Abstract
Purpose
Bortezomib is an important agent in multiple myeloma treatment, but resistance in cell lines and patients has been described. The main mechanisms of resistance described in cancer fall into one of two categories, pharmacokinetic resistance (PK), e.g. over expression of drug efflux pumps and pharmacodynamic resistance, e.g. apoptosis resistance or altered survival pathways, where the agent reaches an appropriate concentration, but this fails to propagate an appropriate cell death response. Of the known pump mechanisms, P-glycoprotein (P-gp) is the best studied and considered to be the most important in contributing to general PK drug resistance. Resistance to bortezomib is multifactorial and there are conflicting indications that cellular overexpression of P-gp may contribute to resistance agent. Hence, better characterization of the interactions of this drug with classical resistance mechanisms should identify improved treatment applications.
Methods
Cell lines with different P-gp expression levels were used to determine the relationship between bortezomib and P-gp. Coculture system with stromal cells was used to determine the effect of the local microenvironment on the bortezomib–elacridar combination. To further assess P-gp function, intracellular accumulation of P-gp probe rhodamine-123 was utilised.
Results
In the present study, we show that bortezomib is a substrate for P-gp, but not for the other drug efflux transporters. Bortezomib activity is affected by P-gp expression and conversely, the expression of P-gp affect bortezomib’s ability to act as a P-gp substrate. The local microenvironment did not alter the cellular response to bortezomib. We also demonstrate that bortezomib directly affects the expression and function of P-gp.
Conclusions
Our findings strongly support a role for P-gp in bortezomib resistance and, therefore, suggest that combination of a P-gp inhibitor and bortezomib in P-gp positive myeloma would be a reasonable treatment combination to extend efficacy of this important drug.
Keywords: P-glycoprotein, MDR protein, Bortezomib, Elacridar, Co-culture
Introduction
Multiple myeloma (MM) is an incurable plasma cell disorder with a median age at diagnosis of 71 years. MM patients tend to initially respond to treatment but inevitably relapse with a median survival duration of 3–5 years [1]. In common with other malignancies, clinical drug resistance has always been a major treatment obstacle. Cancer resistance mechanisms can be categorized as pharmacokinetic resistance (PK), for example, over expression of drug efflux pumps or pharmacodynamic resistance, for example, apoptosis resistance or altered survival pathways, where the agent reaches an appropriate concentration, but this fails to propagate an appropriate cell death response [2]. The cell membrane is the major determinant of cancer drug penetration to sub-cellular targets. Cells have evolved complex chemical defence mechanisms to regulate the entry of foreign substances into and out of the cell. Of the known pump mechanisms, P-glycoprotein (P-gp; MDR-1; ABCB1), multidrug-resistant protein-1 (MRP-1; ABCC1) and breast cancer resistance protein (BCRP; MXR; ABCG2) have the broadest substrate specificity and a strong correlation with drug resistance in vitro and in vivo in many forms of cancer [3]. Of all these drug efflux transporters, P-gp is the best studied and considered to be the most important in contributing to general drug resistance.
The role of ABC transport proteins in drug-resistant cancers is still an active area of research. High expression of P-gp has been observed prior to chemotherapy treatment in many different tumour types, including kidney, colon, liver, breast and ovarian cancers. In haematological malignancies, such as leukaemias, lymphomas and MM, the low levels of P-gp expression observed initially are often markedly increased after chemotherapy treatment and relapse. Grogan et al. [4] have shown that previous treatments with anthracyclines and vinca alkaloids can induce expression of P-gp in MM patients. However, clinical trials that used a combination of vincristine, adriamycin and dexamethasone (VAD) with P-gp inhibitors such as cyclosporine [5], verapamil [6] or PS-833 [7] showed no clinical benefit in terms of increased overall survival or progression-free survival. The failure of these trials can be related to poor inhibition of P-gp function by the P-gp inhibitors; additionally, generalized inhibition of P-gp can reduce the elimination of cytotoxic agents and in some trials this necessitated dose reduction to compensate for increases in toxicity evident in the P-gp treated patients [7, 8].
Bortezomib, a proteasome inhibitor, is an effective treatment for MM. Resistance to bortezomib is multifactorial and while little is known about the interaction of bortezomib with P-gp, there are indications that overexpression of this pump may contribute to resistance to this agent. Rumpold et al. [9] showed that knockdown of P-gp resensitizes P-gp-expressing cells to proteasome inhibitors. Another strategy to overcome P-gp-induced resistance is to prevent P-gp from reaching the cell surface after synthesis in the endoplasmic reticulum. Proteosome inhibitors, lactacystin and MG-132, have been shown to inhibit the maturation of P-gp [10]. Bortezomib may be able to do the same if this is a class effect. Hence, better characterization of the interactions of this drug with classical resistance mechanisms should identify improved treatment applications. In the present study, we characterize the interaction of bortezomib with multidrug transporters; P-gp, BCRP and MRP1 and explore the potential for this interaction to play a role in resistance. We show that bortezomib is a substrate for P-gp but not for the other drug efflux transporters and that bortezomib is not a P-gp inhibitor. We also demonstrate that bortezomib directly affects the expression and function of P-gp.
Materials and methods
Cell lines
We employed a panel of human cell lines that overexpress MDR proteins. We specifically evaluated the squamous lung carcinoma cell line DLKP, which has some overexpression of MRP-1 [11], and its isogenic lines DLKP-A, which highly overexpresses P-gp [12] and DLKP-SQ-Mitox, which highly overexpresses BCRP [13]; the non-small cell lung cancer cell line A549 and its isogenic line A549-taxol which has a limited amount of P-gp overexpression [14], the MM cell line RPMI8226 and its subline RPMI-Dox40 which highly overexpresses P-gp [15]; the ovarian carcinoma cell line NCI/Adr-res which highly overexpresses P-gp [16]; as well as the human immortalized bone marrow stromal cell (BMSC) line, HS-5. MM cell lines were grown in Roswell Park Memorial Institute (RPMI)-1640 medium (Cellgro, Mediatech, Manassas, VA, USA) with 100 U/mL penicillin, 100 μg/mL streptomycin and 10 % foetal calf serum (FCS) (GIBCO/BRL, Gaithersburg, MD, USA). Non-MM cell lines were grown in Dulbecco’s Modified Eagle Medium (DMEM) (Cellgro, Mediatech, Manassas, VA, USA) with 100 U/mL penicillin, 100 μg/mL streptomycin and 10 % FCS (GIBCO/BRL, Gaithersburg, MD, USA). All cell lines used in experiments were at low passage number, ranging from 3 to 10, post-thawing of stocks. The MM cell lines were semi-adherent and subcultured by removing from the flask with a cell scraper, whereas the non-MM cell lines were adherent and required trypsin for subculturing. All cell lines were fed with medium approximately every 2–3 days (depending on their growth stage) and cultured to approximately 70–80 % confluence prior to splitting and application in the cell-based assays employed, and cells were never allowed to reach confluency.
Reagents
Elacridar was purchased from Sequoia Research (Pangbourne, UK). Doxorubicin and verapamil were purchased from Sigma-Aldrich (St. Louis, MO, USA). Bortezomib was kindly provided by Millennium Pharmaceuticals (Cambridge, MA, USA). The primary antibodies used for immunoblotting were purchased from Abcam (GADPH-Hrp conjugate; cat. ab9482) or Alexis Biochemicals, Plymouth Meeting, PA, USA (P-gp; cat. 801-002). The secondary horseradish peroxidase (HRP)-labelled mouse antibody was purchased from Jackson ImmunoResearch (West Grove, PA, USA).
Cell viability assessment
Cell lines were plated in 96-well plates at a density of 1,000–2,000 cells/well. Adherent cells were allowed to attach overnight before compound(s) indicated were added at the concentrations indicated and compared to vehicle treated controls. Cultures were then incubated for 5 days in a 37 °C incubator with 5 % CO2. Cell viability was measured using an acid phosphatase assay as previously described [17].
Stromal cell co-culture and measurement of cell viability using the compartment-specific bioluminescence imaging (CS-BLI)
For co-culture experiments, RPMI-Dox40 cells stably expressing a luciferase (luc) vector were cultured in the presence or absence of the BMSC line HS-5 in 96-well optical bottom tissue culture plates (Nunc). This co-culture method has been previously described, robustly validated and employed in several studies originating from our laboratories [18–20]. Previous research has shown that the HS-5 immortalized stromal line possesses characteristics compatible with normal bone marrow stromal cells [21]. The luc-expressing cell line RPMI-Dox40-MCherry/luc was generated by retroviral transduction with the pFUW–Luc–Ch–puro vector. Briefly, HS-5 stromal cells were plated in 96-well plates and allowed to attach overnight. Tumour cells were then plated and treated with bortezomib, with or without elacridar for 5 days in a 37 °C incubator with 5 % CO2 at the indicated concentrations. At the end of the incubation period, luciferin substrate (Xenogen Corp.) was then added to the culture, and the resulting bioluminescence signal was measured using a Luminos-kanluminometer (Labsystems).
Cellvue dye staining
Flow cytometry-based evaluation of MM cell subsets in the presence of BMSCs was performed by labelling MM cells with the CellVue® cell linker kit (Polysciences, Warrington, PA, USA). 1 × 106/mL of cells were stained at final concentrations of 2 μM CellVue® dye in a final staining volume of 2 mL. The cells were incubated for 2–5 min with periodic mixing. After incubation, an equal volume (2 mL) of serum was added to the sample followed by 1 min incubation. Cells were then washed three times and centrifuged at 400×g for 10 min at room temperature before resuspension in complete medium to ensure removal of unbound dye. BMSCs were plated and allowed to attach overnight before the addition of MM cells. Labelled cells were then cultured in the presence or absence of stromal cells. After incubation period, MM cells stained with CellVue dye were analysed on FACS Canto II (Becton–Dickinson, CA, USA) and data analysed using FlowJo software.
Functional drug accumulation assay using flow cytometry
The P-gp functional activity was determined by Rhodamine 123 (Rh-123) (Sigma) efflux, as this fluorescent dye is a substrate for P-gp. 1 × 105 RPMI-Dox40 cells were seeded in 6-well plates and treated with bortezomib at the concentrations indicated for 72 h. The cells were then pelleted and incubated with 200 ng/mL of Rh-123 dye in the presence or absence of the P-gp inhibitor, verapamil (Sigma) at a concentration of 10 μM for 30 min at 37 °C in a humidified atmosphere of air and 5 % CO2. After washing, cells were incubated in a Rh-123-free medium supplemented with 10 % FCS, in the presence or absence of verapamil and aliquots were removed for analysis at 30, 60 and 120 min, respectively. Prior to analysis, cells were washed and incubated with 7AAD antibody (BD), to exclude non-viable cells, in 0.2 % BSA/PBS for 5 min at room temperature. Data acquisition and analysis were performed using a FACS Canto II (Becton–Dickinson) equipped with a 488-nm argon laser and data analysed using FlowJo software. Only 7AAD-negative, that is, viable cells were included in the analysis. The results were reported as the mean of the median Rh-123 fluorescence intensity relative to control at each time point. To investigate dye efflux in RPMI-Dox40 cells when cocultured with stroma cells, this cell subset was prelabelled with Cellvue dye as described above to distinguish it from stroma cells.
Immunoblotting analysis
For immunoblotting analyses, cells (1 × 107 cells per condition) were plated in RPMI-1640 medium with 10 % FCS, penicillin and streptomycin as previously described. Bortezomib 4 nM was added for 0–72 h. Cell pellets were collected and treated with Triton X-100 lysis buffer containing 1 X PBS, Triton X-100 (1 % v/v), sodium deoxycholate (0.5 % w/v), SDS (0.1 %w/v), EDTA (1 mmol/L), 1 mmol/L phenylmethylsulfonyl fluoride, 1 mmol/L sodium fluoride, 1 mmol/L sodium orthovanadate, 1 μg/mL aprotinin, 5 μg/mL leupeptin and 5 μg/mL pepstatin A. The samples were cleared by centrifugation (16,000×g, 30 min, 4 °C) and assessed for protein concentration by Bradford assay (Sigma). SDS–polyacrylamide gel electrophoresis (4–12 %) was performed (20 μg of protein per lane) using a prestained SDS-PAGE MWmarker (Biorad, cat. 161-0309), and proteins were electroblotted onto PVDF membranes using semi-dry transfer technique at 0.4amps for 1 h. After 1 h incubation in blocking solution [5 % milk in TBS-Tween20 buffer (TBS-T)], membranes were exposed to primary antibody overnight at 4 °C (P-gp antibody (170 kDa) was used at a dilution of 1:250 and, GAPDH-Hrp conjugate (40 kDa) was used at a dilution of 1:10,000). Following 3 washes in TBS-T, a mouse secondary HRP-labelled antibody was added at 1:10,000 dilutions for 1 h at room temperature to P-gp-incubated membranes. The membrane was then washed with TBS-T for 15 min with 5–7 changes of the wash buffer, and the protein expression was visualized using chemiluminescence and developed on film (Kodak Scientific Imaging). Densitometry readings were measured using Image J software (National Institutes of Health, USA). In brief, a common box size was used and the densitometric readings of the target proteins were divided by their corresponding GAPDH controls.
Statistical analysis
In the cell viability assays, each experimental point was set up in triplicate wells and each assay was repeated identically and independently at least once. The final data were expressed as a percentage of the proliferation that took place in control wells where cells were not exposed to any drugs. IC50 values were determined for each experiment using the regression model in the Graphpad Prism software (CA, USA). To evaluate the differences across various experimental conditions, a Student’s t test was employed. In all analyses, P < 0.05 was considered statistically significant and P < 0.001 highly statistically significant.
The additive, synergistic or antagonistic nature of the interaction between two drug combinations was evaluated using the combination index (CIN) method of Chou and Talalay [22, 23]. Calcusyn software (version 1.1, Biosoft, Cambridge, UK), which is based on this method and takes into account both potency [median dose (Dm) or IC50] and the shape of the dose–effect curve (the m value), was used to calculate the CIN. CIN values were interpreted as follows: antagonistic effect when CIN > 1.1, additive effect when CIN = 0.9–1.1, slight synergism when CIN = 0.7–0.9, synergism when CIN = 0.3–0.7, strong synergism when CIN = 0.1–0.3 and very strong synergism when CIN < 0.1.
Results
Bortezomib acts as a substrate and a weak inhibitor of P-glycoprotein: bortezomib is also not a substrate or inhibitor of other drug efflux pumps
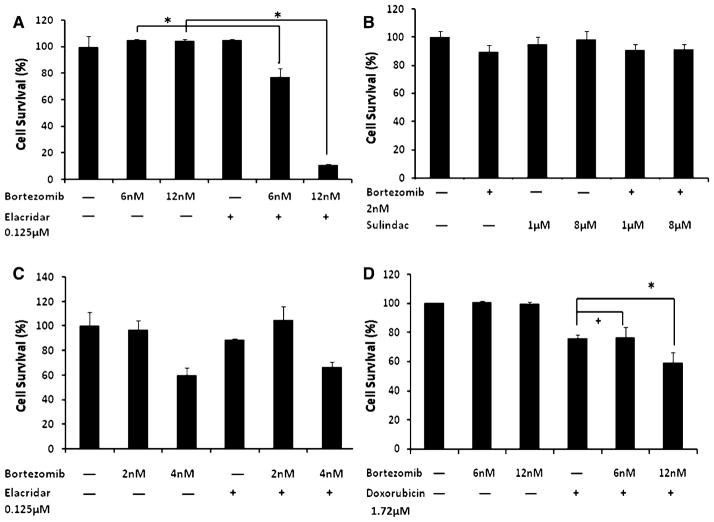
To study the effects of bortezomib on MDR transporter function, we used several isogenic lung cancer cell lines that had been developed by our institute; DLKP which overexpresses MRP-1; DLKP-A which only overexpresses P-gp; and DLKP-SQ/Mitox which only overexpresses BCRP. Figure 1a demonstrated that bortezomib was likely a substrate of P-gp by showing synergistic cytotoxicity in the presence of a dual P-gp/BCRP inhibitor, elacridar, in DLKP-A cells (P-gp overexpressing). In contrast, inhibition of the other 2 drug efflux pumps, MRP-1 (with sulindac sulphide) and BCRP (with the P-gp/BCRP inhibitorelacridar), had no synergistic activity with bortezomib in DLKP cells (Fig. 1b) or DLKP-SQ/Mitox (BCRP overexpressing) cells, respectively (Fig. 1c).
Fig. 1.
Bortezomib acts as a P-glycoprotein substrate and a weak inhibitor. Bortezomib is also not a substrate or inhibitor of other drug efflux pumps. Using isogenic lung cancer cell lines, DLKP which overexpresses MRP-1; DLKP-A which overexpresses P-gp; and DLKP-SQ/Mitox which overexpresses BCRP, we were able to demonstrate that bortezomib was a substrate of P-gp by showing synergistic cytotoxicity in the presence of a P-gp inhibitor, elacridar in DLKP-A cells. a Bortezomib had no synergistic activity when combined with sulindac sulphide, a MRP-1 inhibitor in DLKP cells (b) and elacridar, a BCRP inhibitor in DLKP-SQ/Mitox (c). In DLKP-A cells, when tested in the presence of doxorubicin, a known P-gp substrate, bortezomib was shown to be an ineffective P-gp inhibitor as enhanced cytotoxicity was only demonstrated at the highest dose of bortezomib. All experiments were incubated at the doses indicated for 5 days. Cell survival assessed by acid phosphatase assay was expressed as percentage (mean ± SD) compared to vehicle treated control. +Statistically not significant; *statistically significantly compared to control (P < 0.05)
As bortezomib appeared to be a P-gp substrate, we wanted to examine whether it had any inhibitory actions on P-gp. Using the same P-gp-overexpressing cell line DLKP-A, we observed that bortezomib was only a weak P-gp inhibitor since synergistic cytotoxicity in the presence of a known P-gp substrate, doxorubicin, was demonstrated only at the very highest dose of bortezomib, 12 nM; see Fig. 1d. To determine whether bortezomib had any inhibitory effect on BCRP, we used DLKP-SQ/Mitox cells (BCRP over expressing) and synergistic cytotoxicity was not seen, therefore, bortezomib was not a BCRP inhibitor (Supp Fig 1).
Bortezomib activity is affected by P-glycoprotein expression
To investigate whether the activity of bortezomib was affected by P-gp expression, we tested its single-agent cytotoxicity in two cell lines that overexpress P-gp, DLKP-A, which expresses a high level of P-gp, and A549-taxol, which expresses a much lower level of the transporter. The activity of bortezomib was reduced in the DLKP-A cell line compared to DLKP (Supp Fig. 2A), whereas there was no reduction in activity in A549-taxol cell line compared to its parental cell line (Supp Fig. 2B). This data suggest that the different responses to bortezomib for these cell lines may be related, at least in part, to differences in P-gp expression.
The activity of bortezomib is influenced by the expression levels of P-glycoprotein
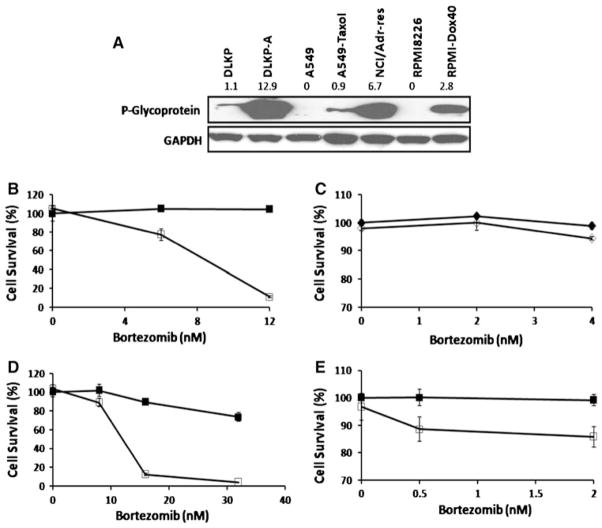
P-gp expression was analysed in the panel of cell lines by Western blot (Fig. 2a). Consistent with previous results in our laboratories, the highest level of P-gp was seen in DLKP-A and NCI-Adr/res followed by RPMI-Dox40 and with the least amount of P-gp expressed by A549-taxol.
Fig. 2.
The effectiveness of bortezomib to act as a P-glycoprotein substrate is dependent on the expression level of the transporter. Using immunoblot analysis, we were able to determine the baseline P-gp expression level of different cell lines that are known to overexpress P-gp with the parental lines as a negative control (a). The densitometry readings are shown on top of the relevant cell lines. This image was representative of findings from three experiments. The cell lines expression level of P-gp corresponded to the degree of synergy seen when bortezomib was combined with elacridar. The cell lines that exhibit the highest levels of P-gp showed the most synergy when bortezomib is combined with elacridar for 5 days. DLKP-A (b) treated with Bortezomib (filled square without elacridar, open square with elacridar 0.125 μM). A549-taxol (c) treated with bortezomib (filled diamond without elacridar, open diamond with elacridar 0.4 μM). NCI/Adr-res (d) and RPMI-Dox40 (e) treated with bortezomib (filled square without elacridar, open square with elacridar 0.125 μM). All experiments were incubated at the doses indicated for 5 days. Cell survival was expressed as percentage (mean ± SD) compared to vehicle treated control
The expression level of P-gp in each cell line corresponded to the degree of synergy seen when bortezomib was combined with elacridar, with cell lines that exhibited the highest levels of P-gp showing the most synergy. Thus, DLKP-A and NCI-Adr/res had the most synergy when bortezomib was combined with elacridar, followed by RPMI-Dox40 and the least amount of synergy was seen with A549-taxol cells (Fig. 2b–e). A representative combination dose for each cell line was calculated using Calcusyn software. The CIN values reflected that the cell lines with high P-gp expression had a higher degree of synergy with the combination (Supp Table 1).
Bortezomib and elacridar combination overcomes the stromal-derived protection of MM cells
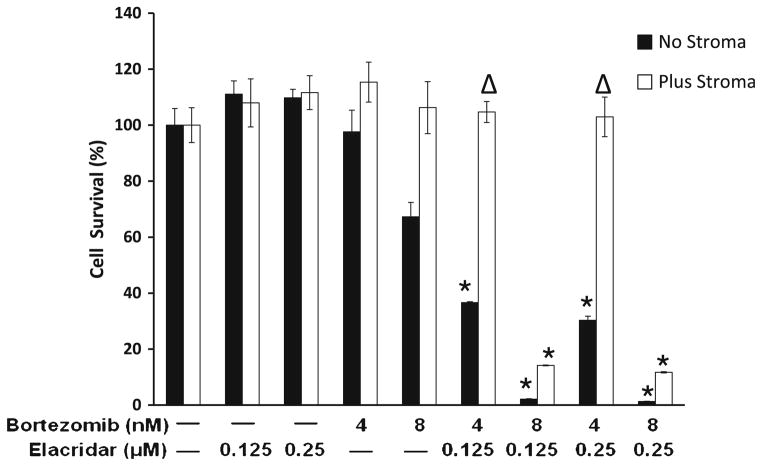
The MM cell line RPMI-Dox40-MCherry/luc was cultured in the presence of HS-5 stromal cells for 5 days, which triggered a 150–180 % increase in cell proliferation (data not shown). Under these same conditions, MM cells were treated with bortezomib and elacridar combination and cell viability using the CS-BLI approach was measured at 5 days. Cell viability was normalized to each respective drug-free control, and synergistic cytotoxicity was evident at all combination doses in the absence of stroma as indicated by a P value of <0.05 for bortezomib 4 nM and <0.001 for bortezomib 8 nM, although the co-culture with HS-5 cells tended to decrease the synergy (Fig. 3).
Fig. 3.
Synergy between bortezomib and elacridar persists in the protective effect of the BM microenvironment. Using the compartment- specific bioluminescence imaging (CS-BLI) approach, we evaluated the combination of bortezomib and elacridar on MM RPMI-Dox40-MCherry/luc cells cultured in the presence versus absence of HS-5 stromal cells and treated with bortezomib 4 and 8 nM and elacridar 0.125, 0.25 μM for 5 days. RPMI-Dox40-MCherry/luc viability was expressed as percentage (mean ± SD) compared to vehicle treated control. Experiment was repeated in triplicate. Synergistic cytotoxicity of the combination of bortezomib and elacridar persisted despite coculture with BMSCs (Δp < 0.05; *p < 0.001)
Bortezomib affects P-glycoprotein expression and function
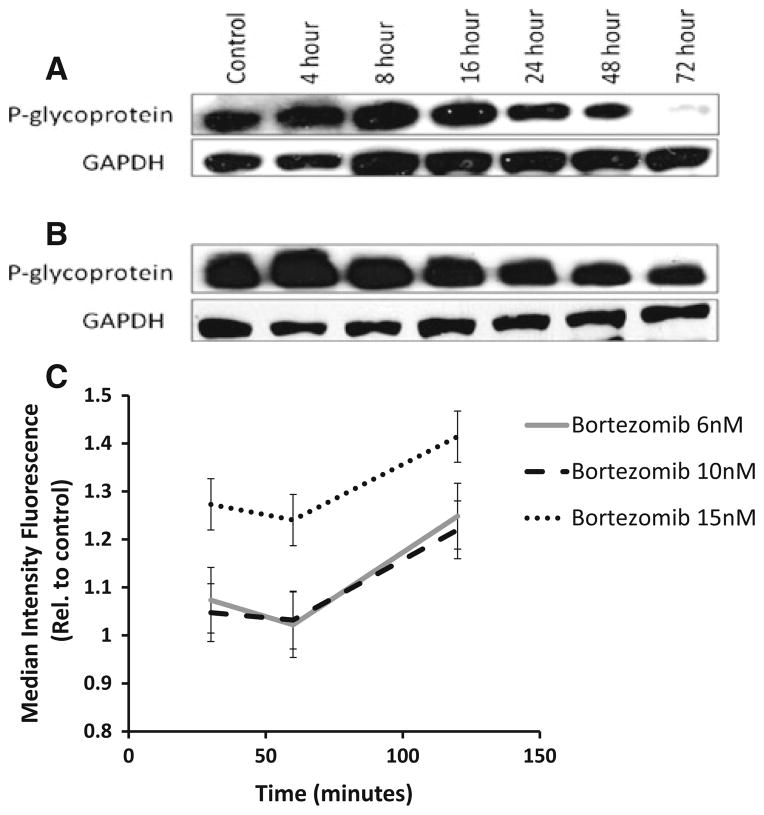
We next investigated the direct effect of bortezomib on P-gp expression. RPMI-Dox40 cells and DLKP-A cells with a modest and high expression of P-gp, respectively, were treated with 4 and 16 nM of bortezomib for 0–72 h. P-gp expression was analysed by Western blot and demonstrated a reduction in P-gp levels with bortezomib treatment by 24 h for both cell lines (Fig. 4a, b).
Fig. 4.
Bortezomib is able to reduce the expression and function of P-glycoprotein. When RPMI-Dox40 (a) and DLKP-A (b) cells were treated with 4 and 16 nM bortezomib, respectively, immunoblot analysis demonstrated a reduction in the level of P-gp expression by 24 h. These images were representative of three experiments. GAPDH was employed as a loading control. Bortezomib treatment inhibited rhodamine-123 efflux in RPMI-Dox40 cells with maximum inhibition at 120 min for all doses tested (c). The experiment was performed in triplicate, and results were expressed relative to control at each time point (mean of the median intensity of rhodamine-123 fluorescence ± SEM)
To determine whether the P-gp function was similarly inhibited, we performed a rhodamine-123 (Rh-123) efflux assay using flow cytometry. The results were expressed as the mean of the median Rh-123 fluorescence intensity as shown in Fig. 4c. RPMI-Dox40 cells treated with bortezomib demonstrated a reduction in Rh-123 efflux indicating a reduction in P-gp function at all doses tested with maximal inhibition of Rh-123 efflux at 120 min.
P-gp expression and function is affected by the local microenvironment
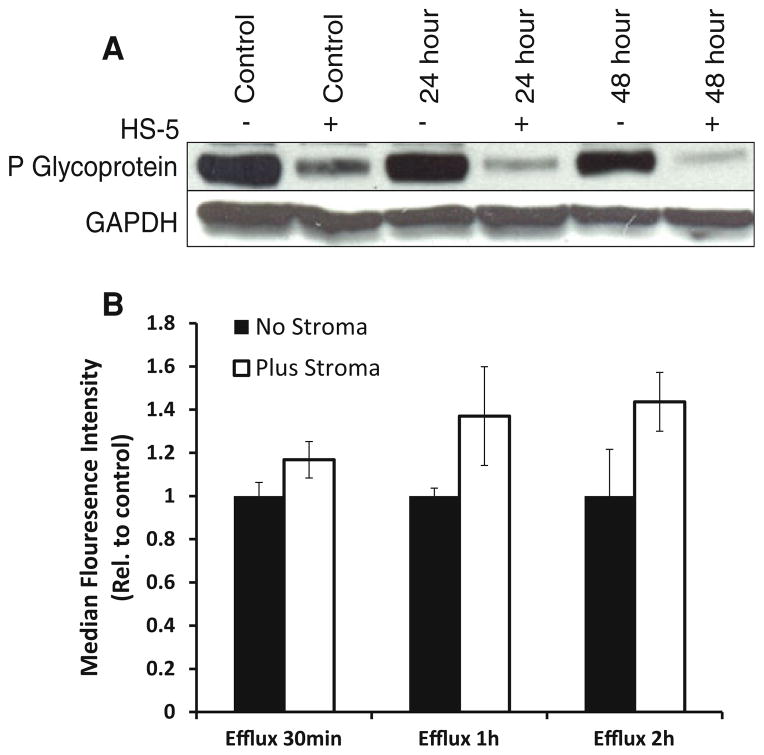
As the BM microenvironment confers protection to MM cells [24], we hypothesized that P-gp expression might be upregulated when MM cells are co-cultured with BMSCs. This is because doxorubicin is known to be less sensitive in the presence of BMSCs. RPMI-Dox40 cells were co-cultured with BMSCs for up to 48 h and cell lysate collected. P-gp expression was analysed by Western blot, unexpectedly, a reduction in P-gp levels were seen in the co-cultured MM cells, compared to MM cells alone, at each time point (Fig. 5a). Interestingly, this data suggest that the attenuation of doxorubicin toxicity when co-cultured with BMSCs was unrelated to P-gp expression in the MM cells.
Fig. 5.
MM cells co-cultured with stroma shows reduced expression and function of P-glycoprotein. RPMI-Dox40 cells were co-cultured with BMSCs HS-5. Immunoblot analysis demonstrated a reduction in P-gp expression in the MM cells that were co-cultured with BMSCs (a). GAPDH was employed as a loading control. Inhibition of rhodamine-123 efflux was demonstrated in RPMI-Dox40 cells when co-cultured with BMSCs (b). The experiment was performed in triplicate, and results were expressed relative to absence of BMSCs control at each time point (mean of the median intensity of rhodamine-123 fluorescence ± SEM)
To determine whether the P-gp-mediated efflux was similarly reduced in MM cells when co-cultured with BMSCs, a Rh-123 assay was performed. We used RPMI-Dox40 cells and co-cultured them with BMSCs HS-5. The RPMI-Dox40 cells were distinguished from the stromal cells by prelabelling with Cellvue claret dye. RPMI-Dox40 cells co-cultured with stroma demonstrated a reduction in Rh-123 efflux indicating a reduction in P-gp function at all doses tested with maximal inhibition of Rh-123 efflux at 2 h.
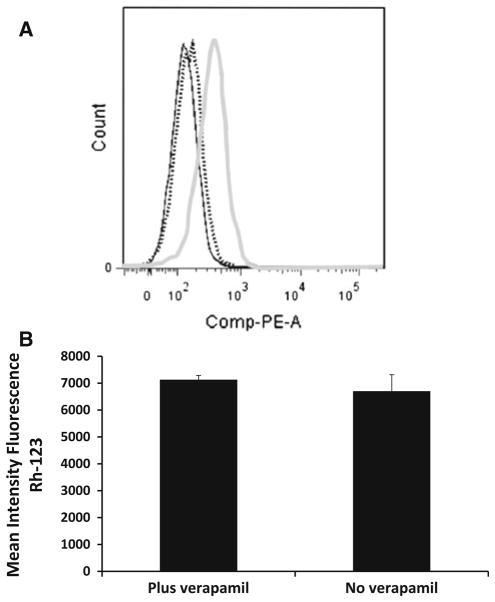
To further probe the relationship between P-gp and the BM microenvironment, we looked at the BMSCs fraction after co-culturing with P-gp-positive RPMI-Dox40 cells. We know that BMSCs do not express P-gp. Figure 6a demonstrated that the previously P-gp-negative BMSCs became P-gp-positive after co-culturing with RPMI-Dox40 for 72 h. However, this P-gp-positive status did not appear to be associated with functional P-gp activity as there was no extrusion of Rh-123 in the absence of verapamil (Fig. 6b).
Fig. 6.
Characterization of stromal cells co-cultured with MM cells. RPMI-Dox40 cells were co-cultured with BMSCs HS-5. Using flow cytometry, we demonstrated that the previously P-gp-negative stroma cells acquired P-gp expression after co-culturing with P-gp-positive RPMI-Dox40 cells; however, this P-gp-positive status was not functional. a RPMI-Dox40 cells prelabelled with CellVue claret were co-cultured with BMSCs HS-5 for 72 h. Analysing only the stroma cell population, there was an increase in the P-gp expression in the previously P-gp-negative stromal cells. Shown in black solid line BMSCs alone with a P-gp–PE antibody. The stroma fraction after co-culture with a IgG-PE (dotted line) and P-gp-PE (grey line) antibody. b RPMI-Dox40 cells prelabelled with CellVue claret were co-cultured with stroma for up to 72 h and Rh-123 assay was performed. The MM cell population was distinguished from the stromal cells, and only the stromal fraction was analysed. The P-gp-positive stromal fraction was not functional as evidenced by the lack of rhodamine 123 extrusion in the absence of verapamil. The experiment was performed in triplicate (average of the mean intensity of rhodamine-123 fluorescence ± SD)
Discussion
Bortezomib is central to the current treatment for MM. Resistance to this drug is multifactorial, and there is now emerging evidence that proteasome inhibitors may be a substrate or inhibitor of P-gp. However, studies in this field are conflicting. Some literature suggests that overexpression of this pump may contribute to resistance to bortezomib. Rumpold et al. [9] showed that knockdown of P-gp resensitizes P-gp-expressing cells to proteasome inhibitors. In Ewing’s sarcoma, P-gp-expressing clones that have shown resistance to substrates of P-gp (e.g. adriamycin, vincristine, etoposide and actinomycin D) have also shown a cross-resistance to bortezomib [25] and the authors proceeded to show that combination treatment with bortezomib and a P-gp or MRP-1 inhibitor reduced the resistance seen with single-agent bortezomib treatment suggesting that bortezomib is a P-gp substrate. A similar study by Iijima et al. [26] confirmed this finding. On the other hand, two other studies have concluded that bortezomib is not an MDR substrate on the basis that there is little cross-resistance to bortezomib in the cells that are known to be P-gp or MRP-1 overexpressors [27, 28]. Thus far, there is no conclusive evidence one way or the other about the interaction of bortezomib with MDR proteins. In our research, we wanted to examine this interaction of bortezomib with MDR proteins and explore its potential in playing a role in resistance. To establish whether bortezomib interacts with the MDR protein P-gp, we utilized cell lines that have differential expression of P-gp; ranging from low (A549-taxol) to high (DLKP-A).
The P-gp-overexpressing cell line, DLKP-A, was less sensitive to bortezomib than its parental non-P-gp overexpressing line, DLKP (suppl. Figure 2) and the combination of a potent P-gp inhibitor, elacridar, with bortezomib produced strong synergistic toxicity in the DLKP-A cell model. This suggests that P-gp can play an important role in bortezomib resistance. To see whether this synergistic cytotoxicity was maintained across different cancer types, we tested the bortezomib and elacridar combination in several different P-gp-resistant cancer cell lines including, DLKP-A (lung cancer) NCI-Adr/res (ovarian cancer) and RPMI-Dox40 (MM) cells, and confirmed that there was synergistic increase in cytotoxicity when elacridar and bortezomib were combined. These experiments demonstrate that bortezomib is a substrate of P-gp, and this resistance was greatly reduced when P-gp was inhibited by elacridar. This finding that bortezomib is a P-gp substrate confirms the results of some other P-gp-bortezomib studies [9, 25, 26].
In the literature, results using one breast cancer cell line study suggests that bortezomib is a P-gp inhibitor [29], whereas other studies in leukaemia-derived cell lines refuted that finding [30, 31]. Here, we used the P-gp-overexpressing cell line DLKP-A and concluded that bortezomib is a weak inhibitor, at best and only at supra pharmacological concentrations. Collectively, these reports suggest that findings with regard to interactions between bortezomib and anthracyclines might depend on the choice of drug combination used, the cell line studied and the method used to assess the drug interactions. Another reason that bortezomib synergizes with doxorubicin may be related to apoptotic mechanisms rather than P-gp. In the treatment of MM, Mitsiades et al. [32] investigated the mechanism of the chemosensitizing activity of bortezomib with oligonucleotide gene microarray analysis. This work demonstrated that bortezomib could downregulate the transcripts of several effectors of the protective cellular response to genotoxic stress and concluded that this was the reason for synergy between bortezomib and doxorubicin. In support of this, we found that bortezomib and doxorubicin synergize in a P-gp-negative cell line (data not shown) implying that factors other than P-gp status can result in bortezomib and doxorubicin synergy. As mentioned earlier, P-gp is not usually expressed in early disease, but there can be upregulation of this protein with exposure to anthracyclines and vincaalkaloids [4, 33]. In this study, we have shown that bortezomib is a good P-gp substrate and a weak inhibitor. The cross-resistance seen in P-gp-expressing cells to bortezomib may be overcome by combining a P-gp inhibitor such as elacridar with bortezomib. To investigate the in vivo relevance of these findings, a clinical trial of bortezomib with or without a P-gp inhibitor would be required.
MRP expression is not a common phenomenon in MM as it has only been detected in about 20 % of 88 myeloma samples [33]. However, MRP-1 is a known multidrug-resistant protein, and we wanted to investigate its interaction with bortezomib. We used the DLKP cell line which is known to have MRP-1 expression [11]. We combined bortezomib with sulindac sulphide, an MRP-1 inhibitor, and failed to yield any synergy. Even though MRP-1 is only expressed in a small minority of MM resistance, we found little evidence that MRP-1 resistance will affect bortezomib treatment. This was also seen in a leukaemia cell line where there was no cross-resistance to bortezomib [31]. BCRP is another broadly important multidrug resistance protein. In our study, treatment of DLKP-SQ/Mitox (which overexpresses BCRP) did not show any synergistic cytotoxicity when bortezomib was combined with elacridar (which also acts as a BCRP inhibitor). This is evidence that bortezomib is not a substrate for BCRP. SN38 is an irinotecan metabolite which is a known substrate for BCRP. Bortezomib was combined with SN38 and DLKP-SQ/Mitox cells were treated with this combination. There was no synergistic killing demonstrating that bortezomib is not a BCRP inhibitor. This result is in agreement with another study using leukaemia cells which also demonstrated that bortezomib is not a BCRP substrate [31].
We found that a cell line with a higher level of P-gp (DLKP-A) demonstrated attenuation of bortezomib activity compared to its parental cell line, whereas A549-taxol, which expresses a low P-gp level, had minimal attenuation of bortezomib activity. This suggested that P-gp expression levels play a part in bortezomib resistance. We then demonstrated that a cell’s P-gp expression correlates with how effective bortezomib is able function as a substrate for P-gp. DLKP-A, which expressed the highest level of P-gp, demonstrated the most synergy in the bortezomib and elacridar combination, whereas A549-taxol which had the lowest level of P-gp produced a mainly additive effect. This result suggests that the better synergy between bortezomib and elacridar is dependent upon P-gp concentration. We can therefore hypothesize that combining a P-gp inhibitor with bortezomib in patients who develop P-gp expression or an increase in P-gp expression should be able to overcome any P-gp-related resistance to bortezomib.
Previous studies have demonstrated that MDR1 activation occurs through NF-κB activation that requires a NF-κB-binding site located distal to the MDR1 promoter [34, 35]. Further publications have implicated NF-kB in upregulation of P-gp expression, which controls drug efflux in cancer cells [36]. Therefore, it is likely that increased P-gp expression participates in NF-kB-related cancer cell resistance to treatment. In this study, we demonstrated that P-gp expression levels in RPMI-Dox40 and DLKP-A cells were downregulated upon bortezomib treatment. These results were also seen by Fujita and colleagues [29], who demonstrated that proteasome inhibitors decreased the expression of MDR1 for both mRNA and protein levels in breast cancer cell line MCF7. This was also shown using MG132 by Zhang et al. [37] in gastric cancer. Using the Rh-123 assay, we have demonstrated that bortezomib treatment of RPMI-Dox40 cells caused a reduction in P-gp function by showing an accumulation of Rh-123 in bortezomib treated cells. Bentires-Alj et al. [38], demonstrated that inhibition of NF-kB through transfection of a plasmid coding for a mutated IkBα inhibitor increased daunomycin cell uptake and reduced MDR1 mRNA and P-gp expression in P-gp-overexpressing colon cancer cells. They showed a role for NF-kB in the regulation of the MDR1 gene expression in cancer cells and drug resistance. Therefore, inhibiting the NF-kB pathway with bortezomib will downregulate MDR1 activation and P-gp expression.
In the context of normal BM physiology, BMSCs are believed to function as an accessory cell population that supports the survival, cell division and differentiation of normal hematopoietic stem cells and progenitors [39]. When MM cells are co-cultured in the presence of BMSCs in vitro, the same phenomenon of cell proliferation is seen. MM cell adhesion to BMSCs triggers the NF-κB-dependent transcription and secretion of cytokines such as IL-6 in BMSCs, which further stimulate MM growth, survival and drug resistance [40]. Furthermore, MM cells secrete other cytokines such as TNF-α, TGFβ and VEGF which further upregulate IL-6 secretion from BMSCs [41–43]. Other than stimulation by growth factors, Jakubikova et al. [44] have shown that MM cell adherence to BMSCs increased the percentage, viability and proliferation potential of MM stem cells further increasing the MM cells in a co-culture.
Conventional chemotherapeutics such as dexamethasone and doxorubicin as well as newer pharmacological agents have been shown to be attenuated in the presence of BMSCs [24, 40, 45, 46]. The anti-apoptotic molecular pathways triggered in MM cells by the interaction with BMSCs are quite pleiotropic and several of them could, individually or in combination, lead to decreased response to doxorubicin. BMSCs have also been proposed to play a detoxifying role with other cytotoxic anticancer agents [47]. We had hypothesized that P-gp expression in MM cells might be upregulated when co-cultured with BMSCs. In fact, a recent paper by Perez et al. [48] showed that stroma-released factor(s)-induced NF-kB activation. If NF-kB is able to upregulate P-gp [36, 38, 49], then co-culture with stroma should induce P-gp expression in MM cells; however, when we investigated this question, we did not observe expression of P-gp when MM cells were cocultured with BMSCs. In fact, we observed that P-gp expression was downregulated when RPMI-Dox40 cells were co-cultured with BMSCs. Ongoing studies will be evaluating the mechanistic basis of this observation. One possible hypothesis that merits further investigation is whether the co-culture of MM cells with BMSCs and the direct contact between them facilitates transference of P-gp protein to the BMSCs, leading to less measurable P-gp in the RPMI-Dox40 cells. This has also been shown in solid tumours where an increase in the proportion of cells expressing P-gp occurs after exposure to a combination chemotherapy programme containing drugs known to select for P-gp expression in vitro [50]. In MM cells, we showed that the downregulation of P-gp expression was associated with a reduction in P-gp function. However, the P-gp present on stromal cells after their interaction with MM cells was not associated with increased P-gp function. This may be due to presence of P-gp on stromal cells in amounts not sufficient to produce a phenotype in the stomal cells or due to the presence of a non-functional form of the protein in these cells.
In summary, we have demonstrated that bortezomib is able to reduce the expression and function of P-gp. We also showed that bortezomib is a P-gp substrate, since its action is enhanced by co-incubation with a P-gp inhibitor and cells with higher P-gp expression show greater synergy with co-administration of a P-gp inhibitor than cells with less P-gp overexpression providing a rationale for potential combination strategies as therapy [51].
Supplementary Material
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s00280-013-2136-7) contains supplementary material, which is available to authorized users.
Conflict of interest Dr. Kenneth Anderson disclosed the following relevant financial relationships: Served as a consultant for Celgene Corporation, Millennium Pharmaceuticals, Inc., Onyx Pharmaceuticals, Inc., Sanofi and Gilead. Stock Ownership in Oncopep and Acetylon Pharmaceuticals; Dr. Paul Richardson disclosed the following relevant financial relationships: Served as a consultant for Millennium Pharmaceuticals, Inc., and Johnson & Johnson Pharmaceutical Research & Development, LLC; Dr. Constantine Mitsiades disclosed the following relevant financial relationships: Received funding for clinical research from Amgen Inc., AVEO Pharma, Genzyme Corporation, Johnson & Johnson Pharmaceutical Research & Development, LLC. Served as a consultant or received honoraria from Bristol-Myers Squibb company, Millennium Pharmaceuticals, Inc., Celgene Corporation, Centocor Research & Development, Inc, Merck & Co., Inc., Novartis Pharmaceuticals Corporation; Dr. Steffen Klippel disclosed the following relevant financial relationships: Stock Ownership in Novartis Pharmaceuticals. The other authors have no disclosures.
Contributor Information
Robert O’Connor, School of Nursing and Human Sciences, Dublin City University, Dublin 9, Ireland. National Institute for Cellular Biotechnology, Dublin City University, Glasnevin, Dublin 9, Ireland.
Melissa G. Ooi, Email: mgooi20@gmail.com, National Institute for Cellular Biotechnology, Dublin City University, Glasnevin, Dublin 9, Ireland. Jerome Lipper Multiple Myeloma Center, Dana-Farber Cancer Institute, Harvard Medical School, 450 Brookline Avenue, Mayer 555, Boston, MA 02215, USA
Justine Meiller, National Institute for Cellular Biotechnology, Dublin City University, Glasnevin, Dublin 9, Ireland.
Jana Jakubikova, Jerome Lipper Multiple Myeloma Center, Dana-Farber Cancer Institute, Harvard Medical School, 450 Brookline Avenue, Mayer 555, Boston, MA 02215, USA.
Steffen Klippel, Jerome Lipper Multiple Myeloma Center, Dana-Farber Cancer Institute, Harvard Medical School, 450 Brookline Avenue, Mayer 555, Boston, MA 02215, USA.
Jake Delmore, Jerome Lipper Multiple Myeloma Center, Dana-Farber Cancer Institute, Harvard Medical School, 450 Brookline Avenue, Mayer 555, Boston, MA 02215, USA.
Paul Richardson, Jerome Lipper Multiple Myeloma Center, Dana-Farber Cancer Institute, Harvard Medical School, 450 Brookline Avenue, Mayer 555, Boston, MA 02215, USA.
Kenneth Anderson, Jerome Lipper Multiple Myeloma Center, Dana-Farber Cancer Institute, Harvard Medical School, 450 Brookline Avenue, Mayer 555, Boston, MA 02215, USA.
Martin Clynes, National Institute for Cellular Biotechnology, Dublin City University, Glasnevin, Dublin 9, Ireland.
Constantine S. Mitsiades, Jerome Lipper Multiple Myeloma Center, Dana-Farber Cancer Institute, Harvard Medical School, 450 Brookline Avenue, Mayer 555, Boston, MA 02215, USA
Peter O’Gorman, National Institute for Cellular Biotechnology, Dublin City University, Glasnevin, Dublin 9, Ireland. Department of Haematology, Mater Misericordiae University Hospital, Eccles Street, Dublin 7, Ireland.
References
- 1.Richardson PG, Mitsiades C, Schlossman R, Munshi N, Anderson K. New drugs for myeloma. Oncologist. 2007;12:664–689. doi: 10.1634/theoncologist.12-6-664. [DOI] [PubMed] [Google Scholar]
- 2.O’Connor R. The pharmacology of cancer resistance. Anticancer Res. 2007;27:1267–1272. [PubMed] [Google Scholar]
- 3.Yang HH, Ma MH, Vescio RA, Berenson JR. Overcoming drug resistance in multiple myeloma: the emergence of therapeutic approaches to induce apoptosis. J Clin Oncol. 2003;21:4239–4247. doi: 10.1200/JCO.2003.06.001. [DOI] [PubMed] [Google Scholar]
- 4.Grogan TM, Spier CM, Salmon SE, et al. P-glycoprotein expression in human plasma cell myeloma: correlation with prior chemotherapy. Blood. 1993;81:490–495. [PubMed] [Google Scholar]
- 5.Sonneveld P, Suciu S, Weijermans P, et al. Cyclosporin A combined with vincristine, doxorubicin and dexamethasone (VAD) compared with VAD alone in patients with advanced refractory multiple myeloma: an EORTC–HOVON randomized phase III study (06914) Br J Haematol. 2001;115:895–902. doi: 10.1046/j.1365-2141.2001.03171.x. [DOI] [PubMed] [Google Scholar]
- 6.Dalton WS, Crowley JJ, Salmon SS, et al. A phase III randomized study of oral verapamil as a chemosensitizer to reverse drug resistance in patients with refractory myeloma. A Southwest Oncology Group study. Cancer. 1995;75:815–820. doi: 10.1002/1097-0142(19950201)75:3<815::aid-cncr2820750311>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 7.Friedenberg WR, Rue M, Blood EA, et al. Phase III study of PSC-833 (valspodar) in combination with vincristine, doxorubicin, and dexamethasone (valspodar/VAD) versus VAD alone in patients with recurring or refractory multiple myeloma (E1A95): a trial of the Eastern Cooperative Oncology Group. Cancer. 2006;106:830–838. doi: 10.1002/cncr.21666. [DOI] [PubMed] [Google Scholar]
- 8.Sonneveld P, Marie JP, Huisman C, et al. Reversal of multidrug resistance by SDZ PSC 833, combined with VAD (vincristine, doxorubicin, dexamethasone) in refractory multiple myeloma. A phase I study. Leukemia. 1996;10:1741–1750. [PubMed] [Google Scholar]
- 9.Rumpold H, Salvador C, Wolf AM, Tilg H, Gastl G, Wolf D. Knockdown of PgP resensitizes leukemic cells to proteasome inhibitors. Biochem Biophys Res Commun. 2007;361:549–554. doi: 10.1016/j.bbrc.2007.07.049. [DOI] [PubMed] [Google Scholar]
- 10.Loo TW, Clarke DM. The human multidrug resistance P-glycoprotein is inactive when its maturation is inhibited: potential for a role in cancer chemotherapy. FASEB J. 1999;13:1724–1732. doi: 10.1096/fasebj.13.13.1724. [DOI] [PubMed] [Google Scholar]
- 11.Duffy CP, Elliott CJ, O’Connor RA, et al. Enhancement of chemotherapeutic drug toxicity to human tumour cells in vitro by a subset of non-steroidal anti-inflammatory drugs (NSAIDs) Eur J Cancer. 1998;34:1250–1259. doi: 10.1016/s0959-8049(98)00045-8. [DOI] [PubMed] [Google Scholar]
- 12.Clynes M, Redmond A, Moran E, Gilvarry U. Multiple drug-resistance in variant of a human non-small cell lung carcinoma cell line, DLKP-A. Cytotechnology. 1992;10:75–89. doi: 10.1007/BF00376102. [DOI] [PubMed] [Google Scholar]
- 13.Murphy L, Clynes M, Keenan J. Proteomic analysis to dissect mitoxantrone resistance-associated proteins in a squamous lung carcinoma. Anticancer Res. 2007;27:1277–1284. [PubMed] [Google Scholar]
- 14.Collins DM, Crown J, O’Donovan N, et al. Tyrosine kinase inhibitors potentiate the cytotoxicity of MDR-substrate anticancer agents independent of growth factor receptor status in lung cancer cell lines. Invest New Drugs. 2010;28(4):433–444. doi: 10.1007/s10637-009-9266-0. [DOI] [PubMed] [Google Scholar]
- 15.Roovers DJ, van Vliet M, Bloem AC, Lokhorst HM. Idarubicin overcomes P-glycoprotein-related multidrug resistance: comparison with doxorubicin and daunorubicin in human multiple myeloma cell lines. Leuk Res. 1999;23:539–548. doi: 10.1016/s0145-2126(99)00041-7. [DOI] [PubMed] [Google Scholar]
- 16.Sarver JG, Klis WA, Byers JP, Erhardt PW. Microplate screening of the differential effects of test agents on Hoechst 33342, rhodamine 123, and rhodamine 6G accumulation in breast cancer cells that overexpress P-glycoprotein. J Biomol Screen. 2002;7:29–34. doi: 10.1177/108705710200700105. [DOI] [PubMed] [Google Scholar]
- 17.Martin A, Clynes M. Acid phosphatase: endpoint for in vitro toxicity tests. In Vitro Cell Dev Biol. 1991;27A:183–184. doi: 10.1007/BF02630912. [DOI] [PubMed] [Google Scholar]
- 18.McMillin DW, Delmore J, Weisberg E, et al. Tumor cell-specific bioluminescence platform to identify stroma-induced changes to anticancer drug activity. Nat Med. 2010;16:483–489. doi: 10.1038/nm.2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McMillin DW, Ooi M, Delmore J, et al. Antimyeloma activity of the orally bioavailable dual phosphatidylinositol 3-kinase/mammalian target of rapamycin inhibitor NVP-BEZ235. Cancer Res. 2009;69:5835–5842. doi: 10.1158/0008-5472.CAN-08-4285. [DOI] [PubMed] [Google Scholar]
- 20.Ooi MG, Hayden PJ, Kotoula V, et al. Interactions of the Hdm2/p53 and proteasome pathways may enhance the antitumor activity of bortezomib. Clin Cancer Res. 2009;15(23):7153–7160. doi: 10.1158/1078-0432.CCR-09-1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schmidmaier R, Baumann P, Meinhardt G. Cell-cell contact mediated signalling—no fear of contact. Exp Oncol. 2006;28:12–15. [PubMed] [Google Scholar]
- 22.Chou TC, Talalay P. Analysis of combined drug effects: a new look at a very old problem. Trends Pharmacol Sci. 1983;4:450–454. [Google Scholar]
- 23.Chou TC, Talalay P. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzym Regul. 1984;22:27–55. doi: 10.1016/0065-2571(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 24.Hideshima T, Mitsiades C, Tonon G, Richardson PG, Anderson KC. Understanding multiple myeloma pathogenesis in the bone marrow to identify new therapeutic targets. Nat Rev Cancer. 2007;7:585–598. doi: 10.1038/nrc2189. [DOI] [PubMed] [Google Scholar]
- 25.Nakamura T, Tanaka K, Matsunobu T, et al. The mechanism of cross-resistance to proteasome inhibitor bortezomib and overcoming resistance in Ewing’s family tumor cells. Int J Oncol. 2007;31:803–811. [PubMed] [Google Scholar]
- 26.Iijima M, Momose I, Ikeda D. Increased ABCB1 expression in TP-110-resistant RPMI-8226 cells. Biosci Biotechnol Biochem. 2010;74:1913–1919. doi: 10.1271/bbb.100325. [DOI] [PubMed] [Google Scholar]
- 27.Styczynski J, Olszewska-Slonina D, Kolodziej B, Napieraj M, Wysocki M. Activity of bortezomib in glioblastoma. Anticancer Res. 2006;26:4499–4503. [PubMed] [Google Scholar]
- 28.Wiberg K, Carlson K, Aleskog A, Larsson R, Nygren P, Lindhagen E. In vitro activity of bortezomib in cultures of patient tumour cells—potential utility in haematological malignancies. Med Oncol. 2009;26:193–201. doi: 10.1007/s12032-008-9107-6. [DOI] [PubMed] [Google Scholar]
- 29.Fujita T, Washio K, Takabatake D, et al. Proteasome inhibitors can alter the signaling pathways and attenuate the P-glycoprotein-mediated multidrug resistance. Int J Cancer. 2005;117:670–682. doi: 10.1002/ijc.21063. [DOI] [PubMed] [Google Scholar]
- 30.Lu S, Chen Z, Yang J, et al. The effects of proteasome inhibitor bortezomib on a P-gp positive leukemia cell line K562/A02. Int J Lab Hematol. 2010;32(1 pt 1):e123–131. doi: 10.1111/j.1751-553X.2009.01145.x. [DOI] [PubMed] [Google Scholar]
- 31.Minderman H, Zhou Y, O’Loughlin KL, Baer MR. Bortezomib activity and in vitro interactions with anthracyclines and cytarabine in acute myeloid leukemia cells are independent of multidrug resistance mechanisms and p53 status. Cancer Chemother Pharmacol. 2007;60:245–255. doi: 10.1007/s00280-006-0367-6. [DOI] [PubMed] [Google Scholar]
- 32.Mitsiades N, Mitsiades CS, Richardson PG, et al. The proteasome inhibitor PS-341 potentiates sensitivity of multiple myeloma cells to conventional chemotherapeutic agents: therapeutic applications. Blood. 2003;101:2377–2380. doi: 10.1182/blood-2002-06-1768. [DOI] [PubMed] [Google Scholar]
- 33.Schwarzenbach H. Expression of MDR1/P-glycoprotein, the multidrug resistance protein MRP, and the lung-resistance protein LRP in multiple myeloma. Med Oncol. 2002;19:87–104. doi: 10.1385/MO:19:2:87. [DOI] [PubMed] [Google Scholar]
- 34.Kuo MT, Liu Z, Wei Y, et al. Induction of human MDR1 gene expression by 2-acetylaminofluorene is mediated by effectors of the phosphoinositide 3-kinase pathway that activate NF-kappaB signaling. Oncogene. 2002;21:1945–1954. doi: 10.1038/sj.onc.1205117. [DOI] [PubMed] [Google Scholar]
- 35.Thevenod F, Friedmann JM, Katsen AD, Hauser IA. Upregulation of multidrug resistance P-glycoprotein via nuclear factor-kappaB activation protects kidney proximal tubule cells from cadmium- and reactive oxygen species-induced apoptosis. J Biol Chem. 2000;275:1887–1896. doi: 10.1074/jbc.275.3.1887. [DOI] [PubMed] [Google Scholar]
- 36.Ros JE, Schuetz JD, Geuken M, et al. Induction of Mdr1b expression by tumor necrosis factor-alpha in rat liver cells is independent of p53 but requires NF-kappaB signaling. Hepatology. 2001;33:1425–1431. doi: 10.1053/jhep.2001.24667. [DOI] [PubMed] [Google Scholar]
- 37.Zhang Y, Shi Y, Li X, et al. Proteasome inhibitor MG132 reverses multidrug resistance of gastric cancer through enhancing apoptosis and inhibiting P-gp. Cancer Biol Ther. 2008;7:540–546. doi: 10.4161/cbt.7.4.5483. [DOI] [PubMed] [Google Scholar]
- 38.Bentires-Alj M, Barbu V, Fillet M, et al. NF-kappaB transcription factor induces drug resistance through MDR1 expression in cancer cells. Oncogene. 2003;22:90–97. doi: 10.1038/sj.onc.1206056. [DOI] [PubMed] [Google Scholar]
- 39.Werts ED, DeGowin RL, Knapp SK, Gibson DP. Characterization of marrow stromal (fibroblastoid) cells and their association with erythropoiesis. Exp Hematol. 1980;8:423–433. [PubMed] [Google Scholar]
- 40.Chauhan D, Uchiyama H, Akbarali Y, et al. Multiple myeloma cell adhesion-induced interleukin-6 expression in bone marrow stromal cells involves activation of NF-kappa B. Blood. 1996;87:1104–1112. [PubMed] [Google Scholar]
- 41.Hideshima T, Chauhan D, Schlossman R, Richardson P, Anderson KC. The role of tumor necrosis factor alpha in the pathophysiology of human multiple myeloma: therapeutic applications. Oncogene. 2001;20:4519–4527. doi: 10.1038/sj.onc.1204623. [DOI] [PubMed] [Google Scholar]
- 42.Urashima M, Ogata A, Chauhan D, et al. Transforming growth factor-beta1: differential effects on multiple myeloma versus normal B cells. Blood. 1996;87:1928–1938. [PubMed] [Google Scholar]
- 43.Podar K, Anderson KC. The pathophysiologic role of VEGF in hematologic malignancies: therapeutic implications. Blood. 2005;105:1383–1395. doi: 10.1182/blood-2004-07-2909. [DOI] [PubMed] [Google Scholar]
- 44.Jakubikova J, Adamia S, Kost-Alimova M, et al. Lenalidomide targets clonogenic side population in multiple myeloma: pathophysiologic and clinical implications. Blood. 2011;117:4409–4419. doi: 10.1182/blood-2010-02-267344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McMillin DW, Negri JM, Mitsiades CS. The role of tumour-stromal interactions in modifying drug response: challenges and oppurtunities. Nat Rev Drug Discov. 2013;12(3):217–228. doi: 10.1038/nrd3870. [DOI] [PubMed] [Google Scholar]
- 46.McMillin DW, Delmore J, Negri J, Ooi M, Klippel S, Miduturu CV, Gray NS, Richardson PG, Anderson KC, Kung AL, Mitsiades CS. Microenvironmental influence on pre-clinical activity of polo-like kinase inhibition in multiple myeloma: implications for clinical translation. PLoS One. 2011;6(7):e2022647. doi: 10.1371/journal.pone.0020226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pessina A, Piccirillo M, Mineo E, et al. Role of SR-4987 stromal cells in the modulation of doxorubicin toxicity to in vitro granulocyte-macrophage progenitors (CFU-GM) Life Sci. 1999;65:513–523. doi: 10.1016/s0024-3205(99)00272-6. [DOI] [PubMed] [Google Scholar]
- 48.Perez LE, Parquet N, Meads M, Anasetti C, Dalton W. Bortezomib restores stroma-mediated APO2L/TRAIL apoptosis resistance in multiple myeloma. Eur J Haematol. 2010;84(3):212–222. doi: 10.1111/j.1600-0609.2009.01381.x. [DOI] [PubMed] [Google Scholar]
- 49.Garcia MG, Alaniz LD, Cordo Russo RI, Alvarez E, Hajos SE. PI3K/Akt inhibition modulates multidrug resistance and activates NF-kappaB in murine lymphoma cell lines. Leuk Res. 2009;33:288–296. doi: 10.1016/j.leukres.2008.06.010. [DOI] [PubMed] [Google Scholar]
- 50.Petrylak DP, Scher HI, Reuter V, O’Brien JP, Cordon-Cardo C. P-glycoprotein expression in primary and metastatic transitional cell carcinoma of the bladder. Ann Oncol. 1994;5:835–840. doi: 10.1093/oxfordjournals.annonc.a059013. [DOI] [PubMed] [Google Scholar]
- 51.Mitsiades CS, Davies FE, Laubach JP, et al. Future directions of next-generation novel therapies, combination approaches, and the development of personalized medicine in myeloma. J Clin Oncol. 2011;29:1916–1923. doi: 10.1200/JCO.2010.34.0760. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.