Abstract
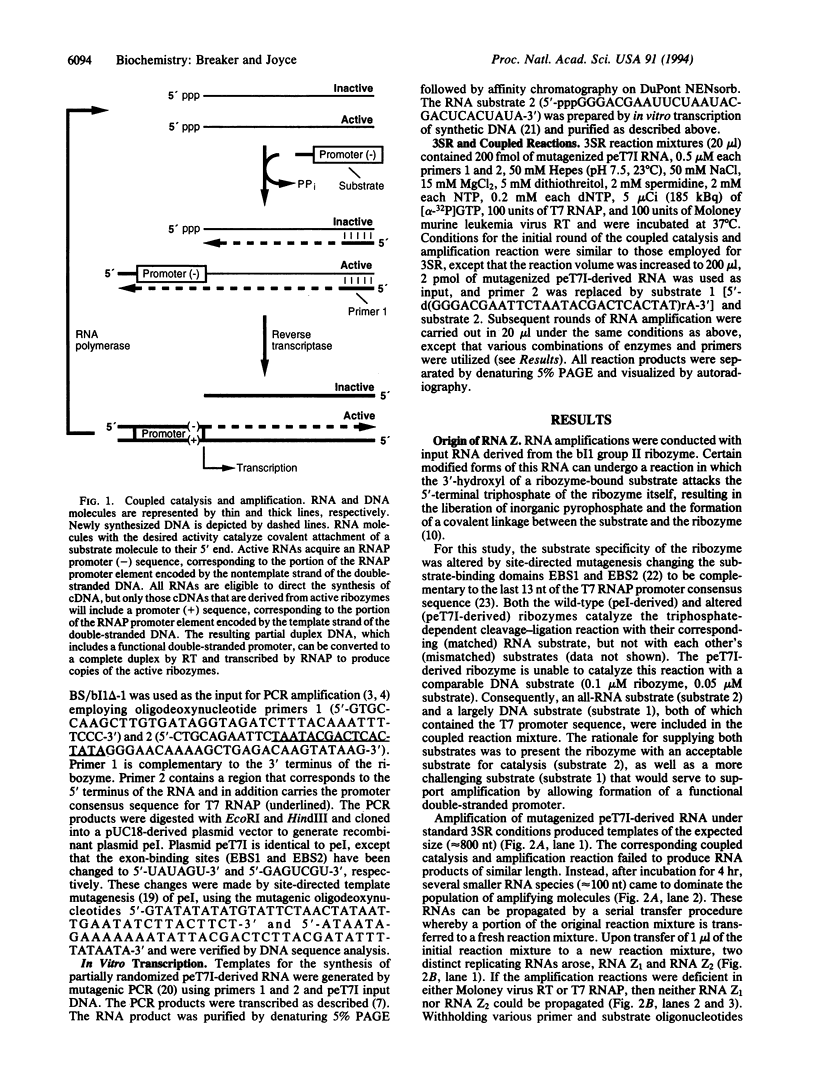
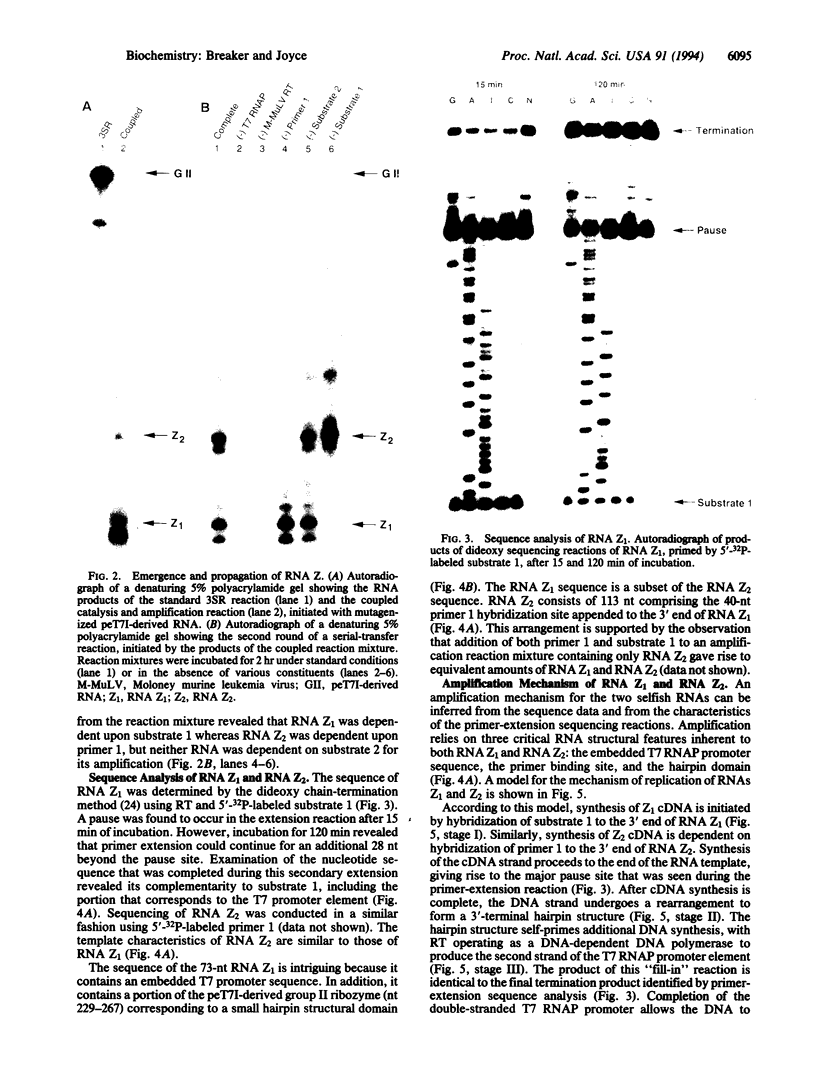
The technique of self-sustained sequence replication allows isothermal amplification of DNA and RNA molecules in vitro. This method relies on the activities of a reverse transcriptase and a DNA-dependent RNA polymerase to amplify specific nucleic acid sequences. We have modified this protocol to allow selective amplification of RNAs that catalyze a particular chemical reaction. During an in vitro RNA evolution experiment employing this modified system, a unique class of "selfish" RNAs emerged and replicated to the exclusion of the intended RNAs. Members of this class of selfish molecules, termed RNA Z, amplify efficiently despite their inability to catalyze the target chemical reaction. Their amplification requires the action of both reverse transcriptase and RNA polymerase and involves the synthesis of both DNA and RNA replication intermediates. The proposed amplification mechanism for RNA Z involves the formation of a DNA hairpin that functions as a template for transcription by RNA polymerase. This arrangement links the two strands of the DNA, resulting in the production of RNA transcripts that contain an embedded RNA polymerase promoter sequence.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bartel D. P., Szostak J. W. Isolation of new ribozymes from a large pool of random sequences [see comment]. Science. 1993 Sep 10;261(5127):1411–1418. doi: 10.1126/science.7690155. [DOI] [PubMed] [Google Scholar]
- Beaudry A. A., Joyce G. F. Directed evolution of an RNA enzyme. Science. 1992 Jul 31;257(5070):635–641. doi: 10.1126/science.1496376. [DOI] [PubMed] [Google Scholar]
- Biebricher C. K., Orgel L. E. An RNA that multiplies indefinitely with DNA-dependent RNA polymerase: selection from a random copolymer. Proc Natl Acad Sci U S A. 1973 Mar;70(3):934–938. doi: 10.1073/pnas.70.3.934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler E. T., Chamberlin M. J. Bacteriophage SP6-specific RNA polymerase. I. Isolation and characterization of the enzyme. J Biol Chem. 1982 May 25;257(10):5772–5778. [PubMed] [Google Scholar]
- Cadwell R. C., Joyce G. F. Randomization of genes by PCR mutagenesis. PCR Methods Appl. 1992 Aug;2(1):28–33. doi: 10.1101/gr.2.1.28. [DOI] [PubMed] [Google Scholar]
- Davanloo P., Rosenberg A. H., Dunn J. J., Studier F. W. Cloning and expression of the gene for bacteriophage T7 RNA polymerase. Proc Natl Acad Sci U S A. 1984 Apr;81(7):2035–2039. doi: 10.1073/pnas.81.7.2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn J. J., Studier F. W. Complete nucleotide sequence of bacteriophage T7 DNA and the locations of T7 genetic elements. J Mol Biol. 1983 Jun 5;166(4):477–535. doi: 10.1016/s0022-2836(83)80282-4. [DOI] [PubMed] [Google Scholar]
- Fahy E., Kwoh D. Y., Gingeras T. R. Self-sustained sequence replication (3SR): an isothermal transcription-based amplification system alternative to PCR. PCR Methods Appl. 1991 Aug;1(1):25–33. doi: 10.1101/gr.1.1.25. [DOI] [PubMed] [Google Scholar]
- Guatelli J. C., Whitfield K. M., Kwoh D. Y., Barringer K. J., Richman D. D., Gingeras T. R. Isothermal, in vitro amplification of nucleic acids by a multienzyme reaction modeled after retroviral replication. Proc Natl Acad Sci U S A. 1990 Mar;87(5):1874–1878. doi: 10.1073/pnas.87.5.1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacquier A., Michel F. Multiple exon-binding sites in class II self-splicing introns. Cell. 1987 Jul 3;50(1):17–29. doi: 10.1016/0092-8674(87)90658-1. [DOI] [PubMed] [Google Scholar]
- Joyce G. F. Directed molecular evolution. Sci Am. 1992 Dec;267(6):90–97. doi: 10.1038/scientificamerican1292-90. [DOI] [PubMed] [Google Scholar]
- Joyce G. F., Inoue T. A novel technique for the rapid preparation of mutant RNAs. Nucleic Acids Res. 1989 Jan 25;17(2):711–722. [PMC free article] [PubMed] [Google Scholar]
- Konarska M. M., Sharp P. A. Replication of RNA by the DNA-dependent RNA polymerase of phage T7. Cell. 1989 May 5;57(3):423–431. doi: 10.1016/0092-8674(89)90917-3. [DOI] [PubMed] [Google Scholar]
- Konarska M. M., Sharp P. A. Structure of RNAs replicated by the DNA-dependent T7 RNA polymerase. Cell. 1990 Nov 2;63(3):609–618. doi: 10.1016/0092-8674(90)90456-o. [DOI] [PubMed] [Google Scholar]
- Lehman N., Joyce G. F. Evolution in vitro of an RNA enzyme with altered metal dependence. Nature. 1993 Jan 14;361(6408):182–185. doi: 10.1038/361182a0. [DOI] [PubMed] [Google Scholar]
- Milligan J. F., Groebe D. R., Witherell G. W., Uhlenbeck O. C. Oligoribonucleotide synthesis using T7 RNA polymerase and synthetic DNA templates. Nucleic Acids Res. 1987 Nov 11;15(21):8783–8798. doi: 10.1093/nar/15.21.8783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills D. R., Peterson R. L., Spiegelman S. An extracellular Darwinian experiment with a self-duplicating nucleic acid molecule. Proc Natl Acad Sci U S A. 1967 Jul;58(1):217–224. doi: 10.1073/pnas.58.1.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mörl M., Niemer I., Schmelzer C. New reactions catalyzed by a group II intron ribozyme with RNA and DNA substrates. Cell. 1992 Sep 4;70(5):803–810. doi: 10.1016/0092-8674(92)90313-2. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Scharf S., Faloona F., Mullis K. B., Horn G. T., Erlich H. A., Arnheim N. Enzymatic amplification of beta-globin genomic sequences and restriction site analysis for diagnosis of sickle cell anemia. Science. 1985 Dec 20;230(4732):1350–1354. doi: 10.1126/science.2999980. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szostak J. W. In vitro genetics. Trends Biochem Sci. 1992 Mar;17(3):89–93. doi: 10.1016/0968-0004(92)90242-2. [DOI] [PubMed] [Google Scholar]