6736(89)91306-8. [DOI] [PubMed] [Google Scholar]
- Tillil H., Frank B. H., Pekar A. H., Broelsch C., Rubenstein A. H., Polonsky K. S. Hypoglycemic potency and metabolic clearance rate of intravenously administered human proinsulin and metabolites. Endocrinology. 1990 Nov;127(5):2418–2422. doi: 10.1210/endo-127-5-2418. [DOI] [PubMed] [Google Scholar]
- Vindrola O., Lindberg I. Biosynthesis of the prohormone convertase mPC1 in AtT-20 cells. Mol Endocrinol. 1992 Jul;6(7):1088–1094. doi: 10.1210/mend.6.7.1508222. [DOI] [PubMed] [Google Scholar]