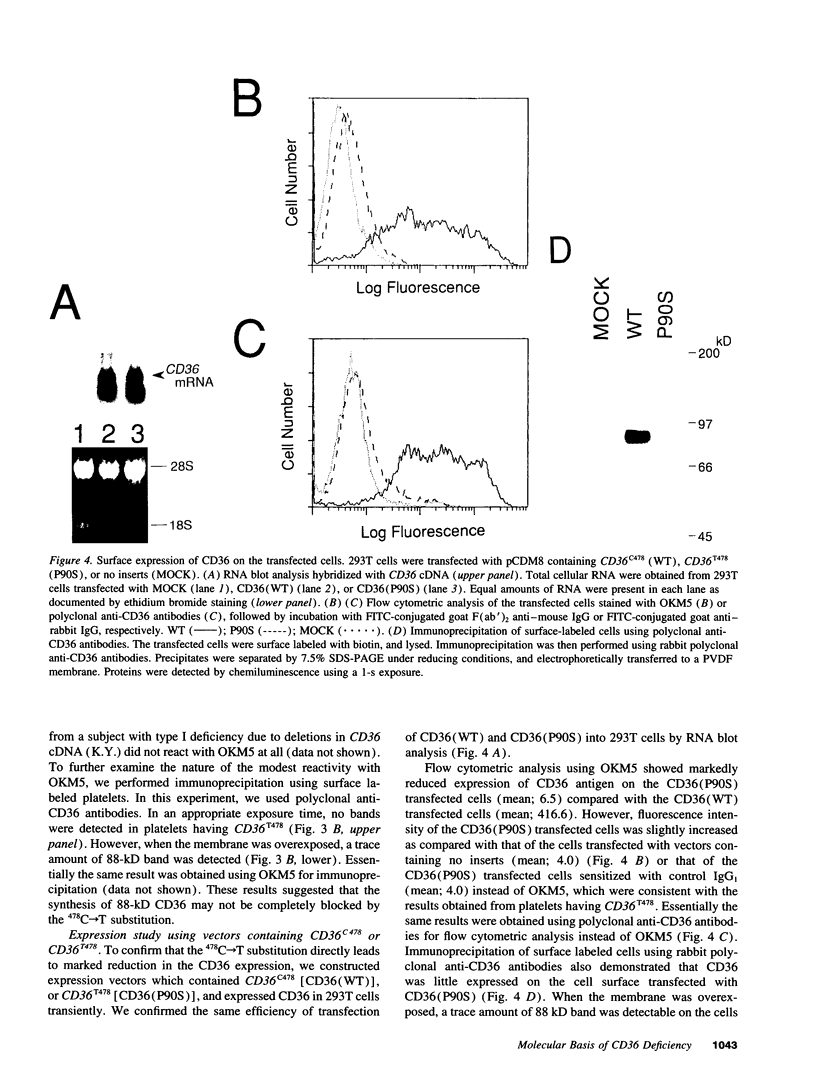
Abstract
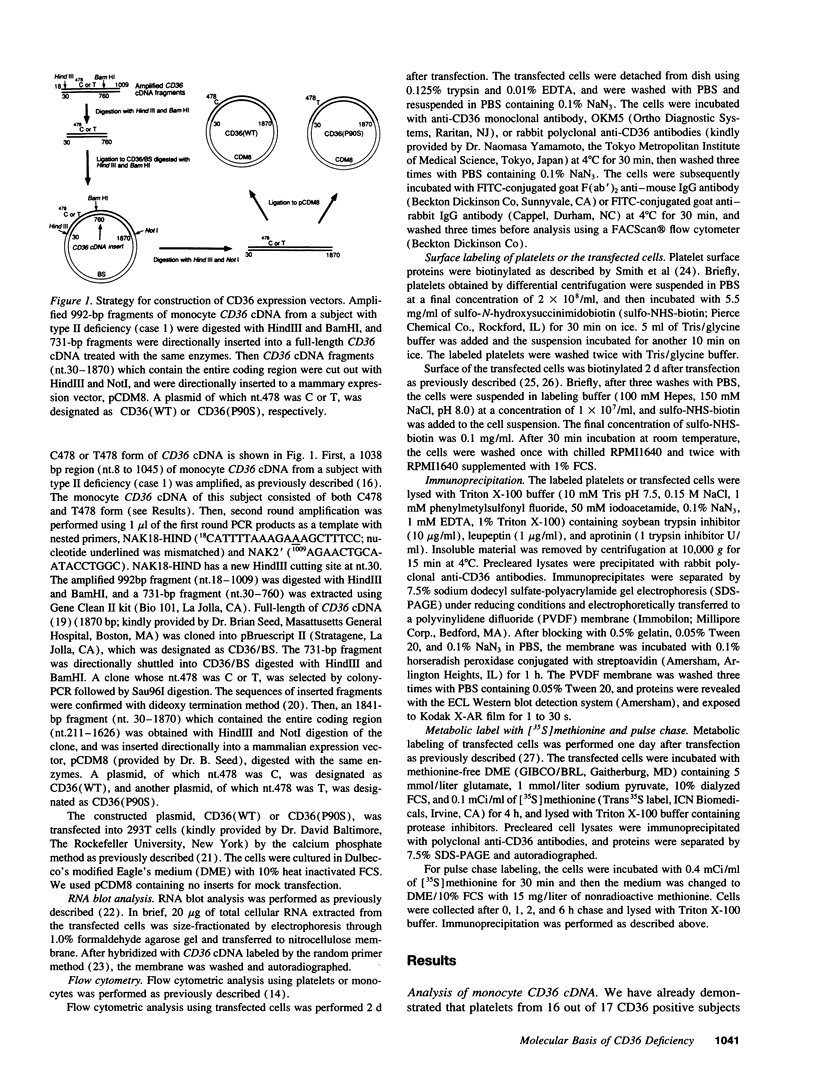
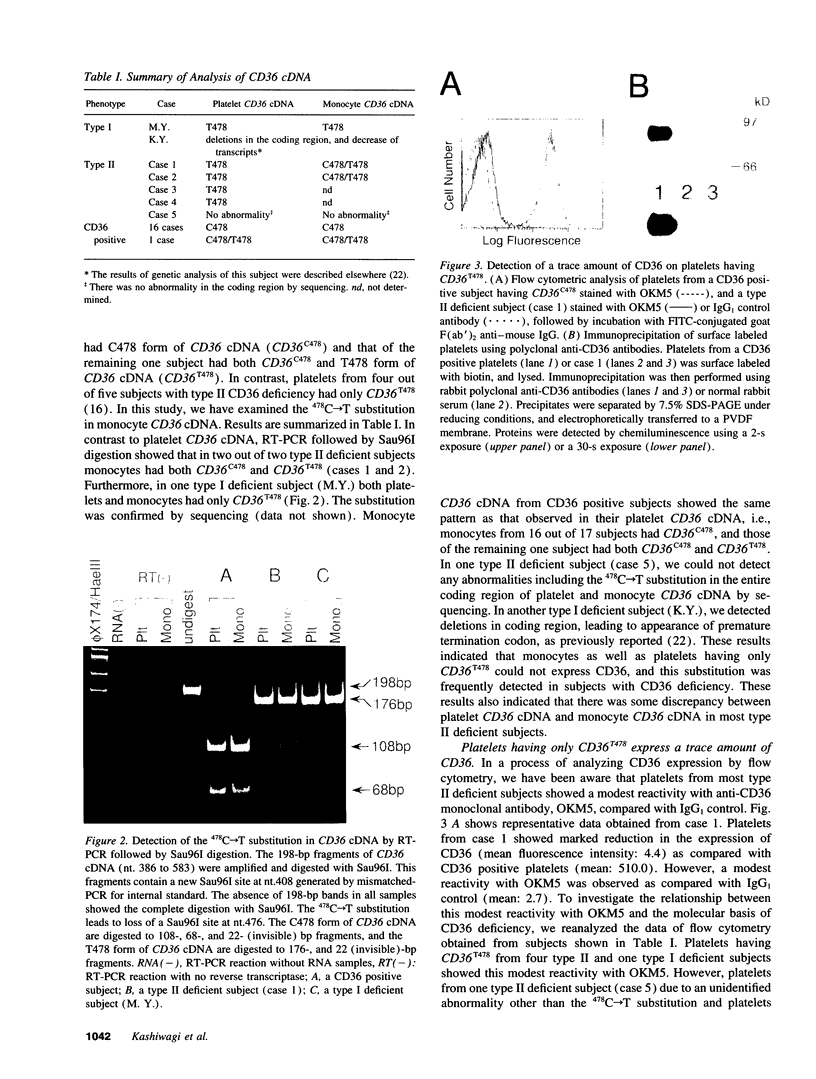
CD36 deficiency is divided into two subgroups: neither platelets nor monocytes express CD36 (type I deficiency), and monocytes express CD36 in spite of the lack of platelet CD36 (type II deficiency). We have already demonstrated that a 478C-->T substitution (proline90-->serine) in platelet CD36 cDNA predominates in type II deficiency (Kashiwagi, H., S. Honda, Y. Tomiyama, H. Mizutani, H. Take, Y. Honda, S. Kosugi, Y. Kanayama, Y. Kurata, and Y. Matsuzawa. 1993. Thromb. Haemostasis. 69:481-484). In this study, we revealed that monocyte CD36 cDNA from two type II deficient subjects was heterozygous for C478 and T478 form, while platelet CD36 cDNA of these subjects consisted of only T478 form. In a type I deficient subject, both platelet and monocyte CD36 cDNA showed only T478 form. Expression assay using C478 or T478 form of CD36 cDNA transfected cells revealed that there was an 81-kD precursor form of CD36, and that the maturation of the 81-kD precursor form to the 88-kD mature form of CD36 was markedly impaired by the substitution. The mutated precursor form of CD36 was subsequently degraded in the cytoplasm. These results indicate that the 478C-->T substitution directly leads to CD36 deficiency via defects in posttranslational modification, and that this substitution is the major defects underlying CD36 deficiency.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abumrad N. A., el-Maghrabi M. R., Amri E. Z., Lopez E., Grimaldi P. A. Cloning of a rat adipocyte membrane protein implicated in binding or transport of long-chain fatty acids that is induced during preadipocyte differentiation. Homology with human CD36. J Biol Chem. 1993 Aug 25;268(24):17665–17668. [PubMed] [Google Scholar]
- Asch A. S., Barnwell J., Silverstein R. L., Nachman R. L. Isolation of the thrombospondin membrane receptor. J Clin Invest. 1987 Apr;79(4):1054–1061. doi: 10.1172/JCI112918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barriocanal J. G., Bonifacino J. S., Yuan L., Sandoval I. V. Biosynthesis, glycosylation, movement through the Golgi system, and transport to lysosomes by an N-linked carbohydrate-independent mechanism of three lysosomal integral membrane proteins. J Biol Chem. 1986 Dec 15;261(35):16755–16763. [PubMed] [Google Scholar]
- Calvo D., Vega M. A. Identification, primary structure, and distribution of CLA-1, a novel member of the CD36/LIMPII gene family. J Biol Chem. 1993 Sep 5;268(25):18929–18935. [PubMed] [Google Scholar]
- Edelman P., Vinci G., Villeval J. L., Vainchenker W., Henri A., Miglierina R., Rouger P., Reviron J., Breton-Gorius J., Sureau C. A monoclonal antibody against an erythrocyte ontogenic antigen identifies fetal and adult erythroid progenitors. Blood. 1986 Jan;67(1):56–63. [PubMed] [Google Scholar]
- Endemann G., Stanton L. W., Madden K. S., Bryant C. M., White R. T., Protter A. A. CD36 is a receptor for oxidized low density lipoprotein. J Biol Chem. 1993 Jun 5;268(16):11811–11816. [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Furitsu T., Tsujimura T., Tono T., Ikeda H., Kitayama H., Koshimizu U., Sugahara H., Butterfield J. H., Ashman L. K., Kanayama Y. Identification of mutations in the coding sequence of the proto-oncogene c-kit in a human mast cell leukemia cell line causing ligand-independent activation of c-kit product. J Clin Invest. 1993 Oct;92(4):1736–1744. doi: 10.1172/JCI116761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwalt D. E., Lipsky R. H., Ockenhouse C. F., Ikeda H., Tandon N. N., Jamieson G. A. Membrane glycoprotein CD36: a review of its roles in adherence, signal transduction, and transfusion medicine. Blood. 1992 Sep 1;80(5):1105–1115. [PubMed] [Google Scholar]
- Greenwalt D. E., Watt K. W., So O. Y., Jiwani N. PAS IV, an integral membrane protein of mammary epithelial cells, is related to platelet and endothelial cell CD36 (GP IV). Biochemistry. 1990 Jul 31;29(30):7054–7059. doi: 10.1021/bi00482a015. [DOI] [PubMed] [Google Scholar]
- Hart K., Wilcox M. A Drosophila gene encoding an epithelial membrane protein with homology to CD36/LIMP II. J Mol Biol. 1993 Nov 5;234(1):249–253. doi: 10.1006/jmbi.1993.1580. [DOI] [PubMed] [Google Scholar]
- Ikeda H., Mitani T., Ohnuma M., Haga H., Ohtzuka S., Kato T., Nakase T., Sekiguchi S. A new platelet-specific antigen, Naka, involved in the refractoriness of HLA-matched platelet transfusion. Vox Sang. 1989;57(3):213–217. doi: 10.1111/j.1423-0410.1989.tb00826.x. [DOI] [PubMed] [Google Scholar]
- Ishihara K., Medina K., Hayashi S., Pietrangeli C., Namen A. E., Miyake K., Kincade P. W. Stromal-cell and cytokine-dependent lymphocyte clones which span the pre-B- to B-cell transition. Dev Immunol. 1991;1(3):149–161. doi: 10.1155/1991/79721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishihara K., Wood W. J., Jr, Damore M., Hermanson G. G., Wall R., Kincade P. W. B29 gene products complex with immunoglobulins on B lymphocytes. Proc Natl Acad Sci U S A. 1992 Jan 15;89(2):633–637. doi: 10.1073/pnas.89.2.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashiwagi H., Honda S., Take H., Mizutani H., Imai Y., Furubayashi T., Tomiyama Y., Kurata Y., Yonezawa T. Presence of the entire coding region of GP IV mRNA in Nak(a)-negative platelets. Int J Hematol. 1993 Apr;57(2):153–161. [PubMed] [Google Scholar]
- Kashiwagi H., Honda S., Take H., Mizutani H., Imai Y., Tomiyama Y., Kurata Y., Yonezawa T. Detection of Naka antigen and GP IV (CD36) mRNA in monocytes of Naka-negative subjects. Thromb Haemost. 1992 Mar 2;67(3):384–385. [PubMed] [Google Scholar]
- Kashiwagi H., Honda S., Tomiyama Y., Mizutani H., Take H., Honda Y., Kosugi S., Kanayama Y., Kurata Y., Matsuzawa Y. A novel polymorphism in glycoprotein IV (replacement of proline-90 by serine) predominates in subjects with platelet GPIV deficiency. Thromb Haemost. 1993 May 3;69(5):481–484. [PubMed] [Google Scholar]
- Kashiwagi H., Tomiyama Y., Kosugi S., Shiraga M., Lipsky R. H., Kanayama Y., Kurata Y., Matsuzawa Y. Identification of molecular defects in a subject with type I CD36 deficiency. Blood. 1994 Jun 15;83(12):3545–3552. [PubMed] [Google Scholar]
- Knowles D. M., 2nd, Tolidjian B., Marboe C., D'Agati V., Grimes M., Chess L. Monoclonal anti-human monocyte antibodies OKM1 and OKM5 possess distinctive tissue distributions including differential reactivity with vascular endothelium. J Immunol. 1984 May;132(5):2170–2173. [PubMed] [Google Scholar]
- Kornfeld R., Kornfeld S. Assembly of asparagine-linked oligosaccharides. Annu Rev Biochem. 1985;54:631–664. doi: 10.1146/annurev.bi.54.070185.003215. [DOI] [PubMed] [Google Scholar]
- Ockenhouse C. F., Tandon N. N., Magowan C., Jamieson G. A., Chulay J. D. Identification of a platelet membrane glycoprotein as a falciparum malaria sequestration receptor. Science. 1989 Mar 17;243(4897):1469–1471. doi: 10.1126/science.2467377. [DOI] [PubMed] [Google Scholar]
- Oquendo P., Hundt E., Lawler J., Seed B. CD36 directly mediates cytoadherence of Plasmodium falciparum parasitized erythrocytes. Cell. 1989 Jul 14;58(1):95–101. doi: 10.1016/0092-8674(89)90406-6. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J. W., Hayward C. P., Warkentin T. E., Horsewood P., Kelton J. G. Investigation of human platelet alloantigens and glycoproteins using non-radioactive immunoprecipitation. J Immunol Methods. 1993 Jan 14;158(1):77–85. doi: 10.1016/0022-1759(93)90260-e. [DOI] [PubMed] [Google Scholar]
- Take H., Kashiwagi H., Tomiyama Y., Honda S., Honda Y., Mizutani H., Furubayashi T., Karasuno T., Nishiura T., Kanayama Y. Expression of GPIV and N(aka) antigen on monocytes in N(aka)-negative subjects whose platelets lack GPIV. Br J Haematol. 1993 Jul;84(3):387–391. doi: 10.1111/j.1365-2141.1993.tb03091.x. [DOI] [PubMed] [Google Scholar]
- Tandon N. N., Kralisz U., Jamieson G. A. Identification of glycoprotein IV (CD36) as a primary receptor for platelet-collagen adhesion. J Biol Chem. 1989 May 5;264(13):7576–7583. [PubMed] [Google Scholar]
- Tandon N. N., Lipsky R. H., Burgess W. H., Jamieson G. A. Isolation and characterization of platelet glycoprotein IV (CD36). J Biol Chem. 1989 May 5;264(13):7570–7575. [PubMed] [Google Scholar]
- Tang Y., Taylor K. T., Sobieski D. A., Medved E. S., Lipsky R. H. Identification of a human CD36 isoform produced by exon skipping. Conservation of exon organization and pre-mRNA splicing patterns with a CD36 gene family member, CLA-1. J Biol Chem. 1994 Feb 25;269(8):6011–6015. [PubMed] [Google Scholar]
- Taylor K. T., Tang Y., Sobieski D. A., Lipsky R. H. Characterization of two alternatively spliced 5'-untranslated exons of the human CD36 gene in different cell types. Gene. 1993 Nov 15;133(2):205–212. doi: 10.1016/0378-1119(93)90639-k. [DOI] [PubMed] [Google Scholar]
- Tomiyama Y., Take H., Ikeda H., Mitani T., Furubayashi T., Mizutani H., Yamamoto N., Tandon N. N., Sekiguchi S., Jamieson G. A. Identification of the platelet-specific alloantigen, Naka, on platelet membrane glycoprotein IV. Blood. 1990 Feb 1;75(3):684–687. [PubMed] [Google Scholar]
- Tono T., Tsujimura T., Koshimizu U., Kasugai T., Adachi S., Isozaki K., Nishikawa S., Morimoto M., Nishimune Y., Nomura S. c-kit Gene was not transcribed in cultured mast cells of mast cell-deficient Wsh/Wsh mice that have a normal number of erythrocytes and a normal c-kit coding region. Blood. 1992 Sep 15;80(6):1448–1453. [PubMed] [Google Scholar]
- Vega M. A., Seguí-Real B., García J. A., Calés C., Rodríguez F., Vanderkerckhove J., Sandoval I. V. Cloning, sequencing, and expression of a cDNA encoding rat LIMP II, a novel 74-kDa lysosomal membrane protein related to the surface adhesion protein CD36. J Biol Chem. 1991 Sep 5;266(25):16818–16824. [PubMed] [Google Scholar]
- Yamamoto N., Akamatsu N., Sakuraba H., Yamazaki H., Tanoue K. Platelet glycoprotein IV (CD36) deficiency is associated with the absence (type I) or the presence (type II) of glycoprotein IV on monocytes. Blood. 1994 Jan 15;83(2):392–397. [PubMed] [Google Scholar]
- Yamamoto N., Ikeda H., Tandon N. N., Herman J., Tomiyama Y., Mitani T., Sekiguchi S., Lipsky R., Kralisz U., Jamieson G. A. A platelet membrane glycoprotein (GP) deficiency in healthy blood donors: Naka- platelets lack detectable GPIV (CD36). Blood. 1990 Nov 1;76(9):1698–1703. [PubMed] [Google Scholar]