Abstract
Objective
Previous studies exploring the relationship of neighborhood characteristics with metabolic conditions have focused on middle-aged adults but none have comprehensively investigated associations in older adults, a potentially vulnerable population. The aim was to explore the relationship of neighborhood characteristics with metabolic conditions in older women.
Design
Cross-sectional analysis.
Setting/Participants
We studied 384 women aged 70–79 years, representing the two-thirds least disabled women in the community, enrolled in the Women’s Health and Aging Study II at baseline. Neighborhood scores were calculated from census-derived data on median household income, median house value, percent earning interest income, percent completing high school, percent completing college, and percent with managerial or executive occupation. Participants were categorized by quartile of neighborhood score with a higher quartile representing relative neighborhood advantage. Logistic regression models were created to assess the association of neighborhood quartiles to outcomes, adjusting for key covariates.
Measurements
Primary outcomes included metabolic conditions: obesity, diabetes, hypertension, and hyperlipidemia. Secondary outcomes included BMI, HbA1c, blood pressure and lipids.
Results
Higher neighborhood quartile score was associated with a lower prevalence of obesity (highest quartile=13.5% versus lowest quartile=36.5%; p<0.001 for trend). A lower prevalence of diabetes was also observed in highest (6.3%) versus lowest (14.4%) neighborhood quartiles, but was not significantly different (p= 0.24 for trend). Highest versus lowest neighborhood quartile was associated with lower HbA1c (−0.31%, p=0.02) in unadjusted models. Women in the highest versus lowest neighborhood quartile had lower BMI (−2.01 kg/m2, p=0.001) and higher HDL-cholesterol (+6.09 mg/dL, p=0.01) after accounting for age, race, inflammation, and smoking.
Conclusion
Worse neighborhood characteristics are associated with adiposity, hyperglycemia, and low HDL. Further longitudinal studies are needed and can inform future interventions to improve metabolic status in older adults.
Keywords: Neighborhood, elderly, obesity, diabetes, metabolic abnormalities
Introduction
Neighborhood characteristics, such as average educational attainment, employment rate and income level, have been associated with type 2 diabetes and obesity in non-elderly adults (1–4). Yet, elderly adults have a high prevalence of metabolic conditions, such as obesity, diabetes, hypertension, and hyperlipidemia, compared to all other age groups and may represent a particularly vulnerable population (5–8). Thus, identifying potentially modifiable neighborhood factors that are associated with worse metabolic outcomes may have important public health implications, especially in older adults, and facilitate effective prevention strategies in the future.
Previous studies exploring the relationship of neighborhood characteristics with obesity and/or insulin resistance have used either single or composite neighborhood measures (9–14). Composite scores may better characterize the range of potential neighborhood factors that could impact the metabolic status of residents. Many different composite scores have been used that rely on census-derived factors or individually assessed neighborhood characteristics (9–11). An example is the CARDIA study, which used composite scores to explore associations of neighborhood characteristics with insulin resistance (11). This study demonstrated a graded association of progressive neighborhood disadvantage with higher levels of insulin resistance and related metabolic components among patients without diabetes. However, the CARDIA study and other previous studies were performed in primarily young- and middle-aged participants, and the degree to which similar associations are also observed in elderly populations has not been previously investigated (11,15).
In the present study, we hypothesized that relatively disadvantaged neighborhoods, characterized using a composite neighborhood score (11), are associated with greater metabolic abnormalities in older adults. Specifically, our hypotheses were: 1) lower neighborhood scores are associated with a higher likelihood of metabolic conditions (primary outcomes: obesity, diabetes, hypertension, hyperlipidemia); 2) lower neighborhood scores are related to worse metabolic abnormalities (secondary outcomes: body-mass index, hyperglycemia, blood pressure, and lipid components); and 3) associations of neighborhood score with metabolic conditions and abnormalities are independent of potential confounders (i.e. age, race, inflammatory markers, and smoking status). We used baseline data from the Women’s Health and Aging Study II for our study (16).
Materials and Methods
Subjects
The study population for this cross sectional analysis consisted of community-dwelling women enrolled in a longitudinal, population based study, the Women’s Health and Aging Study II (WHAS II) (16). Using a random sample from the Health Care Financing Administration’s Medicare Eligibility list for Baltimore, Maryland, women older than 65 years were recruited for the study population. Eligibility for WHAS II required women to be 70–79 years and have difficulty in zero or one of four domains of physical function. Four hundred and thirty six women representative of the two-thirds least disabled older women living in the community enrolled. Seven study visits occurred from 1994–2008 where standardized evaluations, interviews, and physical examinations were conducted at the Johns Hopkins Functional Laboratory. The current study uses data from the initial study visit.
Of the 436 women enrolled in WHAS II, the six components needed to derive the composite neighborhood Z-scores were unavailable for 52 women who did not have addresses that could be matched to known census blocks (17). Women missing Z-scores were similar to those with Z-scores available but with relatively fewer Caucasians. Given the similarities, 384 women were included in the present study.
The Johns Hopkins University Institutional Review Board approved the study and all participants gave informed consent.
Neighborhood Scores
Census derived block groups with a mean population of 1,000 were used as proxies for neighborhoods. Each study participant was linked to their census defined area of reference using their baseline study visit address. The 1990 United States Census data was used to derive the neighborhood scores as it was the most recent census data available that also closely approximated the timing of the initial study visit. The census was used to provide six measures of data that comprised the neighborhood score. These components included (1) median household income, (2) median house value, (3) percent earning interest income, (4) percent completing high school, (5) percent completing college, and (6) percent with managerial or executive occupation. For each of these six census variables, a Z-score was estimated by subtracting the mean for the total sample of block groups and dividing by the standard deviation. The six variables were then summed into a neighborhood composite Z-score with higher scores representing a relatively better neighborhood advantage (17). The neighborhood composite Z-scores in our study were calculated using similar methods as the CARDIA study (11), and participants were categorized according to quartile of Z-scores (n=96 in each quartile). The range of Z-scores in each quartile was as follows: quartile 1 (Z-scores −6.78 to −1.70), quartile 2 (Z-scores −1.70 to 0.09), quartile 3 (Z-scores 0.09 to 2.37), and quartile 4 (Z-scores 2.37 to 6.27). The higher quartiles (Z-scores) represent the most advantaged neighborhoods.
Metabolic Outcomes
At the initial study visit, questionnaires were completed for demographics, medical history and smoking status. Blood pressure, weight and height were measured. Laboratory collection was performed for non-fasting lipids, hemoglobin A1c, CRP, and IL-6. Medicine adjudication was also performed for anti-hypertensive, lipid-lowering, and estrogen use.
The primary outcomes were metabolic conditions that included obesity (body-mass index ≥30 kg/m2), diabetes (self-reported history and/or use of glucose-lowering medication), hypertension (self-reported history, antihypertensive medicine use, systolic blood pressure ≥140 mmHg, and/or diastolic blood pressure ≥90 mm Hg), and hyperlipidemia (total cholesterol ≥200 mg/dl and/or lipid- lowering medication use). In addition, secondary outcomes were continuous metabolic abnormalities including body-mass index (BMI), HbA1c, total cholesterol, HDL-cholesterol, LDL-cholesterol, triglycerides, systolic and diastolic blood pressure.
Statistical Analysis
Baseline characteristics were compared by neighborhood Z-score quartile with chi-squared test for binary variables and ANOVA for continuous variables. For skewed variables (CRP, IL-6), medians and interquartile range were compared. Logistic regression models were created to graphically explore associations of neighborhood Z-score with metabolic conditions (obesity, diabetes, hypertension, hyperlipidemia) after adjustment for age and race. Next, linear regression models were created to explore the association of neighborhood Z-scores with levels of continuous metabolic outcomes. Beta coefficients and 95% confidence intervals are shown. Model 1 was the unadjusted model. Model 2 included adjustment for age and race. Model 3 had additional adjustment for log CRP, log IL-6, and smoking.
In sensitivity analysis, HbA1c ≥7% as a dichotomous outcome was explored in logistic regression models to more specifically assess the association of neighborhood characteristics with the presence of hyperglycemia. In additional sensitivity analyses, BMI ≥ 25 kg/m2 was explored in logistic regression models to assess association of neighborhood characteristics with the presence of either overweight or obesity. Sensitivity analyses stratified by estrogen use were also done among the subset of women with available data (n=338).
We used a multilevel model in sensitivity analysis to take into account neighborhood cluster effects that may be present from participants living in the same geographic census block. This accounts for similar personal behavior that participants who live within the same geographic census block may display. Accounting for the clustering effect prevents standard errors from being underestimated and potential overestimation of the significance of the associations (18). This was done using linear mixed effects models.
All analyses were performed using SAS software (version 9.3, SAS Institute) and R (R 3.01, public free software).
Results
Among the 384 women in our study, mean age did not differ by quartile of neighborhood Z-score (Table 1). The proportion of Caucasians and level of education completed increased with higher quartile of neighborhood Z-score (p<0.001 for both). Inflammatory factors including CRP and IL-6 decreased with higher quartile of neighborhood Z-score but were not significantly different. Mean BMI significantly decreased with higher quartiles of neighborhood Z-score (p=0.001). Hemoglobin A1c did not significantly differ by quartile of Z-score. HDL was the only component of the non-fasting lipid panel that significantly decreased with higher quartiles of neighborhood Z-scores (p=0.03). In addition, the percentage of participants with obesity also decreased significantly with increasing quartiles of neighborhood Z-score (p<0.001). The percentage of participants with diabetes decreased with higher quartiles of neighborhood Z-score, but differences were not statistically significant. The overall study population had a very high prevalence of hypertension and hyperlipidemia (78.9% and 83.1% respectively). There were no significant differences in the percentage of participants with these two conditions across neighborhood Z-score quartiles.
Table 1.
Baseline and Clinical characteristics of WHAS II Women by Neighborhood Z-score Quartile*, n=384
| Neighborhood Z score | ||||||
|---|---|---|---|---|---|---|
| All | Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | p | |
| Demographics | ||||||
| Age (years) | 74.1 (2.8) | 74.0 (3.0) | 74.0 (2.6) | 74.0 (2.8) | 74.4 (3.0) | 0.66 |
| Caucasian (%) | 83.1 | 67.0 | 83.2 | 86.5 | 95.8 | <0.001 |
| Education (years completed) | 12.6 (3.2) | 10.7 (2.8) | 12.2 (2.7) | 13.0 (3.2) | 14.6 (3.0) | <0.001 |
| Smoking (%) | 9.7 | 13.4 | 9.5 | 12.5 | 3.2 | 0.25 |
| Physical and Laboratory Assessments | ||||||
| BMI (kg/m2) | 26.7 (5.2) | 28.3 (5.7) | 27.0 (5.4) | 26.1 (4.6) | 25.4 (4.0) | 0.001 |
| HbA1c (%) | 6.0 (0.9) | 6.2 (1.1) | 6.0 (0.8) | 6.1 (0.9) | 5.9 (0.8) | 0.27 |
| Cholesterol (mg/dL) | 233.6 (38.5) | 233.1 (36.8) | 235.1 (36.5) | 228.9 (37.9) | 238.9 (40.2) | 0.65 |
| HDL (mg/dL) | 56.7 (16.6) | 55.1 (17.6) | 54.9 (15.1) | 54.4 (14.6) | 61.2 (18.2) | 0.03 |
| LDL (mg/dL) | 146.3 (35.1) | 147.7 (35.4) | 147.1 (34.1) | 143.6 (34.1) | 147.3 (35.6) | 0.97 |
| Triglycerides (mg/dL) | 153.2 (88.9) | 151.9 (96.5) | 165.5 (90.2) | 154.9 (86.3) | 152.4 (94.5) | 0.42 |
| CRP (mg/L) | 2.0 (3.9) | 3.5 (5.9) | 2.8 (4.1) | 2.0 (3.3) | 2.0 (3.2) | 0.17 |
| IL-6 (pg/mL) | 3.1 (2.2) | 3.5 (2.4) | 3.1 (2.4) | 2.9 (2.8) | 2.9 (1.6) | 0.11 |
| Clinical Metabolic Conditions | ||||||
| Obesity (%) | 22.3 | 36.5 | 24.5 | 14.6 | 13.5 | <0.001 |
| Diabetes (%) | 9.4 | 14.4 | 8.4 | 8.4 | 6.3 | 0.24 |
| Hypertension (%) | 78.9 | 78.4 | 77.9 | 81.3 | 78.1 | 0.93 |
| Dyslipidemia (%) | 83.1 | 82.1 | 83.7 | 80.0 | 86.5 | 0.68 |
Data are expressed as means (standard deviation) except for CRP and IL-6 which are expressed as median (interquartile range).
Metabolic Conditions
In logistic regression models adjusted for age and race, we graphically explored the association of neighborhood Z-score quartile with the likelihood of metabolic conditions (obesity, diabetes, hypertension, hyperlipidemia). Using neighborhood Z-score quartile 1 as a reference, the odds ratios of metabolic outcomes for neighborhood Z-score quartiles 2, 3, and 4 are shown in Figure 1. For obesity, the odds ratio was significantly lower for both quartile 4 (OR=0.37, 95% CI 0.18–0.79) and quartile 3 (OR=0.35, 95% CI 0.17–0.73) compared to reference. The p-value for trend of obesity across higher neighborhood quartiles was significant (p=0.002). For diabetes, the highest neighborhood Z-score (quartile 4) had a 53% reduced odds of diabetes compared to quartile 1, but the difference was not statistically significant (OR=0.47, 95% CI 0.17–1.34). The likelihood of hypertension and hyperlipidemia did not differ by neighborhood Z-scores.
Figure 1.
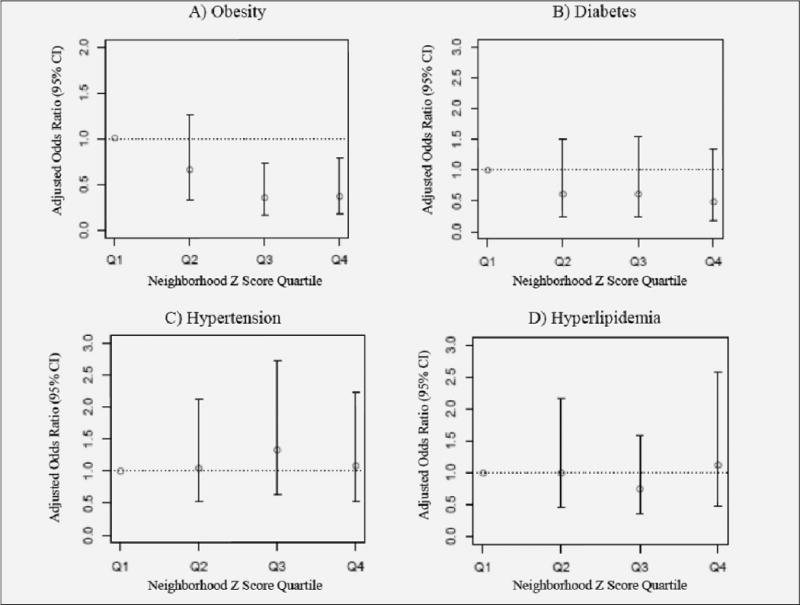
Association of Neighborhood Z-score Quartiles with Metabolic Conditions
The adjusted odds ratios (95% CI) for the presence of metabolic conditions comparing higher neighborhood Z-score quartiles to quartile 1 (reference). Metabolic conditions include: obesity (A), diabetes (B), hypertension (C), and hyperlipidemia (D). The odds ratios are adjusted for age and race. The likelihood of obesity in older women decreased across higher neighborhood Z-score quartiles; the p-value for trend was significant (p=0.002). The likelihood of diabetes in older women also decreased across higher neighborhood Z-score quartiles but did not reach statistical significance (p>0.05). Hypertension and hyperlipidemia were both highly prevalent conditions in older women that did not differ by neighborhood Z-score quartile.
In sensitivity analysis, the likelihood of hyperglycemia (HbA1c ≥7%) was lower by 56% in the highest (quartile 4) versus lowest neighborhood quartile (quartile 1) but was not significantly different (OR=0.44, 95% CI 0.13–1.57). We also examined the likelihood of being either overweight or obese (BMI category ≥25 kg/m2). The findings were similar to the results for obesity alone. In age- and race-adjusted models, the odds ratio was significantly lower for both quartile 4 (OR= 0.46, 95% CI 0.25–0.86) and quartile 3 (OR=0.48, 95% CI 0.26–0.89) for the presence of overweight or obesity.
Metabolic Abnormalities
Next, we created linear regression models investigating associations of the independent variable (neighborhood Z-score quartile) with continuous metabolic outcomes (BMI, HbA1c, HDL, LDL, total cholesterol, triglycerides, systolic and diastolic blood pressure) in Table 2. In unadjusted models, neighborhood Z-score quartile 4 had a lower BMI of −2.88 kg/m2 (95% CI −4.28 to −1.49) and quartile 3 had a lower BMI of −2.19 kg/m2 (95% CI −3.59 to −0.79) compared to neighborhood Z-score quartile 1. Neighborhood Z-score quartile 2 had a lower BMI compared to neighborhood Z-score quartile 1 but was not statistically significant (−1.24 kg/m2, 95% CI −2.65 to 0.16). In model 2, after additional adjustment for age and race, these results were largely unchanged. In the fully adjusted model also accounting for inflammatory factors and smoking (model 3), the results were minimally attenuated and BMI remained significantly lower for quartile 4 (−2.01 kg/m2, 95% CI −3.37 to −0.66) and quartile 3 (−1.47 kg/m2, 95% CI − 2.80 to −0.14) compared to neighborhood Z-score quartile 1.
Table 2.
Linear Regression Analyses Exploring the Association of Neighborhood Z-score with Metabolic Abnormalities (WHAS II)§
| Metabolic Outcome | Quartile Z Score | Model 1* | Model 2** | Model 3*** |
|---|---|---|---|---|
| BMI (kg/m2) | Q1 | Reference | Reference | Reference |
| Q2 | −1.24 (−2.65, 0.16) | −0.83 (−2.22, 0.57) | −0.71 (−2.03, 0.62) | |
| Q3 | −2.19 (−3.59, −0.79)† | −1.68 (−3.08, −0.29)† | −1.47 (−2.80, −0.14)† | |
| Q4 | −2.88 (−4.28, −1.49)‡ | −2.11 (−3.54, −0.68)† | −2.01 (−3.37, −0.66)† | |
| HbA1c (%) | Q1 | Reference | Reference | Reference |
| Q2 | −0.19 (−0.46, 0.08) | −0.13 (−0.40, 0.14) | −0.08 (−0.35, 0.19) | |
| Q3 | −0.14 (−0.42, 0.13) | −0.06 (−0.34, 0.22) | 0.00 (−0.28, 0.28) | |
| Q4 | −0.31 (-0.57, −0.05)† | −0.20 (−0.47, 0.07) | −0.14 (−0.41, 0.14) | |
| HDL (mg/dL) | Q1 | Reference | Reference | Reference |
| Q2 | −0.14 (−4.82, 4.54) | 0.60 (−4.12, 5.31) | −0.71 (−5.33, 3.92) | |
| Q3 | −0.71 (−5.36, 3.93) | 0.19 (−4.52, 4.90) | −0.88 (−5.52, 3.77) | |
| Q4 | 6.08 (1.45, 10.71)† | 7.39 (2.60, 12.18)† | 6.09 (1.35, 10.84)† | |
| LDL (mg/dL) | Q1 | Reference | Reference | Reference |
| Q2 | −0.68 (−10.60, 9.25) | −0.57 (−10.63, 9.48) | −1.10 (−11.25, 9.05) | |
| Q3 | −4.16 (−14.01, 5.68) | −4.02 (−14.06, 6.02) | −3.93 (−14.13, 6.27) | |
| Q4 | −0.47 (−10.29, 9.35) | −0.23 (−10.45, 9.99) | −1.32 (−11.73, 9.09) | |
| Triglycerides | Q1 | Reference | Reference | Reference |
| (mg/dL) | Q2 | 13.63 (−12.60, 39.86) | 5.99 (−20.13, 32.11) | 8.43 (−17.59, 34.45) |
| Q3 | 2.97 (−23.05, 28.99) | −6.24 (−32.32, 19.84) | −1.49 (−27.64, 24.65) | |
| Q4 | 0.51 (−25.44, 26.46) | −11.98 (−38.53, 14.57) | −9.10 (−35.79, 17.59) |
beta coefficient and 95% CI shown
crude unadjusted
adjusted for age and race
adjusted for age, race, log CRP, log IL-6, and smoking
p<0.05
p<0.001.
For HbA1c, in the unadjusted model 1 (Table 2), the difference between quartile 4 and quartile 1 was significantly different (−0.31%, 95% CI −0.57 to −0.05). This difference was attenuated with additional adjustments for age and race in model 2 such that results were no longer statistically significant (−0.20%, 95% CI −0.47 to 0.07). There were no differences in HbA1c level in other neighborhood Z-score quartiles compared to neighborhood Z-score quartile 1.
HDL cholesterol was significantly higher comparing neighborhood Z-score quartile 4 to quartile 1 in the unadjusted model 1 (+6.08 mg/dL, 95% CI 1.45–10.71, Table 2). In model 2, after additional adjustment for age and race, these results were largely unchanged. These finding persisted in the fully adjusted model for quartile 4 versus quartile 1 (+6.09 mg/dL, 95% CI 1.35–10.84). There were no major differences in HDL level when comparing other neighborhood Z-score quartiles to quartile 1. In sensitivity analysis, of the 338 women with available estrogen use data, 86.4% were using estrogen and 13.6% were not. The significant trends in HDL cholesterol persisted after stratifying by estrogen use status.
No significant differences were seen in higher versus lower neighborhood Z-score quartiles for triglycerides or LDL cholesterol in either unadjusted or fully adjusted models (Table 2). Also, total cholesterol, systolic and diastolic blood pressures were similar comparing higher and lower neighborhood Z-score quartiles in unadjusted and fully adjusted models [data not shown].
In sensitivity analyses accounting for potential effects of neighborhood clustering, the results were unchanged [data not shown].
Discussion
Older women living in disadvantaged neighborhoods as determined using census-derived data were significantly more likely to be obese with average BMI that decreased as neighborhood status improved. In addition, as neighborhood characteristics improved, the likelihood of diabetes decreased as did hemoglobin A1c level, but these differences were not statistically significant. Interestingly, HDL cholesterol was significantly higher among women in the more advantaged neighborhoods compared to those living in relatively worse neighborhoods, after accounting for potential confounders.
The results of our study are similar to findings described in other younger populations. The Baltimore Memory Study investigated the association of neighborhood characteristics with obesity (9). This study population had a mean age of 59 years and included both men and women. Using a 12-item scoring system to develop a composite neighborhood score, investigators showed that obesity was more common in participants from the lowest neighborhood quartile compared to those from the highest neighborhood quartile (52.5% versus 27.4%, p<0.001). Further, the mean BMI in the lowest quartile was 31.2 kg/m2 compared to 27.5 kg/m2 in the highest quartile of neighborhood (p<0.001). In the Black Women’s Health study (2010), a younger population (age 21–69 years) of African American women were followed for 10 years (10). Using similar census-derived neighborhood characteristics as those in our study, the authors demonstrated that lower neighborhood status was associated with 10-year weight gain and incident obesity (OR=1.32, 95% CI 1.1–1.59 for lowest versus highest neighborhood quintile). The CARDIA study (2002) used the same neighborhood composite score as in our study but focused on a younger population of patients without diabetes (aged 28 to 40 years). The authors showed that living in neighborhoods from the most disadvantaged quartiles was associated with a higher insulin resistance score (combination of BMI, fasting HDL, triglycerides, insulin, glucose, and blood pressure) compared to living in more advantaged or higher neighborhood quartiles (11). In contrast to these previous reports, our cross-sectional study included older women with an average age of ~74 years. Yet, we still found consistency of associations in our population, with lower neighborhood scores related to significantly greater likelihood of obesity and higher BMI, independent of potential confounders. Interestingly, our study had a lower overall prevalence of obesity compared to previous studies such as the Baltimore Memory Study (22% versus 38%). In contrast to our study, the Baltimore Memory Study included relatively younger participants, fewer Caucasians (54%), and approximately one-third were men, which may have contributed to differences in the prevalence of obesity we observed.
Observational studies of non-elderly adults have shown that neighborhood characteristics are associated with diabetes (19, 20) and more recently, a prospective, randomized study demonstrated that the opportunity to move from neighborhoods of high poverty to those with low poverty reduced the prevalence of diabetes (21). Our study further suggests that the likelihood of diabetes and average HbA1c level was lower in the most versus least advantaged neighborhoods of older women but these findings were, in part, related to differences in age and race between neighborhoods.
There may be multiple pathways by which worse neighborhood characteristics associate with a greater burden of metabolic conditions and abnormalities. One example may be the relationship of neighborhood characteristics to healthy food options. Previous investigators have shown a high prevalence of “food deserts” or areas of unhealthy food environments clustered in low-income neighborhoods with relatively more minorities. By contrast, “food deserts” are relatively less commonly clustered among middle-to-upper income, primarily Caucasian neighborhoods (22). Availability of healthy food options may relate to the association of worse neighborhood characteristics with obesity and other metabolic conditions. Other potential explanations include the availability of resources within a neighborhood that promote walking and, thus, exercise. Previous literature has demonstrated that neighborhood resources for physical activity are related to metabolic outcomes (3, 23). More disadvantaged neighborhoods may have higher crime rates promoting an unsafe outside environment. This may dissuade residents from outdoor leisure activities, such as walking. Neighborhoods with a lack of physical activity resources or a more sedentary lifestyle may be related to a worse metabolic profile. Further, more disadvantaged neighborhoods may be less likely to have commercial shops, stores or recreation facilities, creating an environment where walking among older adults, in particular, is not promoted (24). Availability of healthy food options and resources for physical activity in different neighborhoods were not assessed in our participants but should be explored in future studies. Finally, clustering of obesity in more disadvantaged neighborhoods may be related to role modeling and social networking factors. Residents may be surrounded by neighbors who do not exercise and make poor food choices. These behaviors may be role modeled and dissuade a healthy lifestyle (25). These dietary choices may have important consequences on metabolic outcomes, such as hypertension, in older adults (26).
Our study demonstrates that more disadvantaged neighborhoods are associated with worse metabolic abnormalities. The implication of these findings is that neighborhood characteristics, which may be potentially modifiable, may represent an opportunity for future preventive efforts to target elderly patients at high-risk of poor metabolic outcomes. The hypothesis of whether moving out of a relatively less to more advantaged neighborhood reduces the burden of metabolic conditions such as diabetes has already been demonstrated in one study of non-elderly adults (21). Further studies are needed to investigate if modifying neighborhood characteristics can reduce the likelihood of obesity and other metabolic derangements in older adults.
The strengths of our study include the ability to uniquely investigate associations of neighborhood characteristics with metabolic abnormalities in a potentially vulnerable study population of older women. We were also able to investigate a range of metabolic conditions and abnormalities in this population. Additionally, we used a composite neighborhood score which allowed us to potentially better characterize neighborhood status compared to other studies that used a single neighborhood factor (27).
Our study has limitations. The cross-sectional design does not allow us to infer temporality of associations and our study is limited by the relatively small study population. Data used to characterize neighborhoods in our study was from the initial visit. It is possible our study population may not reflect the characteristics of modern day neighborhoods with the now widespread use of technology; however, our findings of relatively greater neighborhood advantage associated with better metabolic profile in older adults remain relevant to current day knowledge. The use of BMI cut-offs to define obesity in older adults may not appropriately account for relative adiposity given age-related loss of skeletal muscle, as well (28, 29). Dual energy X-ray absorptiometry or other imaging modalities (i.e. CT) may better characterize body composition in older adults and should be explored in future studies. Finally, individual-level characteristics such as socioeconomic status or education may differ from the median values of the overall census block in which a particular individual resides (30, 31). However, in the present study, we were interested in investigating the association of neighborhood-level characteristics to metabolic outcomes as a potential target for prevention strategies in the future.
In summary, we showed that living in a relatively more advantaged neighborhood was associated with a ~65% lower likelihood of obesity and lower average BMI among older women compared to those living in the least advantaged neighborhoods. HDL cholesterol was also significantly higher in individuals from more advantaged neighborhoods, and diabetes and hyperglycemia were relatively less likely. Our findings should be confirmed in larger populations in the future. Further longitudinal studies are needed to confirm the directionality of these associations. A better understanding of the relationship between neighborhood characteristics and metabolic abnormalities can, ultimately, inform future interventions targeted at improving health outcomes in older adults.
Acknowledgments
This study was supported by the National Institute of Diabetes and Digestive and Kidney Diseases (K23-DK093583 and T32 DK062707) and the Johns Hopkins Older Americans Independence Center (P30-AG021334).
Footnotes
Disclosures: The authors have nothing to disclose.
References
- 1.Mujahid MS, Diez Roux AV, Shen M, Gowda D, Sanchez B, Shea S, et al. Relation between neighborhood environments and obesity in the Multi-Ethnic Study of Atherosclerosis. Am J Epidemiol. 2008 Jun 1;167(11):1349–1357. doi: 10.1093/aje/kwn047. [DOI] [PubMed] [Google Scholar]
- 2.Morland K, Diez Roux AV, Wing S. Supermarkets, other food stores, and obesity: the atherosclerosis risk in communities study. Am J Prev Med. 2006 Apr;30(4):333–339. doi: 10.1016/j.amepre.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 3.Auchincloss AH, Diez Roux AV, Mujahid MS, Shen M, Bertoni AG, Carnethon MR. Neighborhood resources for physical activity and healthy foods and incidence of type 2 diabetes mellitus: the Multi-Ethnic study of Atherosclerosis. Arch Intern Med. 2009 Oct 12;169(18):1698–1704. doi: 10.1001/archinternmed.2009.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ross NA, Gilmour H, Dasgupta K. 14-Year Diabetes Incidence: the Role of Socio-Economic Status. Health Rep. 2010 Sep;21(3):19–28. [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention (CDC) Vital signs: prevalence, treatment, and control of high levels of low-density lipoprotein cholesterol–United States, 1999–2002 and 2005-200. MMWR Morb Mortal Wkly Rep. 2011 Feb 4;60(4):109–114. [PubMed] [Google Scholar]
- 6.Wright JD, Hughes JP, Ostchega Y, Yoon SS, Nwankwo T. Mean systolic and diastolic blood pressure in adults aged 18 and over in the United States, 2001–2008. Natl Health Stat Report. 2011 Mar 25;(35):1–22. 24. [PubMed] [Google Scholar]
- 7.Cowie CC, Rust KF, Ford ES, Eberhardt MS, Byrd-Holt DD, Li C, et al. Full accounting of diabetes and pre-diabetes in the U.S. population in 1988–1994 and 2005–2006. Diabetes Care. 2009 Feb;32(2):287–294. doi: 10.2337/dc08-1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999–2010. JAMA. 2012 Feb 1;307(5):491–497. doi: 10.1001/jama.2012.39. [DOI] [PubMed] [Google Scholar]
- 9.Glass TA, Rasmussen MD, Schwartz BS. Neighborhoods and obesity in older adults: the Baltimore Memory Study. Am J Prev Med. 2006 Dec;31(6):455–463. doi: 10.1016/j.amepre.2006.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coogan PF, Cozier YC, Krishnan S, Wise LA, Adams-Campbell LL, Rosenberg L, et al. Neighborhood socioeconomic status in relation to 10-year weight gain in the Black Women’s Health Study. Obesity (Silver Spring) 2010 Oct;18(10):2064–2065. doi: 10.1038/oby.2010.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Diez Roux AV, Jacobs DR, Kiefe CI, Coronary Artery Risk Developoment in Young Adults (CARDIA) Study Neighborhood characteristics and components of the insulin resistance syndrome in young adults: the coronary artery risk development in young adults (CARDIA) study. Diabetes Care. 2002 Nov;25(11):1976–1982. doi: 10.2337/diacare.25.11.1976. [DOI] [PubMed] [Google Scholar]
- 12.Bird CE, Seeman T, Escarce JJ, Basurto-Davila R, Finch BK, Dubowitz T, et al. Neighbourhood socioeconomic status and biological ‘wear and tear’ in a nationally representative sample of US adults. J Epidemiol Community Health. 2010 Oct;64(10):860–865. doi: 10.1136/jech.2008.084814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Loucks EB, Magnusson KT, Cook S, Rehkopf DH, Ford ES, Berkman LF. Socioeconomic position and the metabolic syndrome in early, middle, and late life: evidence from NHANES 1999–2002. Ann Epidemiol. 2007 Oct;17(10):782–790. doi: 10.1016/j.annepidem.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 14.Ngo AD, Paquet C, Howard NJ, Coffee NT, Adams R, Taylor A, et al. Area-level socioeconomic characteristics and incidence of metabolic syndrome: a prospective cohort study. BMC Public Health. 2013 Jul 25;13(1):681. doi: 10.1186/1471-2458-13-681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Murakami K, Sasaki S, Takahashi Y, Uenishi K, Japan Dietetic Students’ Study for Nutrition and Biomarkers Group Neighborhood socioeconomic status in relation to dietary intake and insulin resistance syndrome in female Japanese dietetic students. Nutrition. 2010 May;26(5):508–514. doi: 10.1016/j.nut.2009.08.025. [DOI] [PubMed] [Google Scholar]
- 16.Fried LP, Bandeen-Roche K, Chaves PH, Johnson BA. Preclinical mobility disability predicts incident mobility disability in older women. J Gerontol A Biol Sci Med Sci. 2000 Jan;55(1):M43–52. doi: 10.1093/gerona/55.1.m43. [DOI] [PubMed] [Google Scholar]
- 17.Nicklett EJ, Szanton S, Sun K, Ferrucci L, Fried LP, Guralnik JM, et al. Neighborhood socioeconomic status is associated with serum carotenoid concentrations in older, community-dwelling women. J Nutr. 2011 Feb;141(2):284–289. doi: 10.3945/jn.110.129684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kreft I, de Leeuw J. Introducing Multilevel Modeling. 1. Thousand Oaks, California: SAGE Publications Inc; 1998. [Google Scholar]
- 19.LaVeist TA, Thorpe RJ, Jr, Galarraga JE, Bower KM, Gary-Webb TL. Environmental and socio-economic factors as contributors to racial disparities in diabetes prevalence. J Gen Intern Med. 2009 Oct;24(10):1144–1148. doi: 10.1007/s11606-009-1085-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Krishnan S, Cozier YC, Rosenberg L, Palmer JR. Socioeconomic status and incidence of type 2 diabetes: results from the Black Women’s Health Study. Am J Epidemiol. 2010 Mar 1;171(5):564–570. doi: 10.1093/aje/kwp443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ludwig J, Sanbonmatsu L, Gennetian L, Adam E, Duncan GJ, Katz LF, et al. Neighborhoods, obesity, and diabetes–a randomized social experiment. N Engl J Med. 2011 Oct 20;365(16):1509–1519. doi: 10.1056/NEJMsa1103216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gordon C, Purciel-Hill M, Ghai NR, Kaufman L, Graham R, Van Wye G. Measuring food deserts in New York City’s low-income neighborhoods. Health Place. 2011 Mar;17(2):696–700. doi: 10.1016/j.healthplace.2010.12.012. [DOI] [PubMed] [Google Scholar]
- 23.Auchincloss AH, Diez Roux AV, Brown DG, Erdmann CA, Bertoni AG. Neighborhood resources for physical activity and healthy foods and their association with insulin resistance. Epidemiology. 2008 Jan;19(1):146–157. doi: 10.1097/EDE.0b013e31815c480. [DOI] [PubMed] [Google Scholar]
- 24.Shigematsu R, Sallis JF, Conway TL, Saelens BE, Frank LD, Cain KL, et al. Age differences in the relation of perceived neighborhood environment to walking. Med Sci Sports Exerc. 2009 Feb;41(2):314–321. doi: 10.1249/MSS.0b013e318185496c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Christakis NA, Fowler JH. The spread of obesity in a large social network over 32 years. N Engl J Med. 2007 Jul 26;357(4):370–379. doi: 10.1056/NEJMsa066082. [DOI] [PubMed] [Google Scholar]
- 26.Hsiao PY, Mitchell DC, Coffman DL, Craig Wood G, Hartman TJ, Still C, et al. Dietary patterns and relationship to obesity-related health outcomes and mortality in adults 75 years of age or greater. J Nutr Health Aging. 2013;17(6):566–572. doi: 10.1007/s12603-013-0014-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lysy Z, Booth GL, Shah BR, Austin PC, Luo J, Lipscombe LL. The impact of income on the incidence of diabetes: a population-based study. Diabetes Res Clin Pract. 2013 Mar;99(3):372–379. doi: 10.1016/j.diabres.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 28.Sayer AA, Robinson SM, Patel HP, Shavlakadze T, Cooper C, Grounds MD. New horizons in the pathogenesis, diagnosis and management of sarcopenia. Age Ageing. 2013 Mar;42(2):145–150. doi: 10.1093/ageing/afs191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rosas-Carrasco O, Juarez-Cedillo T, Ruiz-Arregui L, Garcia Pena C, Vargas-Alarcon G, Sanchez-Garcia S. Overweight and obesity as markers for the evaluation of disease risk in older adults. J Nutr Health Aging. 2012 Jan;16(1):14–20. doi: 10.1007/s12603-012-0001-8. [DOI] [PubMed] [Google Scholar]
- 30.Szanton SL, Allen JK, Thorpe RJ, Jr, Seeman T, Bandeen-Roche K, Fried LP. Effect of financial strain on mortality in community-dwelling older women. J Gerontol B Psychol Sci Soc Sci. 2008 Nov;63(6):S369–74. doi: 10.1093/geronb/63.6.s369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chichlowska KL, Rose KM, Diez-Roux AV, Golden SH, McNeill AM, Heiss G. Individual and neighborhood socioeconomic status characteristics and prevalence of metabolic syndrome: the Atherosclerosis Risk in Communities (ARIC) Study. Psychosom Med. 2008 Nov;70(9):986–992. doi: 10.1097/PSY.0b013e318183a491. [DOI] [PMC free article] [PubMed] [Google Scholar]


