Abstract
Background
Administration of exogenous progesterone for luteal phase support has become a standard of practice. Intramuscular (IM) injections of progesterone in oil (PIO) and vaginal administration of progesterone are the primary routes of administration. This report describes the administration preferences expressed by women with infertility that were given progesterone vaginal insert (PVI) or progesterone in oil injections (PIO) for luteal phase support during fresh IVF cycles.
Methods
A questionnaire to assess the tolerability, convenience, and ease of administration of PVI and PIO given for luteal phase support was completed by infertile women diagnosed with PCOS and planning to undergo IVF. The women participated in an open-label study of highly purified human menopausal gonadotropins (HP-hMG) compared with recombinant FSH (rFSH) given for stimulation of ovulation.
Results
Most women commented on the convenience and ease of administration of PVI, while a majority of women who administered IM PIO described experiencing pain. In addition, their partners often indicated that they had experienced at least some anxiety regarding the administration of PIO. The most distinguishing difference between PVI and PIO in this study was the overall patient preference for PVI. Despite the need to administer PVI either twice a day or three times a day, 82.6% of the patients in the PVI group found it “very” or “somewhat convenient” compared with 44.9% of women in the PIO group.
Conclusions
The results of this comprehensive, prospective patient survey, along with findings from other similar reports, suggest that PVI provides an easy-to-use and convenient method for providing the necessary luteal phase support for IVF cycles without the pain and inconvenience of daily IM PIO. Moreover, ongoing pregnancy rates with the well-tolerated PVI were as good as the pregnancy rates with PIO.
Trial registration
ClinicalTrial.gov, NCT00805935
Electronic supplementary material
The online version of this article (doi:10.1186/1742-4755-11-78) contains supplementary material, which is available to authorized users.
Keywords: Tolerability, Vaginal progesterone, Luteal support, Endometrium, IVF, Polycystic ovary syndrome
Background
Progesterone produced by the corpus luteum during the luteal phase of the menstrual cycle promotes secretory transformation of endometrial tissue, which is essential for embryo implantation [1]. Use of gonadotropin-releasing hormone (GnRH) agonists and antagonists administered to patients during cycles of IVF may adversely affect luteal function and compromise the production of progesterone by the corpus luteum [1, 2]. Administration of exogenous progesterone for luteal phase support has become a standard of practice, and its administration has been shown to improve pregnancy rates during cycles of IVF [3–5].
Progesterone is administered by oral, intramuscular (IM), or vaginal routes [3–5]. However, because of poor bioavailability of progesterone administered orally, IM injections of progesterone in oil (PIO) and vaginal administration of progesterone are the primary routes used in clinical practice [6].
Administration of progesterone by IM and vaginal routes appears to result in similar pregnancy rates [3, 6–16]. Notably, a recent retrospective study of a large cohort of women undergoing IVF showed significantly better clinical pregnancy rates when progesterone was given by both the IM and vaginal routes compared with the vaginal route alone for IVF cycles with frozen embryo transfer (P < 0.001); but no difference for IVF cycles with fresh autologous (P = 0.05) or donor oocytes (P = 0.39) [17].
Surveys associated with clinical trials have shown that vaginal administration is preferred over IM administration by most women [15, 18, 19]. Women prefer vaginal over IM administration because vaginal administration is convenient, easy to use, and does not involve the pain and side effects commonly associated with daily IM injections [15, 18, 19].
This report describes the administration preferences expressed by women with polycystic ovarian syndrome (PCOS) who were given progesterone vaginal insert (PVI, Endometrin®, Ferring Pharmaceuticals, Inc, Parsippany, NJ, USA) or IM PIO (Progesterone Injection USP 50 mg/mL, American Reagent, Corp, Shirley, NY, USA) for luteal phase support during fresh IVF cycles.
Methods
Patients
To be eligible for this phase 4 study, participants had to be premenopausal (women aged 18–42 years) with a documented history of infertility (e.g., unable to conceive for at least 1 year, or for 6 months for women aged 38 years or older, or with bilateral tubal occlusion or absence of tubal segments, or male factor) previously diagnosed with PCOS and considered to be a favorable candidate to undergo an assisted reproductive technology (ART) procedure. PCOS was defined using the criteria adopted at the 2003 Rotterdam PCOS Consensus [20].
For study inclusion, women were required to have a body mass index (BMI) of 18–39 kg/m2; an intact uterus adnexa visualized on transvaginal ultrasonography (TVU) performed within 1 year of screening; and 2 of the following 3 conditions present: (1) oligo- or anovulation, (2) clinical and/or biochemical signs of hyperandrogenism, and/or (3) polycystic ovaries. Women were also required to have an early follicular phase (day 3) serum follicle-stimulating hormone (FSH) concentration ≤ 15 IU/L, and serum estradiol (E2) concentration within normal limits for 4 months before screening.
Women were ineligible to participate if they had 2 or more previous failed IVF cycles or poor response to gonadotropins (i.e., development of ≤ 2 mature follicles); planned to use a gestational or surrogate carrier or donor oocytes; had a history of recurrent pregnancy loss (>2); currently had abnormal uterine bleeding of an undetermined origin; or had a male partner with severe male factor infertility who required invasive or surgical sperm retrieval (e.g., microsurgical epididymal sperm aspiration, testicular sperm extraction).
Women were also ineligible if they were diagnosed with clinically relevant systemic disease (e.g., uncontrolled thyroid and adrenal dysfunction, an organic intracranial lesion such as a pituitary tumor, insulin-dependent diabetes mellitus, uterine cancer); had a current or a recent history of substance abuse, including alcohol abuse; smoked 10 or more cigarettes per day; had a hypersensitivity to any of the study drugs; or had any surgical or medical condition that, in the investigator’s or sponsor’s judgment, would interfere with absorption, distribution, metabolism, or excretion of the study drugs.
The study was conducted in accordance with the ethical principles that have their origin in the Declaration of Helsinki, followed the approved protocol, observed International Conference on Harmonization–Good Clinical Practice guidelines, and complied with all applicable regulatory requirements. The protocol and its associated Informed Consent Agreement were reviewed and approved by the appropriate Institutional Review Board (Independent Investigational Review Board, Inc., Women and Infants Hospital of Rhode Island Institutional Review Board and Weill Cornell Medical College Institutional Review Board). Study personnel obtained written informed consent directly from all participants before their entry into the study. This trial is registered under NCT00805935 [https://clinicaltrials.gov/].
Study design
This multicenter, randomized, open-label, phase 4, exploratory, study was conducted to assess the efficacy and safety of highly purified human menopausal gonadotropins (HP-hMG, Menopur® Ferring Pharmaceuticals, Inc, Parsippany, NJ, USA) compared with recombinant FSH (rFSH) during fresh cycles of IVF in otherwise healthy infertile women who were diagnosed with PCOS and planning to undergo IVF. Women were also randomized to PVI vs PIO. Women who participated in the study completed a questionnaire to assess the tolerability, convenience, and ease of administration of PVI and PIO given for luteal phase support.
Women were screened based on the study’s inclusion/exclusion criteria, analysis of medical and infertility history, evaluation of FSH and E2 serum concentrations, results from physical and gynecological examinations, and findings from the center’s standard screening evaluations for IVF participants. TVU was performed and serum was collected for E2 and progesterone concentration measurement from all participants at baseline and periodically throughout the study. All adverse events (AEs) and concomitant medications taken by participants were documented throughout the study. Patients returned to the study center for regularly scheduled clinic visits as required per the IVF protocol and at other specified time periods.
Study treatments
Leuprolide acetate, a GnRH agonist (GnRHa), was given for pituitary downregulation. Participants were allowed to use oral contraceptives before administration of the GnRHa, if this was standard practice at the center. Patients received daily injections of either HP-hMG (Menopur®, Ferring Pharmaceuticals, Inc, Parsippany, NJ, USA) 225 IU (3 vials) subcutaneously (SC) or follitropin beta for injection (rFSH: Follistim®, Merck & Co Inc, Whitehouse Station, NJ, USA) 225 IU SC for a minimum of 5 days for ovarian stimulation. Human chorionic gonadotropin (hCG; Novarel®, Ferring Pharmaceuticals, Inc) was administered to trigger ovulation.
Luteal phase support
Progesterone was administered to support the luteal phase beginning on the day after oocyte retrieval. Patients were randomized to receive PVI 100 mg inserted vaginally 2 or 3 times daily, or PIO 50-mg IM injection once daily until 10-weeks’ gestation or confirmation of a negative pregnancy test. A large, randomized control trial previously demonstrated that there is no significant difference in pregnancy outcomes between 2 and 3 times daily dosing of PVI [21]. Each patient also took estradiol tablets (Estrace®, Barr Laboratories, Inc, Pomona, NY, USA) 2 mg by mouth once daily for the duration of luteal support. Embryo transfer occurred on day 3 or day 5 after insemination or intracytoplasmic sperm injection.
Assessments
Patients completed a survey to assess administration preferences of PVI and PIO at the start of their luteal phase support and at their final visit. Questions included on the PVI and PIO patient surveys are shown in the Assessments subsection under Methods. Responses to the survey assessed convenience and ease of use and other variables such as pain and discomfort associated with study drug administration. Assessment of safety and tolerability included documentation of AEs and serious AEs, results from clinical laboratory evaluations and electrocardiograms, measurements of vital signs, and findings from TVU.
Patient survey assessing administration preferences of progesterone vaginal insert (PVI) and progesterone in oil (PIO)
Section 1*† (PVI and PIO)
For the following questions, please choose the response which best reflects your experience.
-
How would you describe the convenience of administering/dosing [Endometrin /progesterone in oil injections]?
- Very convenient
- Somewhat convenient
- Neither convenient or inconvenient
- Somewhat inconvenient
- Very inconvenient
-
How would you describe the ease of administering/dosing [Endometrin/progesterone in oil injections]?
- Very easy
- Somewhat easy
- Neither easy or difficult
- Somewhat difficult
- Very difficult
-
In a previous ART cycle, were you prescribed progesterone supplementation?
- Yes
- No (Skip questions 4, 5).
-
If you answered ‘Yes’ to question 3: Which of the following progesterones were you prescribed in a previous cycle?
- Progesterone in oil
- Crinone/Prochieve
- Prometrium orally
- Prometrium vaginally
- Pharmacy compounded suppositories
- Endometrin
-
If you answered ‘Yes’ to question 3: Overall, how would you compare [Endometrin/ progesterone in oil injections] to the different progesterone used in a previous cycle?
- Very convenient and easy to use
- Somewhat convenient and easy to use
- Neither convenient or inconvenient and easy to use
- Very inconvenient and difficult to use
-
Please rate your overall satisfaction level with [Endometrin/ progesterone in oil injections]
- Very satisfied
- Somewhat satisfied
- Neither satisfied nor dissatisfied
- Somewhat dissatisfied
- Very dissatisfied
-
If given a choice, which progesterone would you prefer to use?
- Progesterone in oil
- Crinone/Prochieve
- Prometrium orally
- Prometrium vaginally
- Pharmacy compounded suppositories
- Endometrin
Section 2¶ (PIO only)
-
How painful were the progesterone in oil injections?
- Very painful
- Somewhat painful
- Neither painful nor painless
- Somewhat painless
- Very painless
-
How would you describe your partner’s level of anxiety in giving the progesterone in oil injections?.
- Very anxious
- Somewhat anxious
- Neither anxious nor comfortable
- Not anxious
-
Did the progesterone in oil injections make you consider dropping out of treatment?
- Yes
- No
* Separate surveys were administered for PVI and PIO.
† “Endometrin” or “progesterone in oil injection” was included in the questions on each survey, and referred to PVI or PIO, respectively.
‡ Sections 1 and 2 on PVI survey.
¶ Section 2 PIO survey.
Statistical analysis
Fisher’s exact test was used to determine treatment group comparisons for categorical variables. As appropriate, one-way analysis of variance or the Wilcoxon rank sum test were used, to determine treatment group comparisons for continuous variables. Statistical significance was declared if the two-sided P value was ≤ 0.05. Statistical analyses were performed using statistical software (SAS version 9.2; SAS Institute Inc, Cary, NC, USA).
Results
Demographic characteristics were similar for the PVI and PIO treatment groups (Table 1). The majority of women enrolled in the study were Caucasian, with 71.7% and 75.4% in the PVI and PIO groups, respectively. Mean age was 30.9 and 31.5 years in the PVI and PIO groups, respectively.
Table 1.
Patient demographics and baseline characteristics, ITT population
| Parameter | PVI | PIO |
|---|---|---|
| (n = 53) | (n = 57) | |
| Age, y | 30.9 (20–40) | 31.5 (22–41) |
| BMI, kg/m2 | 26.9 (5.2) | 28.9 (6.5) |
| Race | ||
| White | 38 (71.7) | 43 (75.4) |
| Black | 0 | 1 (1.8) |
| Asian | 9 (17.0) | 2 (3.5) |
| Hispanic | 6 (11.3) | 10 (17.5) |
| Other | 0 | 1 (1.8) |
| Duration of infertility, months | 32.2 (19.7) | 49.6 (44.6) |
| 26.0 (10–96) | 34.0 (12–240) | |
| ART History | ||
| Number previous IVF cycles | 0.2 (0.5) | 0.2 (0.4) |
| Number failed IVF cycles | 0.1 (0.3) | 0.1 (0.3) |
| Number previous ovulation induction cycles* | 4.2 (3.1) | 5.3 (6.8) |
| Number failed ovulation induction cycles† | 4.1 (3.1) | 5.2 (6.8) |
| Gonadotropin for ovulation induction | ||
| rFSH | 28 (52.8) | 30 (52.6) |
| HP-hMG | 25 (47.2) | 27 (47.4) |
Values are n (%), median (range), or means (standard deviation).
*Excluding IVF, gamete intrafallopian transfer, and/or pronuclear stage embryo transfer.
†Excluding IVF.
ITT, intent to treat; PVI, progesterone vaginal insert; PIO, progesterone in oil; BMI, body mass index; ART, assisted reproductive technologies; rFSH, recombinant follicle-stimulating hormone; HP-hMG, highly purified human menopausal gonadotropin.
The number of women who received PVI or PIO for luteal phase support, underwent embryo transfer, and completed 10 weeks of luteal phase support are shown in Table 2. A negative result on serum pregnancy test was the most frequent reason for discontinuing luteal phase support in both groups (PVI, 26%; PIO, 21%). PVI was administered twice daily in 23.1% and 3 times daily in 76.9% of women randomized to PVI.
Table 2.
Disposition of women randomized to luteal support with progesterone vaginal insert and progesterone in oil
| Parameter | PVI | PIO |
|---|---|---|
| (n = 53) | (n = 57) | |
| Received luteal phase support | 48 (90.6) | 50 (87.7) |
| Underwent embryo transfer | 46 (86.8) | 49 (86.0) |
| Biochemical pregnancy* | 32 (60.4) | 37 (64.9) |
| Clinical pregnancy† | 27 (50.9) | 29 (50.9) |
| Ongoing pregnancy‡ | 25 (47.2) | 28 (49.1) |
Values are n (%).
*Positive result on serum β-hCG test 12–14 days after embryo transfer, P = 0.853.
†Gestational sac visualized using transvaginal ultrasonography approximately 4 weeks after embryo transfer, P = 1.0.
‡Fetal heart movements identified using transvaginal ultrasonography at approximately 6 weeks’ gestation, P = 0.851.
PVI, progesterone vaginal insert; PIO, progesterone in oil; β-hCG, beta human chorionic gonadotropin.
Patient-reported outcomes
The convenience/ease of administration and overall patient satisfaction for PVI or PIO based on survey responses at the final study visit are shown in Figure 1. More women commented on the convenience and ease of administration of PVI than IM PIO. Conversely, a majority of patients and their partners described pain and anxiety, respectively, associated with PIO. Progesterone treatment was reported as “very” or “somewhat convenient” to administer by 87.1% in the PVI group compared with 40.9% in the PIO group (Figure 1A). The “ease of use” of progesterone treatment was considered “very” or “somewhat easy” to administer by 97.4% in the PVI group compared with only 56.8% in the PIO group (Figure 1B). In the PIO group, 65.9% found treatment “very” or “somewhat painful”. Overall satisfaction with progesterone treatment was higher for PVI compared to PIO (71.8% of women were “very satisfied” with PVI compared with only 18.2% of women who received PIO) (Figure 1C).In the study, 24% (11/46) and 31% (15/49) of patients in the PVI and IM PIO arms, respectively, had used progesterone in a previous treatment cycle. Of these, 90.9% in the PVI group found vaginal inserts “very” or “somewhat easier” to use than a previous progesterone treatment. For those women in the PIO group, only 33.3% found this treatment “very” or “somewhat easier” to use than any previous progesterone treatment. In addition, 47.7% of patients’ partners in the PIO group reported being “very” or “somewhat anxious” about administering the treatment. No women in the PVI group found the vaginal inserts “difficult to administer” or expressed “dissatisfaction with administration” of the insert (Figure 1B and C). Overall, more patients expressed a preference for PVI than IM PIO.
Figure 1.
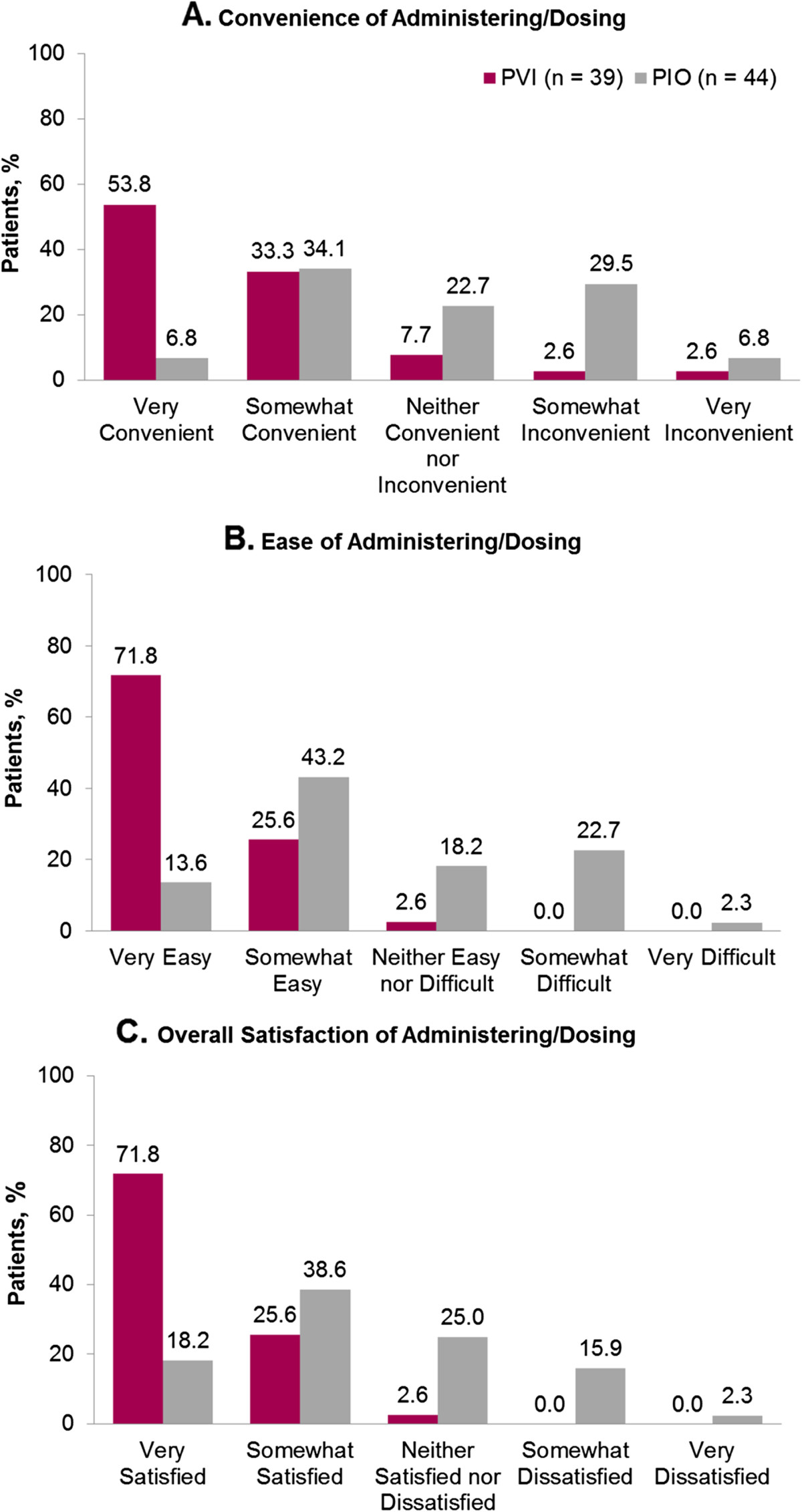
Convenience, ease, and overall patient satisfaction of administering/dosing progesterone vaginal insert (PVI) and progesterone in oil (PIO).
Pregnancy rates
There were no significant differences in the biochemical, clinical, and ongoing pregnancy rates for those patients in the PVI group compared with those rates for patients in the PIO group (see Table 2). Biochemical pregnancy rates were 60.4% and 64.9% for the PVI and PIO groups, respectively (P = 0.853). Clinical pregnancies occurred in 50.9% in both the PVI and PIO groups (P = 1.0). The ongoing pregnancy rates were 47.2% and 49.1% for the PVI and PIO groups, respectively (P = 0.851).
Adverse events
One or more AEs were reported during progesterone treatment by 30.2% in the PVI group and 31.6% in the PIO group. Most AEs were of mild or moderate intensity in both groups. Abdominal distension, lower abdominal pain, nausea, and ovarian hyperstimulation syndrome (OHSS) were the most common AEs reported in both groups.
Discussion
The IM and vaginal routes of administration are used almost exclusively in clinical practice when progesterone is given to patients for luteal phase support during ART [4, 6]. Reports indicate that the vaginal and IM administration of progesterone results in comparable pregnancy outcomes [6, 13, 15].
Moreover, this analysis provides a comprehensive, prospective comparison of patient-reported convenience and ease of use, overall patient satisfaction, and tolerability for PVI compared to IM PIO from a population of women undergoing cycles of IVF. The most distinguishing difference between PVI and PIO in this study was the overall patient preference for PVI. Despite the need to administer PVI either twice a day or 3 times a day, 82.6% of the patients in the PVI group found it “very” or “somewhat convenient” compared with 44.9% of women in the PIO group. Furthermore, 90% of those in the PVI group found this treatment to be “very” or “somewhat easier” to use than a previous progesterone treatment. This is in contrast to patient response in the PIO group, where 33.3% found PIO “very” or “somewhat easier” to use than a previous progesterone treatment.
Only a few studies with progesterone for luteal phase support during cycles of IVF have attempted to assess patient treatment satisfaction and ease of use. In two previous studies comparing administration of progesterone vaginal gel to IM PIO, patients reported better satisfaction with vaginal gel when compared to IM PIO [15, 18]. In a study by Schoolcraft et al., patients found the vaginal gel easier to use, less painful, and less time consuming to administer when compared to treatment with IM PIO during a previous cycle of IVF [19]. Data on patient satisfaction were obtained prospectively in these studies; however, the data collected were more limited in scope than the data collected in the current study. Results from this survey of patient satisfaction, convenience, ease of use, and tolerability provide a more comprehensive assessment of patient-reported satisfaction than analysis included in previous studies.
No significant difference in biochemical, clinical, or ongoing pregnancy rates were observed among the treatment groups. Pregnancy outcomes in this study were similar to those reported in previous studies comparing vaginal to IM administration of progesterone for luteal support in women of a similar age range [6–11, 14, 15].
Progesterone administered vaginally for luteal phase support has been shown to be well tolerated. The most common treatment-emergent AEs associated with PVI include nausea, abdominal pain, OHSS, and oocyte retrieval pain [22]. Breast tenderness, headache, abdominal pain, abdominal distension, nonspecific muscle spasms, and vaginal discharge have been associated with vaginal gel [23].
It can be difficult to clearly elucidate the true cause-effect relationship of AEs reported during a clinical study. Nausea, OHSS, abdominal distention, and abdominal pain reported most frequently during this study by both PVI and PIO users were also commonly reported AEs when medications given for ovarian stimulation and IVF were used [21, 22, 24, 25]. Injection site irritation and redness have been reported frequently with administration of IM PIO [18, 26].
The results of this comprehensive, prospective patient survey and other similar reports suggest that PVI provides an easy-to-use and convenient method for providing the necessary luteal phase support for IVF cycles without the pain and inconvenience of daily IM injections and with comparable pregnancy rates.
Acknowledgements
This study was supported by Ferring Pharmaceuticals, Inc, Parsippany, NJ, USA. The authors wish to thank Ferring Pharmaceuticals, Inc, for support of this manuscript. We also thank Jill S McCollam, PharmD, of The JB Ashtin Group, Inc., who, on the behalf of Ferring, developed the first draft based on an author-approved outline and assisted in implementing author revisions.
Funding/support
Financial support for this manuscript was provided by Ferring Pharmaceuticals, Inc.
Abbreviations
- AE
Adverse event
- ART
Assisted reproductive technology
- BMI
Body mass index
- E2
Serum estradiol
- FSH
Follicle-stimulating hormone
- GnRH
Gonadotropin-releasing hormone
- GnRHa
Gonadotropin-releasing hormone agonist
- hCG
Human chorionic gonadotropin
- HP-hMG
Highly purified human menopausal gonadotropin
- IM
Intramuscular
- IVF
In vitro fertilization IVF
- OHSS
Ovarian hyperstimulation syndrome
- PCOS
Polycystic ovarian syndrome
- PIO
Progesterone in oil
- PVI
Progesterone vaginal insert
- rFSH
Recombinant FSH
- SC
Subcutaneously
- TVU
Transvaginal ultrasonography.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Footnotes
Competing interests
AB is the executive chairperson of the Midwest Reproductive Symposium and has served on speakers’ bureaus for Ferring Pharmaceuticals, Inc; EMD Serono, Inc; Merck & Co, Inc; and TherapeuticsMD, Inc. MDS has no competing interests. KD is an advisor to Ferring Pharmaceuticals, Inc; Merck & Co, Inc; and Good Start Genetics, Inc, and has served on speakers’ bureaus for Ferring Pharmaceuticals, Inc. MRB has participated in speakers’ bureaus and/or acted as a consultant for Ferring Pharmaceuticals, Inc. ADD participated in the speakers’ bureau for Merck and is a grant recipient of Merck; in addition, ADD acts as a consultant to Johnson and Johnson and Ovascience. MGC is a full-time employee of Ferring Pharmaceuticals, Inc.
Authors’ contributions
ADD contributed to the conception and design of the study. AB, MDS, KD, MRB, and MGC contributed to the conception and design of the study and participated in the collection of data. AB, MDS, KD, MRB, and MGC interpreted the data and participated in revision of the manuscript. AB, MDS, KD, MRB, ADD and MGC read and gave final approval for the version submitted for publication.
Contributor Information
Angeline N Beltsos, Email: angeline.beltsos@integramed.com.
Mark D Sanchez, Email: obdoc@hotmail.com.
Kevin J Doody, Email: kevind@embryo.net.
Mark R Bush, Email: MarkB@conceptionsrepro.com.
Alice D Domar, Email: domar@domarcenter.com.
Michael G Collins, Email: michael.collins@ferring.com.
References
- 1.Check JH. Luteal phase support in assisted reproductive technology treatment: Focus on endometrin(r) (progesterone) vaginal insert. Ther Clin Risk Manag. 2009;5:403–407. doi: 10.2147/TCRM.S4192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.DiLuigi AJ, Nulsen JC. Effects of gonadotropin-releasing hormone agonists and antagonists on luteal function. Curr Opin in Obstet ynecol. 2007;19:258–265. doi: 10.1097/GCO.0b013e3281338874. [DOI] [PubMed] [Google Scholar]
- 3.ASRM Practice Bulletin Progesterone supplementation during the luteal phase and in early pregnancy in the treatment of infertility: an educational bulletin. Fertil Steril. 2008;89:789–792. doi: 10.1016/j.fertnstert.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 4.Fatemi HM, Popovic-Todorovic B, Papanikolaou E, Donoso P, Devroey P. An update of luteal phase support in stimulated IVF cycles. Hum Reprod Update. 2007;13:581–590. doi: 10.1093/humupd/dmm021. [DOI] [PubMed] [Google Scholar]
- 5.Penzias AS, Alper MM. Luteal support with vaginal micronized progesterone gel in assisted reproduction. Reprod Biomed Online. 2003;6:287–295. doi: 10.1016/S1472-6483(10)61847-0. [DOI] [PubMed] [Google Scholar]
- 6.Zarutskie PW, Phillips JA. A meta-analysis of the route of administration of luteal phase support in assisted reproductive technology: vaginal versus intramuscular progesterone. Fert Steril. 2009;92:163–169. doi: 10.1016/j.fertnstert.2009.02.018. [DOI] [PubMed] [Google Scholar]
- 7.Chantilis SJ, Zeitoun KM, Patel SI, Johns DA, Madziar VA, McIntire DD. Use of crinone vaginal progesterone gel for luteal support in in vitro fertilization cycles. Fertil Steril. 1999;72:823–829. doi: 10.1016/S0015-0282(99)00362-3. [DOI] [PubMed] [Google Scholar]
- 8.Dal Prato L, Bianchi L, Cattoli M, Tarozzi N, Flamigni C, Borini A. Vaginal gel versus intramuscular progesterone for luteal phase supplementation: a prospective randomized trial. Reprod Biomed Online. 2008;16:361–367. doi: 10.1016/S1472-6483(10)60597-4. [DOI] [PubMed] [Google Scholar]
- 9.Jobanputra K, Toner JP, Denoncourt R, Gibbons WE. Crinone 8% (90 mg) given once daily for progesterone replacement therapy in donor egg cycles. Fertil Steril. 1999;72:980–984. doi: 10.1016/S0015-0282(99)00390-8. [DOI] [PubMed] [Google Scholar]
- 10.Kahraman S, Karagozoglu SH, Karlikaya G. The efficiency of progesterone vaginal gel versus intramuscular progesterone for luteal phase supplementation in gonadotropin-releasing hormone antagonist cycles: a prospective clinical trial. Fertil Steril. 2010;94:761–763. doi: 10.1016/j.fertnstert.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 11.Khan N, Richter KS, Newsome TL, Blake EJ, Yankov VI. Matched-samples comparison of intramuscular versus vaginal progesterone for luteal phase support after in vitro fertilization and embryo transfer. Fertil Steril. 2009;91:2445–2450. doi: 10.1016/j.fertnstert.2008.03.072. [DOI] [PubMed] [Google Scholar]
- 12.Martin-Johnson M, Beltsos A, Robinson A, Gress N, Elgar C, Byers M. Luteal phase support with endometrin vs. Progesterone in oil in in vitro fertilization cycles. Fertil Steril. 2008;90(Suppl):S459. doi: 10.1016/j.fertnstert.2008.07.457. [DOI] [Google Scholar]
- 13.Mitwally MF, Diamond MP, Abuzeid M. Vaginal micronized progesterone versus intramuscular progesterone for luteal support in women undergoing in vitro fertilization-embryo transfer. Fertil Steril. 2010;93:554–569. doi: 10.1016/j.fertnstert.2009.02.047. [DOI] [PubMed] [Google Scholar]
- 14.Silverberg KM, Vaughn TC, Hansard LJ, Burger NZ, Minter T. Vaginal (crinone 8%) gel vs. Intramuscular progesterone in oil for luteal phase support in in vitro fertilization: a large prospective trial. Fertil Steril. 2012;97:344–348. doi: 10.1016/j.fertnstert.2011.11.018. [DOI] [PubMed] [Google Scholar]
- 15.Yanushpolsky E, Hurwitz S, Greenberg L, Racowsky C, Hornstein M. Crinone vaginal gel is equally effective and better tolerated than intramuscular progesterone for luteal phase support in in vitro fertilization-embryo transfer cycles: a prospective randomized study. Fertil Steril. 2010;94:2596–2599. doi: 10.1016/j.fertnstert.2010.02.033. [DOI] [PubMed] [Google Scholar]
- 16.Beltsos AS, Sanchez M, Doody K, Bush M, Scobey J. Efficacy of vaginal progesterone inserts (Endometrin) compared to intramuscular progesterone in oil (PIO) for luteal phase support in PCOS patients. Fertil Steril. 2011;96(Suppl):S130. doi: 10.1016/j.fertnstert.2011.07.505. [DOI] [Google Scholar]
- 17.Feinberg EC, Beltsos AN, Nicolaou E, Marut EL, Uhler ML. Endometrin as luteal phase support in assisted reproduction. Fertil Steril. 2013;99:174–178. doi: 10.1016/j.fertnstert.2012.09.019. [DOI] [PubMed] [Google Scholar]
- 18.Propst AM, Hill JA, Ginsburg ES, Hurwitz S, Politch J, Yanushpolsky EH. A randomized study comparing crinone 8% and intramuscular progesterone supplementation in in vitro fertilization-embryo transfer cycles. Fertil Steril. 2001;76:1144–1149. doi: 10.1016/S0015-0282(01)02872-2. [DOI] [PubMed] [Google Scholar]
- 19.Schoolcraft WB, Hesla JS, Gee MJ. Experience with progesterone gel for luteal support in a highly successful IVF programme. Hum Reprod. 2000;15:1284–1288. doi: 10.1093/humrep/15.6.1284. [DOI] [PubMed] [Google Scholar]
- 20.Rotterdam EA-SPCWG. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS) Hum Reprod. 2004;19:41–47. doi: 10.1093/humrep/deh098. [DOI] [PubMed] [Google Scholar]
- 21.Doody KJ, Schnell VL, Foulk RA, Miller CE, Kolb BA, Blake EJ, Yankov VI. Endometrin for luteal phase support in a randomized, controlled, open-label, prospective in-vitro fertilization trial using a combination of menopur and bravelle for controlled ovarian hyperstimulation. Fertil Steril. 2009;91:1012–1017. doi: 10.1016/j.fertnstert.2008.01.069. [DOI] [PubMed] [Google Scholar]
- 22.Ferring Pharmaceuticals, Inc . Endometrin. Parsippany, NJ, USA: Ferring Pharmaceuticals, Inc; 2010. [Google Scholar]
- 23.Watson Pharmaceuticals, Inc . Crinone. Parsippany, NJ, USA: Watson Pharmaceuticals, Inc; 2011. [Google Scholar]
- 24.Merck & Co, Inc . Follistim. Kenilworth, NJ, USA: Merck & Co, Inc; 2012. [Google Scholar]
- 25.Ferring Pharmaceuticals, Inc . Menopur. Parsippany, NJ, USA: Ferring Pharmaceuticals, Inc; 2012. [Google Scholar]
- 26.American Regent, Inc . Progesterone. Shirley, NY, USA: American Regent, Inc; 2009. [Google Scholar]


