Abstract
Background/Objectives
Older adults with known diabetes are vulnerable to accelerated loss of lean body mass. However, the relationship of hyperglycemia per se with lean body mass is not fully understood. We sought to examine the independent relationship of hyperglycemia with relative lean body mass in older persons without a reported history of diabetes.
Design
Cross-sectional nationally representative survey.
Setting
United States.
Participants
We studied U.S. adults >50 years without known diabetes (n=5434) in the National Health and Nutrition Examination Survey (1999–2004).
Measurements
In linear regression models, we studied the relationship of measured HbA1c (<5.0%, 5.0–5.4%, 5.5–5.9%, 6.0–6.4%, ≥6.5%) with percent lean body mass, measured by dual-energy x-ray absorptiometry, after accounting for potential confounders.
Results
Among older U.S. men and women, progressively higher HbA1c was associated with relatively lower total, appendicular, and trunk percent lean mass, independent of demographics and height (all p<0.05). Accounting for physical activity, C-reactive protein, and diabetes-related comorbidities (heart disease, peripheral arterial disease, arthritis, neuropathy, hip fracture, amputation, cancer, pulmonary disease), undiagnosed diabetes (i.e. HbA1c ≥6.5%) versus reference (<5.0%) in both men and women was associated with lower total (−3.5±0.8% and −2.9±0.8%), appendicular (−1.8±0.5% and −1.2±0.4%), and trunk percent lean mass (−1.2±0.4% and −1.3±0.5%), respectively (all p<0.05). Persons at increased risk for diabetes (i.e. HbA1c 6.0–6.4%) also had significant decrements at these sites versus reference.
Conclusions
Hyperglycemia is associated with relatively lower lean mass in a nationally representative population of older adults without history of diabetes. Future longitudinal studies are needed to investigate the relationship of hyperglycemia with the accelerated decline of skeletal muscle mass in older persons.
Keywords: Hyperglycemia, muscle function, elderly, diabetes
Introduction
Older adults with diabetes are vulnerable to accelerated loss of skeletal muscle mass compared to adults without diabetes (1). Declines in lower extremity muscle mass, in particular, can be associated with decreased muscle strength and lead to muscle weakness, poor lower-extremity performance, and mobility loss, all of which have been reported in persons with diabetes, as well (2–4). However, the relationship of hyperglycemia per se to lower lean mass has not been fully investigated, yet this information is essential for the development of potentially novel therapeutic strategies to preserve muscle function for older adults in the future.
We have previously reported that longer diabetes duration is associated with proportionally larger decrements in muscle strength, muscle power, and gait speed (5). Nonetheless, it remains unclear if progressively elevated glucose levels are also associated with proportional declines in skeletal muscle mass, particularly in those who do not have a history of diagnosed diabetes. Insulin resistance as assessed using Homeostasis Model of Assessment – Insulin Resistance (HOMA-IR) has been associated with greater loss of total and appendicular lean mass among men without diabetes; in addition, insulin sensitizers may attenuate skeletal muscle loss in older men with diabetes (6–7). However, these previous studies were conducted in quite old adults (average age ~72 years) and the participants were all men. Because other studies suggest that women with diabetes may be at especially high-risk for loss of skeletal muscle mass (1), exploring associations of hyperglycemia with skeletal muscle mass in women is also important but has yet to be investigated.
In comparison to HOMA-IR, a surrogate measure of insulin resistance based upon fasting levels of glucose and insulin, hemoglobin A1c (HbA1c) reflects relatively long-term hyperglycemia over three months in both fasting and postprandial states and may be less subject to measurement error (8). Further, direct associations of hyperglycemia (measured by HbA1c) with skeletal muscle mass have not been previously described. In the present study, we sought to examine the following hypotheses: 1) progressively higher HbA1c levels are associated with relatively decreased skeletal muscle mass in both men and women; 2) persons with undiagnosed diabetes (i.e. HbA1c≥6.5%) have relatively lower lean mass compared to those without diabetes; 3) these associations are independent of potential confounders in a nationally representative population of older (>50 years) U.S. adults.
Methods
Study Design and Population
The National Health and Nutrition Examination Survey (NHANES) used a stratified multistage probability design to provide nationally representative estimates of the U.S. civilian non-institutionalized population (9). The present study was based on NHANES survey data from 1999–2004 which included assessment of dual-energy X-ray absorptiometry (DXA) in participants.
Of 7493 potential participants aged ≥50 years who were screened, 6776 participants attended the examination visit. Participants were not included in the study if they were pregnant (n=3), had highly variable data often due to equipment failure (n=183), or had data that could not be imputed for reasons such as amputation (n=37). The remaining 6553 participants had DXA measurements available (including n=4740 participants with DXA measured at all sites, n=1260 with DXA data for at least one or all regions imputed, and n=553 with all DXA data imputed). A further 3 participants with missing diabetes status and 1116 persons with known history of diabetes and/or use of insulin therapy were excluded, leaving 5434 participants for the present study.
Assessment of Diabetes and Hemoglobin A1c
Participants self-reported a physician diagnosis of diabetes and age of onset. Hemoglobin A1c (HbA1c) measurements were performed using Primus CLC330/CLC 385 (5) and were used to categorize participants without a reported history of diagnosed diabetes.
Measurement of Lean Body Mass (DXA)
The whole body dual-energy X-ray absorptiometry (DXA) examinations in NHANES were acquired according to the procedures recommended by the manufacturer on a QDR-4500A fan beam densitometer (Hologic, Inc., Bedford, MA). All subjects changed into paper gowns and were asked to remove all jewelry and other personal effects that could interfere with the DXA examination. The DXA examinations were reviewed and analyzed by the University of California, San Francisco Department of Radiology Bone Density Group using industry standard techniques. Analysis of all examinations was performed using Hologic Discovery software version 12.1. Examinations that contained artifacts which could affect the accuracy of the DXA results, such as prosthetic devices, implants or other extraneous objects had the regional and global DXA results for these examinations set to missing in the dataset. The precision of the DXA instrument used in the NHANES study has been reported previously and measurements for lean body mass from DXA instruments used in the NHANES survey were calibrated as described (10).
Fat mass and lean mass not including bone mineral content (BMC) measurements were available for a number of predefined anatomical regions. All measures of lean body mass (kg) were divided by total body weight (kg) and multiplied by 100% to give standardized estimates of % lean mass, similar to previous authors (2), to better account for lean mass relative to body size.
Measurement of Covariates
Demographics and smoking was ascertained from the questionnaire. Height and weight were measured. Participants were asked if they did “any physical activities specifically designed to strengthen their muscles such as lifting weights, push-ups or sit-ups” over the past 30 days. High sensitivity C-reactive protein (CRP) was measured using Behring Nephelometer (11).
History of comorbidities including arthritis, cardiovascular disease (CVD), chronic obstructive pulmonary disease (COPD), and cancer (excluding non-melanoma skin cancer), and hip fracture was self-reported. Peripheral arterial disease (PAD) using ankle-brachial index and peripheral neuropathy using monofilament testing were defined as previously described (12). Lower extremity amputations were documented.
Statistical Analyses
All analyses were performed using SAS software (version 9.3, SAS Institute Inc, Cary, NC) and incorporated population-based sampling weights to obtain unbiased, nationally representative estimates from the complex NHANES sampling design (9). Our analysis used the DXA data sets released by NHANES on the Center for Disease Control website: http://www.cdc.gov/nchs/about/major/nhanes/dxx/dxa.htm. To prevent bias in the survey due to the fact that the missing data was not completely at random, missing data was multiply imputed at the National Center for Health Statistics as described in the technical documentation available on the above referenced website. Five complete records containing valid and/or imputed values were created for each survey participant to allow the assessment of variability due to imputation. For subgroup analysis, the DOMAIN statement was used to provide reliable estimates for survey data (13).
The analysis of variance (ANOVA) test for continuous variables and Rao-Scott chi-square test for categorical variables was used to compare differences in baseline characteristics by HbA1c categories for men as follows: <5.0% (n=255), 5.0–5.4% (n=1028), 5.5–5.9% (n=1022), 6.0–6.4% (n=224), ≥6.5% (n=135), and women as follows: <5.0% (n=210), 5.0–5.4% (n=1161), 5.5–5.9% (n=1073), 6.0–6.4% (n=224), ≥6.5% or undiagnosed diabetes (n=102). The HbA1c categories were chosen similar to previous studies exploring associations of hyperglycemia with other adverse complications (14). We included participants with undiagnosed diabetes in our study to better explore the implications of uncontrolled hyperglycemia on skeletal muscle mass.
The relationship of progressively higher HbA1c categories to % lean body mass at different sites (total, appendicular, trunk) was graphically explored, adjusted for age, race, education, smoking, and height. In these analyses, p-values for trend across higher HbA1c categories are shown.
Linear regression models were created to characterize the association of higher HbA1c categories versus reference (<5.0%) with % lean mass at different sites, after accounting for key covariates including: Model 1: demographic factors (age, race/ethnicity, education, smoking history) + height and Model 2: adjusted for physical activity + CRP + comorbidities (self-reported CVD, PAD, arthritis, hip fracture, lower extremity amputation, cancer, COPD, peripheral neuropathy). We chose to adjust for these covariates given their role as potential confounders of the association between hyperglycemia and muscle outcomes (5). Since higher body-mass index may be due to relatively greater fat or lean mass, we chose instead to standardize lean mass to body weight in our outcome similar to other authors, and adjust for height (2).
Data are shown as mean ± standard error (SE) unless otherwise indicated. A p-value of less than 0.05 was considered statistically significant.
Results
Among U.S. men and women aged 50 years and older (Tables 1A and 1B), those participants in higher HbA1c categories were generally more likely to be older, non-White, less educated, and physically inactive compared to their counterparts with lower HbA1c levels. Body weight was greater, as was prevalence of neuropathy, among those persons in higher versus lower HbA1c categories. Total, appendicular, and trunk lean mass were all significantly different in both men and women by HbA1c categories (both p<0.001). Percent body fat also significantly differed in men and women according to HbA1c categories (p<0.001).
Table 1.
| A: Demographic and clinical characteristics of male U.S. adults (>50 years) without a history of known diabetes (n=2664)*
| |||||||
|---|---|---|---|---|---|---|---|
| All | HbA1c < 5% |
HbA1c 5–5.4% |
HbA1c 5.5–5.9% |
HbA1c 6.0–6.4% |
HbA1c ≥ 6.5% |
p-value | |
| Demographics | |||||||
| Mean Age (years) | 62.7 ± 0.2 | 62.7 ± 0.8 | 61.6 ± 0.4 | 63.4 ± 0.4 | 65.9 ± 0.7 | 63.5 ± 1.2 | < 0.001 |
| Age (years) | < 0.001 | ||||||
| 50–59 | 47.2 ± 1.2 | 46.1 ± 4.7 | 53.0 ± 2.0 | 43.5 ± 2.0 | 34.7 ± 4.0 | 40.1 ± 5.8 | |
| 60–69 | 26.2 ± 0.9 | 27.1 ± 3.2 | 23.8 ± 1.6 | 27.7 ± 1.5 | 27.1 ± 3.1 | 34.5 ± 5.3 | |
| 70–79 | 18.2 ± 0.7 | 18.2 ± 3.0 | 16.2 ± 1.2 | 19.5 ± 1.4 | 25.8 ± 3.1 | 15.9 ± 2.7 | |
| 80+ | 8.4 ± 0.5 | 8.6 ± 1.6 | 7.0 ± 0.7 | 9.3 ± 0.7 | 12.4 ± 2.1 | 9.5 ± 1.9 | |
| Race (%) | < 0.001 | ||||||
| Black | 7.4 ± 0.8 | 17.4 ± 2.5 | 3.6 ± 0.6 | 8.0 ± 0.9 | 16.3 ± 2.4 | 8.5 ± 1.9 | |
| White | 81.6 ± 1.7 | 70.7 ± 2.7 | 86.9 ± 1.6 | 81.9 ± 2.0 | 64.8 ± 4.7 | 71.7 ± 4.6 | |
| Mex-American | 3.4 ± 0.6 | 2.1 ± 0.5 | 3.1 ± 0.6 | 3.5 ± 0.7 | 5.5 ± 1.3 | 5.4 ± 0.8 | |
| Other | 7.6 ± 1.3 | 9.8 ± 2.4 | 6.4 ± 1.2 | 6.6 ± 1.3 | 13.4 ± 3.8 | 14.4 ± 4.7 | |
| Education | |||||||
| > High school (%) | 54.2 ± 1.8 | 55.3 ± 5.7 | 60.3 ± 2.0 | 49.7 ± 2.3 | 45.3 ± 4.0 | 41.2 ± 4.7 | < 0.001 |
| Questionnaire, physical examination, and laboratory measures | |||||||
| Physically active (%)† | 21.2 ± 1.4 | 25.4 ± 4.3 | 23.7 ± 2.2 | 18.5 ± 1.8 | 16.3 ± 3.2 | 17.1 ± 4.4 | < 0.001 |
| Mean weight (kg) | 85.9 ± 0.4 | 80.1 ± 1.1 | 84.7 ± 0.6 | 87.4 ± 0.6 | 89.0 ± 1.9 | 93.2 ± 1.3 | < 0.001 |
| Mean height (cm) | 175.0 ± 0.2 | 174.0 ± 0.5 | 175.9 ± 0.3 | 174.8 ± 0.3 | 173.2 ± 0.7 | 172.3 ± 0.8 | < 0.001 |
| Neuropathy (%) | 12.6 ± 0.7 | 9.6 ± 2.2 | 12.5 ± 1.4 | 11.8 ± 1.0 | 20.0 ± 2.7 | 17.3 ± 4.0 | < 0.001 |
| Mean CRP (mg/dl) | 0.4 ± 0.0 | 0.3 ± 0.0 | 0.3 ± 0.0 | 0.5 ± 0.0 | 0.4 ± 0.0 | 0.7 ± 0.1 | < 0.001 |
| Body composition measures | |||||||
| Total lean mass (%)** | 67.4 ± 1.2 | 68.6 ± 3.9 | 68.1 ± 1.3 | 66.9 ± 1.8 | 65.7 ± 2.0 | 65.0 ± 3.2 | < 0.001 |
| Appendicular lean mass (%)** | 29.4 ± 1.0 | 30.1 ± 2.8 | 29.8 ± 1.3 | 29.1 ± 1.2 | 28.4 ± 2.0 | 27.9 ± 2.2 | < 0.001 |
| Trunk lean mass (%)** | 34.0 ± 1.4 | 34.3 ± 3.5 | 34.2 ± 1.5 | 33.8 ± 1.6 | 33.4 ± 1.7 | 33.3 ± 2.4 | < 0.001 |
| Total body fat (%)** | 30.0 ± 0.2 | 28.7 ± 0.6 | 29.3 ± 0.3 | 30.6 ± 0.3 | 31.8 ± 0.4 | 32.5 ± 0.6 | < 0.001 |
| B: Demographic and clinical characteristics of female U.S. adults (>50 years) without a history of known diabetes (n=2770)*
| |||||||
|---|---|---|---|---|---|---|---|
| All | HbA1c < 5% |
HbA1c 5–5.4% |
HbA1c 5.5–5.9% |
HbA1c 6.0–6.4% |
HbA1c ≥ 6.5% |
p-value | |
| Demographics | |||||||
| Mean Age (years) | 64.2 ± 0.2 | 63.3 ± 0.8 | 63.2 ± 0.4 | 65.5 ± 0.4 | 64.8 ± 0.7 | 66.2 ± 0.9 | < 0.001 |
| Age (years) | < 0.001 | ||||||
| 50–59 | 41.1 ± 1.0 | 44.5 ± 3.8 | 45.4 ± 1.8 | 37.3 ± 2.0 | 31.9 ± 3.5 | 33.3 ± 6.6 | |
| 60–69 | 26.4 ± 1.2 | 27.4 ± 3.1 | 25.9 ± 1.7 | 25.5 ± 1.9 | 33.7 ± 3.7 | 26.5 ± 4.9 | |
| 70–79 | 20.8 ± 0.8 | 15.3 ± 3.0 | 18.9 ± 1.4 | 23.4 ± 1.5 | 24.3 ± 3.2 | 25.3 ± 5.7 | |
| 80+ | 11.7 ± 0.7 | 12.8 ± 2.5 | 9.8 ± 0.8 | 13.8 ± 1.0 | 10.1± 2.0 | 14.8 ± 2.0 | |
| Race (%) | < 0.001 | ||||||
| Black | 8.1 ± 1.0 | 11.2 ± 1.6 | 5.1 ± 0.8 | 8.8 ± 1.2 | 17.0 ± 3.1 | 21.4 ± 3.5 | |
| White | 80.1 ± 1.7 | 76.7 ± 2.9 | 86.2 ± 1.3 | 77.6 ± 2.5 | 63.5 ± 3.6 | 55.9 ± 7.2 | |
| Mex-American | 3.1 ± 0.6 | 3.0 ± 0.9 | 2.6 ± 0.5 | 3.6 ± 0.7 | 3.2 ± 0.8 | 4.9 ± 1.8 | |
| Other | 8.7 ± 1.3 | 9.1 ± 2.6 | 6.1 ± 1.0 | 10.0 ± 1.9 | 16.3 ± 3.3 | 17.8 ± 5.7 | |
| Education | |||||||
| > High school (%) | 47.0 ± 1.5 | 47.4 ± 2.9 | 51.7 ± 2.0 | 43.5 ± 2.3 | 36.6 ± 3.1 | 35.0 ± 5.4 | < 0.001 |
| Questionnaire, physical examination, and laboratory measures | |||||||
| Physically active (%)† | 18.7 ± 1.2 | 19.4 ± 3.7 | 21.7 ± 1.6 | 16.3 ± 1.6 | 11.7 ± 1.9 | 12.7 ± 4.8 | < 0.001 |
| Mean weight (kg) | 72.9 ± 0.5 | 69.2 ± 1.2 | 70.3 ± 0.6 | 75.3 ± 0.6 | 78.2 ± 1.1 | 82.3 ± 1.6 | < 0.001 |
| Mean height (cm) | 160.7 ± 0.2 | 160.4 ± 0.4 | 161.4 ± 0.2 | 160.1 ± 0.3 | 159.1 ± 0.5 | 160.4 ± 0.6 | < 0.001 |
| Neuropathy (%) | 7.1 ± 0.5 | 5.1 ± 1.4 | 6.2 ± 0.8 | 8.3 ± 0.9 | 8.8 ± 2.9 | 7.0 ± 2.2 | < 0.001 |
| Mean CRP (mg/dl) | 0.5 ± 0.0 | 0.5 ± 0.1 | 0.4 ± 0.0 | 0.6 ± 0.0 | 0.7 ± 0.1 | 0.8 ± 0.1 | < 0.001 |
| Body composition measures | |||||||
| Total lean mass (%) ** | 56.0 ± 1.5 | 57.1 ± 4.2 | 56.8 ± 1.8 | 55.3 ± 1.6 | 54.4 ± 4.0 | 54.0 ± 5.6 | < 0.001 |
| Appendicular lean mass (%) ** | 23.1 ± 0.8 | 23.5 ± 2.8 | 23.4 ± 1.2 | 22.8 ± 0.7 | 22.8 ± 2.8 | 22.4 ± 3.8 | < 0.001 |
| Trunk lean mass (%) ** | 28.9 ± 1.4 | 29.4 ± 3.7 | 29.3 ± 1.5 | 28.5 ± 1.5 | 27.9 ± 3.5 | 27.9 ± 4.5 | < 0.001 |
| Total fat mass (%)** | 41.7 ± 0.2 | 40.6 ± 0.6 | 40.9 ± 0.3 | 42.5 ± 0.2 | 43.4 ± 0.5 | 44.0 ± 0.7 | < 0.001 |
Mean values ± SE are shown unless indicated.
DEXA reported values (kg) were divided by body weight (kg) to give % body weight.
Any self-reported muscle strengthening exercises such as lifting weights, push-ups or sit-ups over the past 30 days.
Mean values ± SE are shown unless indicated.
DEXA reported values (kg) were divided by body weight (kg) to give % body weight.
Any self-reported muscle strengthening exercises such as lifting weights, push-ups or sit-ups over the past 30 days.
Figure 1 shows the association of HbA1c category with relative lean mass after adjustment for age, race, education, smoking, and height. Among older men, progressively higher HbA1c categories were associated with significantly lower percent lean mass at the total body (p-value for trend=0.005; Figure 1A), appendicular (p=0.009; Figure 1C), and trunk sites (p-value for trend=0.004; Figure 1E). Among older women, higher HbA1c categories were also associated with significantly lower percent lean mass at the total body (p-value for trend=0.005; Figure 1B), appendicular (p=0.003; Figure 1D), and trunk sites (p-value for trend=0.02; Figure 1F). For both men and women, when HbA1c categories 5.5–5.9%, 6.0– 6.4%, and ≥ 6.5% were each compared to HbA1c <5.0% in Figure 1, the pair-wise statistical tests were significant for percent lean mass at the total body, appendicular, and trunk sites after adjusting for multiple comparisons (all p<0.001).
Figure 1.
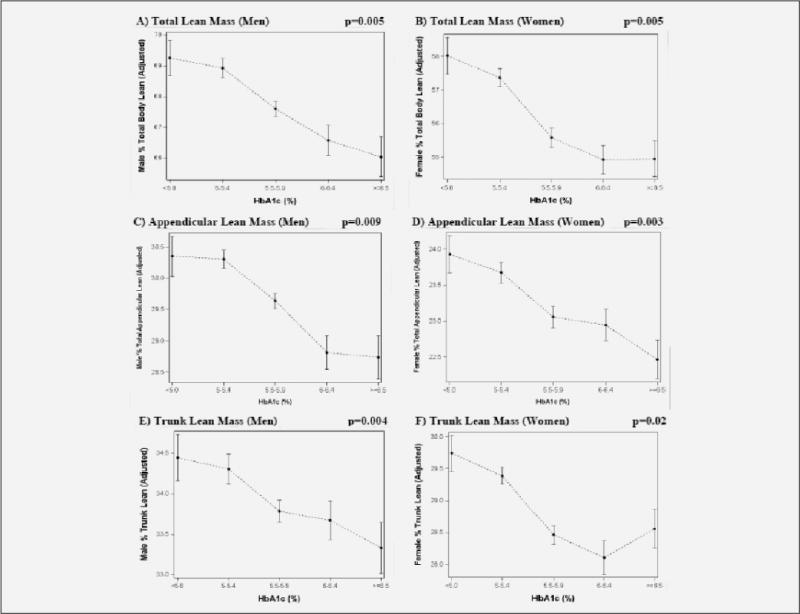
Association of HbA1c category with relatively lower lean body mass in older U.S. adults
Figure 1 shows the significant, negative relationship across higher hemoglobin A1c categories of total percent lean mass in men (1A, p-value for trend=0.005) and women (1B, p-value for trend=0.005), appendicular percent lean mass in men (1C, p-value for trend=0.009) and women (1D, p-value for trend=0.003); and trunk percent lean mass in men (1E, p-value for trend=0.004) and women (1F, p-value for trend=0.02) after adjustment for age, race, education, smoking, and height.
Linear regression models among older U.S. men (Table 2) demonstrated that higher HbA1c categories (5.5–5.9%, 6.0– 6.4%, ≥6.5%) versus reference (<5.0%) were associated with significantly lower percent lean mass at the total body, appendicular, and trunk sites after accounting for age, race, education, smoking, and height (Model 1). After further accounting for physical activity, CRP, and diabetes-related comorbidities in the fully adjusted model (Model 2), these results were minimally changed. In particular, those with undiagnosed diabetes (HbA1c ≥6.5%) versus reference (HbA1c<5.0%) had significantly lower percent lean mass at the total body (−3.5 ± 0.8%, p<0.001), appendicular (−1.8 ± 0.5%; p=0.001), and trunk sites (−1.2 ± 0.4%, p=0.003). Older men with HbA1c levels between 5.5–5.9% or 6.0–6.4% also had significantly lower percent lean mass at these sites compared to reference in fully adjusted models.
Table 2.
Linear regression models for the association of HbA1c category with percent lean body mass among older U.S. adults, NHANES 1999–2004*
| Men | Women | |
|---|---|---|
| Total percent lean mass | ||
| Model 1** | ||
| HbA1c <5.0% | Ref | Ref |
| HbA1c 5.0 – 5.4% | −0.3 ± 0.6 | −0.6 ± 0.5 |
| HbA1c 5.5 – 5.9% | −1.7 ± 0.6† | −2.4 ± 0.5‡ |
| HbA1c 6.0 – 6.4% | −2.7 ± 0.8† | −3.1 ± 0.8‡ |
| HbA1c ≥ 6.5% | −3.2 ± 0.9‡ | −3.1 ± 0.7‡ |
| Model 2** | ||
| HbA1c <5.0% | Ref | |
| HbA1c 5.0 – 5.4% | −0.7 ± 0.6 | −1.0 ± 0.6 |
| HbA1c 5.5 – 5.9% | −2.0 ± 0.6† | −2.4 ± 0.6‡ |
| HbA1c 6.0 – 6.4% | −2.9 ± 0.7‡ | −3.0 ± 0.9‡ |
| HbA1c ≥ 6.5% | −3.5 ± 0.8‡ | −2.9 ± 0.8‡ |
| Appendicular percent lean mass | ||
| Model 1** | ||
| HbA1c <5.0% | Ref | Ref |
| HbA1c 5.0 – 5.4% | −0.1 ± 0.3 | −0.2 ± 0.3 |
| HbA1c 5.5 – 5.9% | −0.7 ± 0.3† | −0.9 ± 0.3‡ |
| HbA1c 6.0 – 6.4% | −1.5 ± 0.4‡ | −1.0 ± 0.4† |
| HbA1c ≥ 6.5% | −1.6 ± 0.5† | −1.5 ± 0.4‡ |
| Model 2** | ||
| HbA1c <5.0% | Ref | Ref |
| HbA1c 5.0 – 5.4% | −0.3 ± 0.3 | −0.3 ± 0.3 |
| HbA1c 5.5 – 5.9% | −0.9 ± 0.4† | −0.7 ± 0.3† |
| HbA1c 6.0 – 6.4% | −1.7 ± 0.4‡ | −0.8 ± 0.4† |
| HbA1c ≥ 6.5% | −1.8 ± 0.5‡ | −1.2 ± 0.4† |
| Trunk percent lean mass | ||
| Model 1** | ||
| HbA1c <5.0% | Ref | Ref |
| HbA1c 5.0 – 5.4% | −0.1 ± 0.3 | −0.3 ± 0.3 |
| HbA1c 5.5 – 5.9% | −0.7 ± 0.2† | −1.3 ± 0.3‡ |
| HbA1c 6.0 – 6.4% | −0.8 ± 0.4 | −1.6 ± 0.4‡ |
| HbA1c ≥ 6.5% | −1.1 ± 0.4† | −1.2 ± 0.4† |
| Model 2** | ||
| HbA1c <5.0% | Ref | Ref |
| HbA1c 5.0 – 5.4% | −0.2 ± 0.3 | −0.6 ± 0.3 |
| HbA1c 5.5 – 5.9% | −0.7 ± 0.3† | −1.4 ± 0.3‡ |
| HbA1c 6.0 – 6.4% | −0.8 ± 0.3† | −1.7 ± 0.5‡ |
| HbA1c ≥ 6.5% | −1.2 ± 0.4† | −1.3 ± 0.5† |
Beta coefficients ± SE are shown
Model 1: demographics (age, race, education, and smoking) + height; Model 2: Model 1 + physical activity + CRP + comorbidities (self-reported CHD, PAD, arthritis, neuropathy, hip fracture, lower extremity amputation, cancer, COPD)
p<0.05 compared to reference group HbA1c <5.0%
p≤0.001 compared to reference group HbA1c <5.0%
Among older U.S. women (Table 2), higher HbA1c categories (5.5–5.9%, 6.0–6.4%, ≥6.5%) versus reference (<5.0%) were also associated with significantly lower percent lean mass at the total body, appendicular, and trunk sites after accounting for age, race, education, smoking, and height (Model 1). After further accounting for physical activity, CRP, and diabetes-related comorbidities in the fully adjusted model (Model 2), these results were minimally changed. In particular, those with undiagnosed diabetes (i.e. HbA1c ≥6.5%) versus (HbA1c<5.0%) had lower percent lean mass at the total body (− 2.9 ± 0.8%, p=0.001), appendicular (−1.2 ± 0.4%; p=0.003), and trunk sites (−1.3 ± 0.5%, p=0.01). Older women with HbA1c levels between 5.5–5.9% or 6.0–6.4% also had significantly lower percent lean mass at these sites compared to reference in fully adjusted models.
Discussion
Among older U.S. men and women without known history of diabetes, hyperglycemia was independently associated with decreased total, appendicular, and trunk lean mass relative to body size and after adjusting for confounders such as demographics, height, inflammation, physical activity, and neuropathy. Of note, those with undiagnosed diabetes had ~ 3– 4% comparatively lower total lean body mass than those with normal glucose levels. These findings raise the intriguing hypothesis that hyperglycemia may represent a modifiable factor associated with lower skeletal muscle mass in older adults, and is potentially related to the increased burden of disability previously reported in persons with diabetes (12).
Our findings are similar to previous groups that described the association of insulin resistance as assessed by fasting HOMA-IR with greater loss of total and appendicular lean mass in men without diabetes (6). However, in contrast, we directly assessed hyperglycemia using HbA1c which reflects relatively longer-term fasting and post-prandial glycemic exposure (8). Our study adds to previous studies by demonstrating that hyperglycemia, itself, is also associated with relatively lower total and appendicular lean mass in older adults without a known history of diabetes. In comparison to studies that examined associations of insulin resistance with lower skeletal muscle mass only in men, we examined associations of hyperglycemia with relatively lower skeletal muscle mass in both men and women and found similar findings by gender.
Similar to other authors (1), we further report that persons with undiagnosed diabetes have relatively lower lean mass compared to those without diabetes. While previous authors used a 75-gram oral glucose tolerance test to characterize glucose status, we used HbA1c which is a relatively newer criterion to identify undiagnosed diabetes (15). Interestingly, previous authors found that persons with undiagnosed diabetes had the greatest loss of skeletal muscle mass, more so than those with diagnosed diabetes (1), suggesting potential protective effects of glucose-lowering treatments but this requires further investigation. We extend these findings in our study by demonstrating that progressively higher hyperglycemia is related to proportionately greater loss of skeletal muscle mass, a novel finding.
Hyperglycemia may be related to relatively decreased skeletal muscle mass through multiple pathways. Potential explanations include the relationship of hyperglycemia with elevated inflammatory factors, decreased physical activity and comorbidities such as neuropathy to the loss of skeletal muscle mass (16–19). However, we found that associations remained independent of these potential confounders in our study. Further, insulin resistance is related to decreased muscle protein synthesis and disinhibition of muscle protein breakdown (20), and may indirectly relate to the lower lean mass we observed among men and women with hyperglycemia in our study. It is possible that persons with hyperglycemia have higher relative body fat which is also connected to the loss of skeletal muscle mass, however, fat mass gain and lean mass are distinct processes not necessarily present to the same degree (6) and require further exploration in longitudinal studies. In addition, glucose may have a direct toxic effect on skeletal muscle mitochondrial activity (21), leading to lower skeletal muscle function. Through multiple pathways, hyperglycemia may be linked to lower lean body mass in older adults.
The presence of relatively lower lean body mass in older adults likely has wide-ranging clinical implications. Previous authors have demonstrated that small changes in skeletal muscle mass are associated with poorer lower extremity performance in older adults (3). Lower skeletal muscle mass may also be associated with an increased risk of mortality (22, 23) but studies are mixed (24). Thus, hyperglycemia may represent a potentially modifiable factor associated with lower skeletal muscle mass that could be targeted to preserve functional status in the future but requires further investigation.
Strengths of our study include the use of a nationally representative U.S. population of both men and women, allowing more directly for generalizability. NHANES also has rigorous and well-documented protocols that include multiply imputed data sets for persons with missing DXA results resulting in a comprehensive data set for analysis. We were also able to more specifically examine the association of hyperglycemia with lean mass given the availability of HbA1c on a large population of older U.S. adults in NHANES that were not on glucose-lowering therapies (i.e. including those with undiagnosed diabetes).
Limitations include the cross-sectional design of our study which limits inferences on temporality. Indeed, the reverse association of relatively low skeletal muscle mass with insulin resistance has been described (25). A cycle may occur in which hyperglycemia leads to low muscle mass, resulting in decreased surface area for glucose uptake, which then further exacerbates hyperglycemia and continues the cycle. However, in the present study, we were interested primarily in hyperglycemia as a potentially modifiable factor related to the outcome of lower lean mass that could be further explored in future intervention studies. We were also interested in exploring the associations of hyperglycemia with lean body mass relative to body size (i.e. percent lean mass); the relationship with absolute lean mass may be distinct. Also, persons with undiagnosed diabetes could have been misclassified into lower HbA1c categories given the lack of oral glucose tolerance testing information available in NHANES, though previous studies have shown reasonable agreement of HbA1c with fasting glucose for diagnosis of diabetes (15, 26). However, this possible misclassification would have biased our findings towards the null, yet we still observed significant associations of lower lean mass across higher categories of HbA1c. Lastly, although screening for peripheral neuropathy with monofilament is useful clinically (27), this test may not be as sensitive as nerve conduction velocities to detect neuropathy, and could have led to residual confounding in our study. Of note, peripheral neuropathy can also be present in those with prediabetes (19).
In summary, our study demonstrates the relationship of progressive hyperglycemia with proportional decrements in skeletal muscle mass among older adults without a known history of diabetes, relative to body size and adjusting for confounders. These findings may be related to the subsequent declines in muscle strength, functional limitations, and physical disability previously reported in persons with diabetes (12). In contrast to studies that report a U-shaped association between HbA1c and other adverse outcomes such as mortality (14), we found a linear association of HbA1c with comparatively lower lean mass in older adults. However, symptoms of hypoglycemia may also occur at relatively higher glucose levels in older patients with diabetes and the relationship to our findings is unclear (28). In addition, those with undiagnosed diabetes had significantly lower lean mass compared to participants with normal glucose status, in both men and women. Further longitudinal studies are needed to confirm the direction of these associations, and explore the potential role of glucose-lowering therapies in preservation of lean mass in older adults.
Acknowledgments
Funding: This study was supported by the National Institute of Diabetes and Digestive and Kidney Diseases (K23-DK093583, K24-DK062222, P60-DK079637), The Johns Hopkins Older Americans Independence Center (P30-AG021334), and the intramural research program of the National Institute on Aging.
Footnotes
Conflict of Interest Disclosures: No
Author Contributions: Drs. Kalyani, Egan, Ferrucci, and Brancati contributed to the study concept and design, methods, data analysis and interpretation of data, and preparation of the manuscript. Drs. Kalyani and Tra contributed to data acquisition. Dr. Tra also contributed to study methods, data analysis, and interpretation of data.
Sponsor’s Role: None.
References
- 1.Park SW, Goodpaster BH, Lee JS, et al. Health, Aging, and Body Composition Study Excessive loss of skeletal muscle mass in older adults with type 2 diabetes. Diabetes Care. 2009;32:1993–7. doi: 10.2337/dc09-0264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Janssen I, Heymsfield SB, Ross R. Low relative skeletal muscle mass (sarcopenia) in older persons is associated with functional impairment and physical disability. J Am Geriatr Soc. 2002;50:889–96. doi: 10.1046/j.1532-5415.2002.50216.x. [DOI] [PubMed] [Google Scholar]
- 3.Visser M, Kritchevsky SB, Goodpaster BH, et al. Leg muscle mass and composition in relation to lower extremity performance in men and women aged 70 to 79: the health, aging and body composition study. J Am Geriatr Soc. 2002;50:897–904. doi: 10.1046/j.1532-5415.2002.50217.x. [DOI] [PubMed] [Google Scholar]
- 4.Park SW, Goodpaster BH, Strotmeyer ES, et al. Health, Aging, and Body Composition Study Decreased muscle strength and quality in older adults with type 2 diabetes: the health, aging, and body composition study. Diabetes. 2006;55:1813–8. doi: 10.2337/db05-1183. [DOI] [PubMed] [Google Scholar]
- 5.Kalyani RR, Tra Yolande, Yeh HC, et al. Quadriceps strength, quadriceps power, and gait speed in older U.S. adults with diabetes: results from the National Health and Nutrition Examination Survey (NHANES), 1999–2002. J Am Geriatr Soc. 2013;61:769–75. doi: 10.1111/jgs.12204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee CG, Boyko EJ, Strotmeyer ES, et al. Association between insulin resistance and lean mass loss and fat mass gain in older men without diabetes mellitus. J Am Geriatr Soc. 2011;59:1217–24. doi: 10.1111/j.1532-5415.2011.03472.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee CG, Boyko EJ, Barrett-Connor E, et al. Osteoporotic Fractures in Men (MrOS) Study Research Group Insulin sensitizers may attenuate lean mass loss in older men with diabetes. Diabetes Care. 2011;34:2381–2386. doi: 10.2337/dc11-1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Selvin E, Crainiceanu CM, Brancati FL, et al. Short-term variability in measures of glycemia and implications for the classification of diabetes. Arch Intern Med. 2007 Jul 23;167:1545–1551. doi: 10.1001/archinte.167.14.1545. [DOI] [PubMed] [Google Scholar]
- 9.National Center for Health Statistics, Center for Disease Control and Prevention, National Health and Nutrition Examination Survey (NHANES) Questionnaire, Exam Protocol, and Analytic Guidelines [on-line] Available at: http://www.cdc.gov/nchs/about/major/nhanes/datalink.htm. Accessed 9 April 2012.
- 10.Kelly TL, Wilson KE, Heymsfield SB. Dual energy X-Ray absorptiometry body composition reference values from NHANES. PLoS One. 2009;4:e7038. doi: 10.1371/journal.pone.0007038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Malik S, Wong ND, Franklin S, et al. Cardiovascular disease in U.S. patients with metabolic syndrome, diabetes, and elevated C-reactive protein. Diabetes Care. 2005;28:690–693. doi: 10.2337/diacare.28.3.690. [DOI] [PubMed] [Google Scholar]
- 12.Kalyani RR, Saudek CD, Brancati FL, et al. The association of diabetes, comorbidities, and hemoglobin A1c with functional disability in older adults: results from the National Health and Nutrition Examination Survey (NHANES), 1999–2006. Diabetes Care. 2010 May;33:1055–1060. doi: 10.2337/dc09-1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.SAS Institute Inc. SAS/STAT 9.2 User’s Guide. North Carolina: SAS Institute Inc; 2008. Chapter 83: The SURVEYFREQ Procedure; pp. 6286–6364. [Google Scholar]
- 14.Selvin E, Steffes MW, Zhu H, et al. Glycated hemoglobin, diabetes, and cardiovascular risk in nondiabetic adults. N Engl J Med. 2010;362:800–811. doi: 10.1056/NEJMoa0908359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Standards of medical care in diabetes–2013. Diabetes Care. 2013;36(Suppl 1):S11–66. doi: 10.2337/dc13-S011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schaap LA, Pluijm SM, Deeg DJ, et al. Health ABC Study Higher inflammatory marker levels in older persons: associations with 5-year change in muscle mass and muscle strength. J Gerontol A Biol Sci Med Sci. 2009;64:1183–9. doi: 10.1093/gerona/glp097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kumar V, Atherton P, Smith K, et al. Human muscle protein synthesis and breakdown during and after exercise. J Appl Physiol. 2009;106:2026–39. doi: 10.1152/japplphysiol.91481.2008. [DOI] [PubMed] [Google Scholar]
- 18.Andersen H, Gjerstad MD, Jakobsen J. Atrophy of foot muscles: a measure of diabetic neuropathy. Diabetes Care. 2004;27:2382–5. doi: 10.2337/diacare.27.10.2382. [DOI] [PubMed] [Google Scholar]
- 19.Rajabally YA. Neuropathy and impaired glucose tolerance: an updated review of the evidence. Acta Neurol Scand. 2011;124:1–8. doi: 10.1111/j.1600-0404.2010.01425.x. [DOI] [PubMed] [Google Scholar]
- 20.Halvatsiotis P, Short KR, Bigelow M, et al. Synthesis rate of muscle proteins, muscle functions, and amino acid kinetics in type 2 diabetes. Diabetes. 2002;51:2395–404. doi: 10.2337/diabetes.51.8.2395. [DOI] [PubMed] [Google Scholar]
- 21.Kelley DE, He J, Menshikova EV, et al. Dysfunction of mitochondria in human skeletal muscle in type 2 diabetes. Diabetes. 2002;51:2944–50. doi: 10.2337/diabetes.51.10.2944. [DOI] [PubMed] [Google Scholar]
- 22.Wijnhoven HA, Snijder MB, van Bokhorst-de van der Schueren MA, et al. Region-specific fat mass and muscle mass and mortality in community-dwelling older men and women. Gerontology. 2012;58:32–40. doi: 10.1159/000324027. [DOI] [PubMed] [Google Scholar]
- 23.Szulc P, Munoz F, Marchand F, et al. Rapid loss of appendicular skeletal muscle mass is associated with higher all-cause mortality in older men: the prospective MINOS study. Am J Clin Nutr. 2010;91:1227–36. doi: 10.3945/ajcn.2009.28256. [DOI] [PubMed] [Google Scholar]
- 24.Newman AB, Kupelian V, Visser M, et al. Strength, but not muscle mass, is associated with mortality in the health, aging and body composition study cohort. J Gerontol A Biol Sci Med Sci. 2006;61:72–7. doi: 10.1093/gerona/61.1.72. [DOI] [PubMed] [Google Scholar]
- 25.Srikanthan P, Karlamangla AS. Relative muscle mass is inversely associated with insulin resistance and prediabetes. Findings from the third National Health and Nutrition Examination Survey. J Clin Endocrinol Metab. 2011;96:2898–903. doi: 10.1210/jc.2011-0435. [DOI] [PubMed] [Google Scholar]
- 26.Carson AP, Reynolds K, Fonseca VA, et al. Comparison of A1C and fasting glucose criteria to diagnose diabetes among U.S. adults. Diabetes Care. 2010;33:95–7. doi: 10.2337/dc09-1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Perkins BA, Olaleye D, Zinman B, et al. Simple screening tests for peripheral neuropathy in the diabetes clinic. Diabetes Care. 2001;24:250–256. doi: 10.2337/diacare.24.2.250. [DOI] [PubMed] [Google Scholar]
- 28.Abdelhafiz AH, Bailey C, Eng Loo B, Sinclair A. Hypoglycaemic symptoms and hypoglycaemia threshold in older people with diabetes-a patient perspective. J Nutr Health Aging. 2013;10:899–802. doi: 10.1007/s12603-013-0351-x. [DOI] [PubMed] [Google Scholar]


