Abstract
Hepatopulmonary syndrome is an uncommon complication of nonacute liver failure, and in rare cases, hypoxia may be the presenting sign of liver dysfunction. The condition, once thought to be a contraindication, is improved in most cases by transplantation. There is a significant risk of postoperative, hypoxia-related morbidity and mortality in patients with hepatopulmonary syndrome. We present a case of life-threatening hypoxia following liver transplantation for liver failure and associated hepatopulmonary syndrome, with successful management using extracorporeal membrane oxygenation.
End-stage liver disease may present with multiple organ dysfunction, which complicates the care of these critically ill patients. Pulmonary manifestations of end-stage liver disease include hepatopulmonary syndrome (HPS), which occurs infrequently and with variable incidence. Clinically, it is manifested by hypoxemia, elevated alveolar-arterial gradient, dyspnea, and platypnea in association with liver dysfunction. The diagnosis of HPS requires 3 elements: liver disease, hypoxemia, and intrapulmonary vascular dilation. The wide variation in reported incidences of HPS is due to the definition of hypoxemia used in the determination.1,2 Some have defined hypoxia using partial pressure of oxygen (PaO2) while the patient is breathing room air,3 whereas other investigators have used alveolar-arterial gradients in the definition.1,4 The published incidence of HPS ranges from 4% to 32%.1,4–11 Hypoxemia has been correlated to risk of mortality for these patients, and this suggests that an arterial PaO2 < 50 mmHg at rest while the patient is breathing room air carries the greatest risk for complications after orthotopic liver transplantation (OLT).12–14 Although hypoxemia was once a contraindication, liver transplantation appears to reverse hypoxemia in >70% of patients and is more effective than current medical therapies.13,15 We present a case of a child with HPS and profound hypoxemia post liver transplantation who was successfully supported with venovenous (V-V) extracorporeal membrane oxygenation (ECMO) until his oxygenation improved enough to allow for standard mechanical ventilation.
CASE PRESENTATION
A previously healthy 12-year-old, 40-kg male presented with shortness of breath and fatigue. He was found to have cirrhosis secondary to autoimmune hepatitis. Liver function tests showed aspartate aminotransferase of 304 IU/L, alanine aminotransferase of 219 IU/L, total bilirubin of 1.4 mg/dL, international normalization ratio of 1.5, serum albumin of 2.2 g/dL, and serum creatinine without dialysis of 0.4 mg/dL; this gave him a lab Model for End-Stage Liver Disease score of 14.
At presentation, he was hypoxemic with arterial oxygen saturation (SaO2) of 86% while breathing room air, with platypnea, orthodeoxia, and digital clubbing on physical examination. Chest radiograph demonstrated diffuse reticular markings at the lung field periphery, without consolidation. Agitated saline contrast echocardiography revealed a right-to-left extracardiac shunt, although computed tomography of the chest did not demonstrate discrete arteriovenous malformations. A ventilation-perfusion scan with technetium-99-m–labeled macroaggregated albumin scintigraphy revealed normal ventilation with marked uptake in the brain, thyroid, spleen, and kidneys, confirming the right-to-left shunt physiology. Pulmonary function testing showed normal spirometric values for his age with diminished carbon monoxide diffusion at 37% of the predicted value. An initial arterial blood gas demonstrated a pH of 7.42, partial pressure of carbon dioxide of 35 mmHg, and PaO2 of 53 mmHg while he was breathing room air.
In the weeks following initial presentation, his clinical status deteriorated with worsening hypoxemia, which rendered him bed-bound. His SaO2 ranged from 88% to 92% while he was breathing 6 liters per minute (Lpm) oxygen via a face mask, and he had significant desaturations with minimal activity. Hence, the United Network for Organ Sharing regional review board was petitioned, and he was listed for transplantation with an exception Model for End-Stage Liver Disease score of 22, including a diagnosis of HPS. Two months after presentation, he underwent uncomplicated deceased donor OLT. The procedure was performed piggyback without V-V bypass. He required 3 units of packed red blood cells, 7 units of fresh frozen plasma, 275 mL of cell saver fluid, and 2 L of crystalloid. On postoperative day 1, the patient had good liver function and no requirement of blood products.
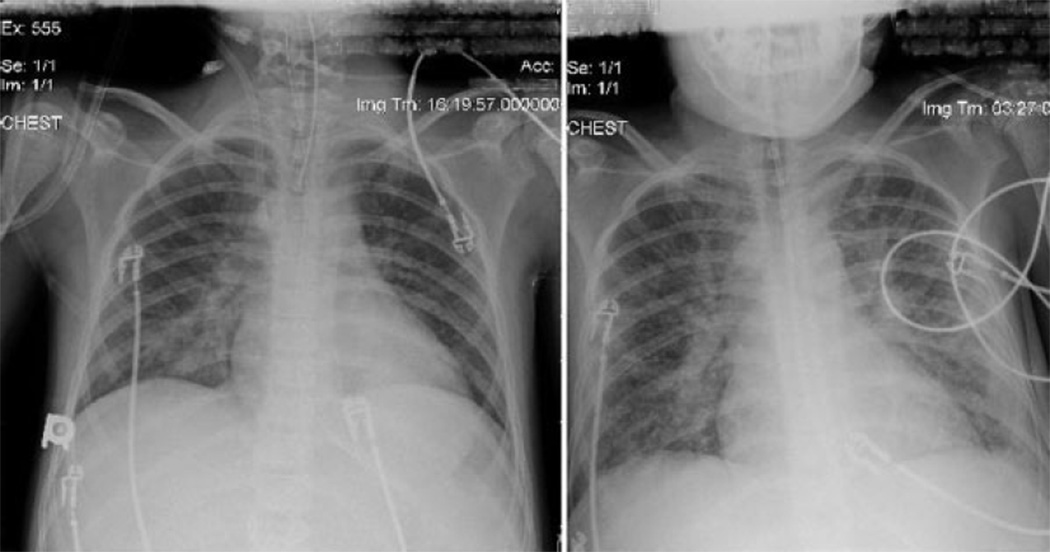
On postoperative day 2, the patient’s trachea was extubated, having met standard local intensive care unit criteria of 4–6 mL/kg tidal breaths on continuous positive airway pressure without significant tachypnea or signs of respiratory distress. However, within 12 hours, because of persistently low SaO2 [75%-85% with PaO2 of 36 mmHg on a fraction of inspired oxygen (FIO2) of 0.8] and respiratory distress, he required urgent reintubation of the trachea and mechanical ventilatory support. His settings on positive pressure ventilation were 10 mL/kg tidal volume (reaching peak inspiratory pressures of 28–30 cmH2O), positive end expiratory pressure of 5 cmH2O, and mean airway pressure of 9–11 cmH2O. This resulted in SaO2 of 90%–95% on an FIO2 of 0.8. Over the ensuing 3 days, his FIO2 and ventilator settings were weaned, and on postoperative day 5, his trachea was again extubated. He was transitioned to a high-flow nasal cannula oxygen delivery device (Vapotherm, Stevensville, MD) at 40 Lpm, and his SaO2 was maintained at 90% on an FIO2 of 0.9–1; however, further decompensation with respiratory distress and hypoxia required him to be transitioned to noninvasive bilevel positive pressure ventilation of 18/8 cmH2O with FIO2 of 0.9. On postoperative day 6, the patient developed fever and tachycardia without leukocytosis, and blood cultures subsequently grew coagulase- negative staphylococcus from a central venous catheter. The central venous catheter was removed, and appropriate broad-spectrum antimicrobial therapy was initiated. Previous and subsequent endotracheal cultures were negative during his course. On postoperative day 8, he had an acute decompensation with SaO2 below 60% and severe respiratory distress. He underwent emergent endotracheal intubation and mechanical ventilatory support. Attempts at lung recruitment with positive pressure ventilation and FIO2 of 1.0 did not improve his SaO2, and his chest radiograph at that juncture showed increased parenchymal opacification (Fig. 1). His total pulmonary compliance had decreased, reaching peak inspiratory pressures of 35 cmH2O on 8–10 mL/kg tidal volumes. Nitric oxide at 40 parts per million was added to his regimen without improvement in oxygenation, PaO2 remaining at 31 mmHg. Because of the failure of conventional ventilatory support, and in the face of satisfactory graft function, he was considered to be a candidate for V-V ECMO.
Figure 1.
Anterior-posterior chest radiographs from postoperative day 1 (left) and postoperative day 8 (right) demonstrating a marked increase in bilateral pulmonary infiltrates.
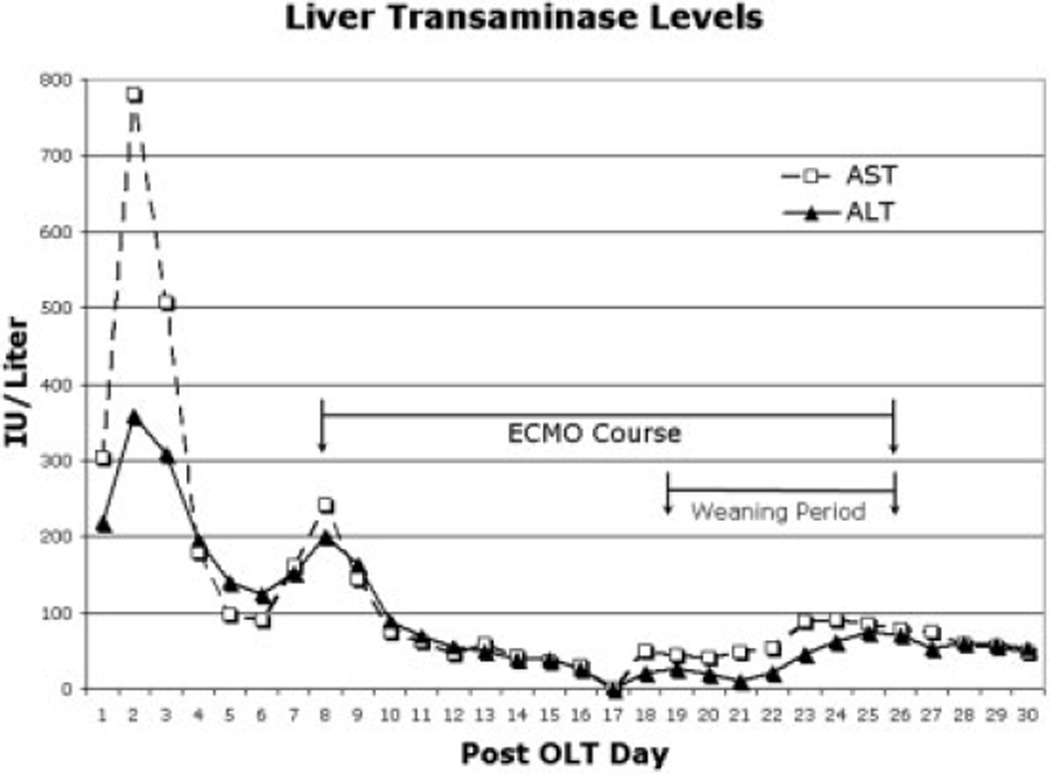
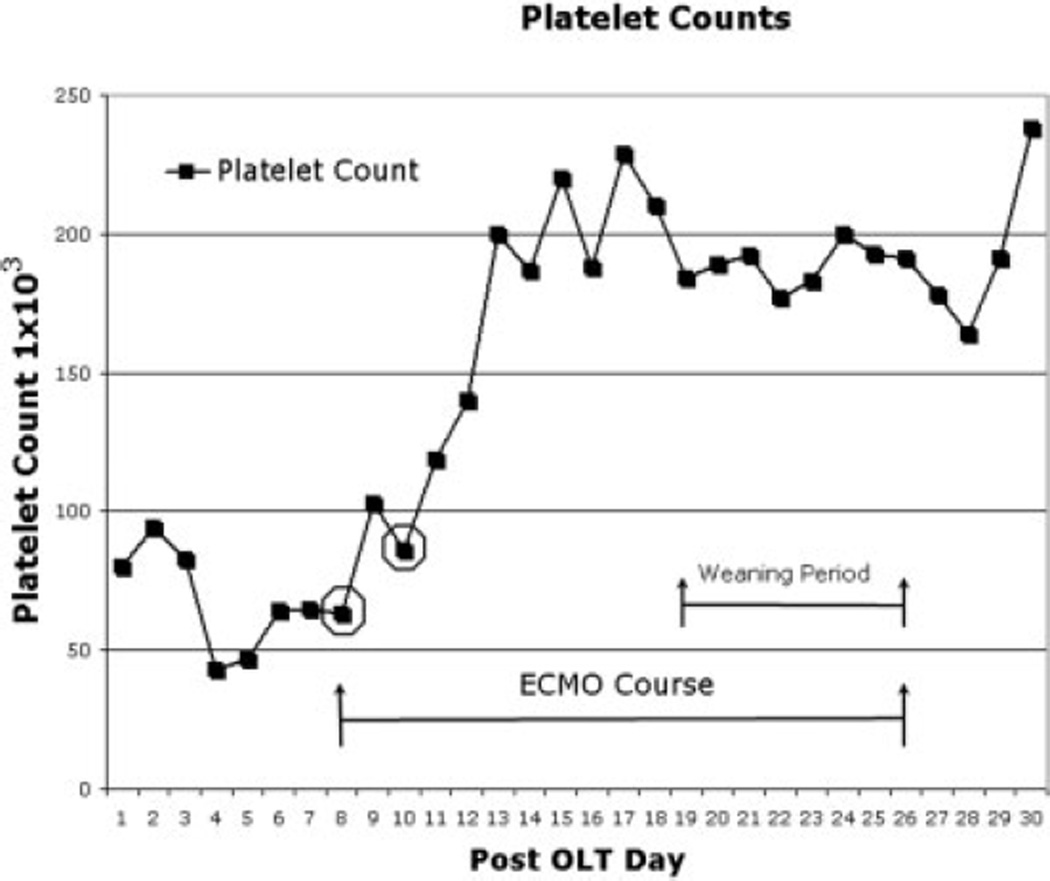
Because currently available double lumen cannula sizes are restricted to 18 French and smaller, a 23-French venous drainage cannula was percutaneously placed in the right internal jugular vein, and a 21-French venous return cannula was similarly placed in the right femoral vein. He remained on V-V ECMO for 18 days, during which time he was placed in the prone position for periods ranging from 6 to 12 hours to optimize lung recruitment. His initial ECMO blood flow rates were 3–3.6 Lpm, and they were slowly decreased to 2 Lpm on ECMO days 12–13. After his ECMO blood flow rate was decreased, his sweep gas FIO2 was slowly weaned from 1.0 to 0.4. As he recovered from his catheter- associated bloodstream infection and his ECMO support was decreased, his SaO2 remained stable, with a peak SaO2 – central venous oxygen saturation difference of 26% and a lowest SaO2 recording of 79%. His liver function remained stable, and liver enzymes continued to normalize; this suggested no adverse effects of his graft during this weaning period (Fig. 2). Our institution practice is to maintain platelet counts > 1 × 105 during ECMO support, and total platelet count data are presented for his entire intensive care unit stay (Fig. 3). Total blood product use for the ECMO course was 1188 mL of platelets and 4550 mL of packed red blood cells, and no cryoprecipitate or fresh frozen plasma was administered. On postoperative day 26 and ECMO day 18, his ECMO cannulae were removed. Five days after separation from V-V ECMO, his trachea was extubated, and he was transitioned to 2 Lpm oxygen by nasal cannula with a PaO2 of 52 mmHg and SaO2 of 90%. By postoperative day 51, his SaO2 was 99% with no supplemental oxygen support. He was subsequently discharged and is currently doing well greater than 1 year post transplantation.
Figure 2.
Liver aminotransferase levels during the post-OLT period. Aspartate aminotransferase (AST) and alanine aminotransferase (ALT) are presented on the y axis in international units per liter for each post-OLT day (x axis). The period of ECMO is delineated with arrows in the graph with the appropriate legend. Additionally, the period of weaning of ECMO support is delineated with arrows in the graph with the appropriate legend.
Figure 3.
The platelet count in thousands (y axis) is presented during the post-OLT period (x axis). The period of ECMO is delineated with arrows in the graph with the appropriate legend. Platelet transfusion is indicated by a circle around data points on ECMO days 1 and 2.
DISCUSSION
For many years, severe HPS was a contraindication to liver transplantation. More recently, however, case reports and series have demonstrated a reversal of this process with liver transplantation.2 Worsening of hypoxemia may occur and contribute to posttransplantation mortality; however, if the posttransplant period is survived, the outcome is generally favorable with reversal of hypoxemia.12–13,15–17 The degree of pre-OLT hypoxia associated with HPS has been felt to further delineate patients at greatest risk for postoperative complications and reduced survival; in one study, a PaO2 < 60 mmHg was associated with an increased mortality,11 whereas a subsequent study reports a threshold for survival with a PaO2 < 50 mmHg.14 Regardless, significant hypoxia associated with HPS appears to portend an increased risk for complications and mortality after OLT.
Although management of severe hypoxia related to acute respiratory distress syndrome (ARDS) in neonatal and pediatric patients using ECMO is not a novel concept, its use after OLT for pulmonary complications is infrequently reported, with no reference to patients with HPS among these cases. Jeng et al.18 reported ECMO support for hypoxia-mediated ARDS that developed after OLT for end-stage liver disease secondary to hemochromatosis and hepatitis B infection. This 37-year-old female was placed on ECMO (no indication of mode) on postoperative day 18 and survived for 10 days until the development of severe intracranial hemorrhage necessitating withdrawal of care. Szocik et al.19 reported ECMO support for a massive pulmonary embolism that developed intraoperatively in a 54-year-old female with biliary cirrhosis undergoing OLT. She was supported with ECMO (no indication of mode) intraoperatively and weaned from support in the operative suite. She died 9 weeks after OLT secondary to multiorgan failure mediated by venacaval thrombosis. Fujita et al.20 reported V-V ECMO support in a 1-month-old female who developed pulmonary hemorrhage on postoperative day 27 after OLT for idiopathic neonatal hepatitis. She was supported for a total of 4 days without complication with repeat OLT performed on ECMO on day 2 of support. She was weaned from ECMO and survived to hospital discharge. Additional reference to ECMO support post liver transplantation was noted within a series documenting pediatric pulmonary complications after OLT.21 Three patients ranging from 4 to 9 months of age were supported with ECMO after OLT for respiratory tract infections with respiratory syncytial virus, parainfluenza virus, and pseudomonas. Two of the 3 patients were reported to survive to hospital discharge.
The use of ECMO to support patients in the posttransplantation period needs to be weighed against the 3 main risks: anticoagulation, cannula placement, and infection. Although systemic anticoagulation is required during ECMO, the bleeding complications are usually manageable even in patients in the postoperative period. A growing population supported on ECMO in this modern era is neonates requiring support after congenital cardiac surgery correction.22 Although bleeding is not uncommon in this group, it is successfully managed by minimization of heparin infusion rates to target activated clotting times in the lowest tolerable range. Cannula placement also requires consideration after OLT, especially in the pediatric population. Current ECMO coaxial or double lumen catheter sizes are restricted to 18 French and smaller, necessitating bicaval cannulation in patients greater than 15 kg for V-V support. Hence, the transplant surgeon will consider the risk of femoral cannulation and proximity to graft anastomosis against the risk for carotid artery sacrifice necessary for venoarterial cannulation. Finally, infectious complications with ECMO are minimized by aseptic technique and nursing care but remain a concern for any patient on ECMO. Immunosuppression associated with solid organ transplant does not appear to be a contraindication to ECMO as experience grows with supporting posttransplantation organ failure.23,24
We present a case report of extracorporeal life support for life-threatening hypoxia complicating HPS after OLT in a pediatric patient. The likely etiology of his respiratory decompensation was exacerbation of severe HPS by sepsis (as outlined by the International Pediatric Sepsis Consensus Conference) -induced ARDS.25 Elimination of the source of bacteremia by removal of the colonized central venous catheter, implementing broad-spectrum antibiotic therapy, and allowing lung parenchymal normalization are what ultimately reversed his condition. However, the severity of his respiratory failure and decompensation required more support than conventional ventilatory strategies could provide. Using V-V ECMO to provide this support while ARDS and bacteremia could be treated was successful, as it gave the management team the ability to carry out effective lung recruitment and pulmonary toileting strategies during the period of improvement in intrapulmonary shunting.
Because of the marked advances in conventional ventilatory management, fewer patients are supported with ECMO for traditional respiratory failure. However, this does not make ECMO obsolete, as it clearly still has a role in complex, physiologically challenging patients. In this case, ECMO provided a safe and viable option for severe hypoxemic respiratory failure in conjunction with HPS in the post–liver transplantation period.
Abbreviations
- ALT
alanine aminotransferase
- ARDS
acute respiratory distress syndrome
- AST
aspartate aminotransferase
- ECMO
extracorporeal membrane oxygenation
- FIO2
fraction of inspired oxygen
- HPS
hepatopulmonary syndrome
- Lpm
liters per minute
- OLT
orthotopic liver transplantation
- PaO2
partial pressure of oxygen
- SaO2
arterial oxygen saturation
- V-V
venovenous
REFERENCES
- 1.Schenk P, Fuhrmann V, Madl C, Funk G, Lehr S, Kandel O, Müller C. Hepatopulmonary syndrome: prevalence and predictive value of various cut offs for arterial oxygenation and their clinical consequences. Gut. 2002;51:853–859. doi: 10.1136/gut.51.6.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mandell MS. The diagnosis and treatment of hepatopulmonary syndrome. Clin Liver Dis. 2006;10:387–405. doi: 10.1016/j.cld.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 3.Krowka MJ. Hepatopulmonary syndromes. Gut. 2000;46:1–4. doi: 10.1136/gut.46.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stoller JK, Lange PA, Westveer MK, Carey MD, Vogt D, Henderson JM. Prevalence and reversibility of the hepatopulmonary syndrome after liver transplantation: the Cleveland Clinic experience. West J Med. 1995;163:133–138. [PMC free article] [PubMed] [Google Scholar]
- 5.Krowka MJ, Tajik J, Dickson ER, Wiesner RH, Cortese DA. Intrapulmonary vascular dilations (IPVD) in liver transplant candidates. Chest. 1990;97:1165–1170. doi: 10.1378/chest.97.5.1165. [DOI] [PubMed] [Google Scholar]
- 6.Hopkins WE, Waggoner AD, Barzilai B. Frequency and significance of intrapulmonary right to left shunting in end-stage hepatic disease. Am J Cardiol. 1992;70:516–519. doi: 10.1016/0002-9149(92)91200-n. [DOI] [PubMed] [Google Scholar]
- 7.Barbé T, Losay J, Grimon G, Devictor D, Sardet A, Gauthier F, et al. Pulmonary arteriovenous shunting in children with liver disease. J Pediatr. 1995;126:571–579. doi: 10.1016/s0022-3476(95)70351-9. [DOI] [PubMed] [Google Scholar]
- 8.Castro M, Krowka MJ. Hepatopulmonary syndrome. Clin Chest Med. 1996;17:35–48. doi: 10.1016/s0272-5231(05)70297-5. [DOI] [PubMed] [Google Scholar]
- 9.Egawa H, Kasahara M, Inomata Y, Uemoto S, Asonuma K, Fujita S, et al. Long-term outcome of living related liver transplantation for patients with intrapulmonary shunting and strategy for complications. Transplantation. 1999;67:712–717. doi: 10.1097/00007890-199903150-00012. [DOI] [PubMed] [Google Scholar]
- 10.Martínez GP, Barberà JA, Visa J, Rimola A, Paré JC, Roca J, et al. Hepatopulmonary syndrome in candidates for liver transplantation. J Hepatol. 2001;34:651–657. doi: 10.1016/s0168-8278(00)00108-2. [DOI] [PubMed] [Google Scholar]
- 11.Schenk P, Schoniger-Hekele M, Fuhrmann V, Madl C, Silberhumer G, Muller C. Prognostic significance of the hepatopulmonary syndrome in patients with cirrhosis. Gastroenterology. 2003;125:1042–1052. doi: 10.1016/s0016-5085(03)01207-1. [DOI] [PubMed] [Google Scholar]
- 12.Hobeika J, Houssin D, Bernard O, Devictor D, Grimon G, Chapuis Y. Orthotopic liver transplantation in children with chronic liver disease and severe hypoxemia. Transplantation. 1994;57:224–228. doi: 10.1097/00007890-199401001-00012. [DOI] [PubMed] [Google Scholar]
- 13.Krowka MJ, Porayko MK, Plevak D, Pappas C, Steers J, Krom R, et al. Hepatopulmonary syndrome with progressive hypoxemia as an indication for liver transplantation: case reports and review of the literature. Mayo Clin Proc. 1997;72:44–53. doi: 10.4065/72.1.44. [DOI] [PubMed] [Google Scholar]
- 14.Swanson KL, Wiesner RG, Krowka MJ. Natural history of hepatopulmonary syndrome: impact of liver transplantation. Hepatology. 2005;41:1122–1129. doi: 10.1002/hep.20658. [DOI] [PubMed] [Google Scholar]
- 15.Taille C, Cadranel J, Bellocq A, Thabut G, Soubrane O, Durand F, et al. Liver transplantation for hepatopulmonary syndrome: a ten year experience in Paris, France. Transplantation. 2003;75:1482–1489. doi: 10.1097/01.TP.0000061612.78954.6C. [DOI] [PubMed] [Google Scholar]
- 16.Uemoto S, Inomata Y, Egawa H, Satomura K, Kiuchi T, Okajima H, et al. Effects of hypoxemia on early postoperative course of liver transplantation in pediatric patients with intrapulmonary shunting. Transplantation. 1997;63:407–414. doi: 10.1097/00007890-199702150-00014. [DOI] [PubMed] [Google Scholar]
- 17.Laberge JM, Brandt ML, Lebecque P, Moulin D, Veykemans F, Paradis K, et al. Reversal of cirrhosis-related pulmonary shunting in two children by orthotopic liver transplantation. Transplantation. 1992;53:1135–1138. [PubMed] [Google Scholar]
- 18.Jeng LB, Cheng MH, Lee WC, Wang CC, Wang KL, Chen SC, et al. Extracorporeal membrane oxygenation therapy for adult respiratory distress syndrome developing post liver transplantation. Transplant Proc. 1994;26:2237–2238. [PubMed] [Google Scholar]
- 19.Szocik J, Rudich S, Csete M. ECMO resuscitation after massive pulmonary embolism during liver transplantation. Anesthesiology. 2002;97:763–764. doi: 10.1097/00000542-200209000-00059. [DOI] [PubMed] [Google Scholar]
- 20.Fujita S, Hemming AW, Fujikawa T, Reed AI, Howard RJ, Langham MR, et al. Expanded efficacy and indication of extracorporeal membrane oxygenation for preoperative pulmonary bleeding on pediatric cadaveric orthotopic liver transplantation. Transplantation. 2005;79:1637. doi: 10.1097/01.tp.0000160815.04784.4a. [DOI] [PubMed] [Google Scholar]
- 21.Mack CL, Millis JM, Whitington PF, Alonso EM. Pulmonary complications following liver transplantation in pediatric patients. Pediatr Transplant. 2000;4:39–44. doi: 10.1034/j.1399-3046.2000.00080.x. [DOI] [PubMed] [Google Scholar]
- 22.Fleming GM, Gurney JG, Donohue JE, Remenapp RT, Annich GM. Neonatal and pediatric ECMO mechanical component failures: analysis of 28,171 circuits from 1987–2006. Abstract presented at: 18th Annual ELSO Conference; September 2007; Nashville TN. [Google Scholar]
- 23.Burch M, Aurora P. Current status of paediatric heart, lung, and heart-lung transplantation. Arch Dis Child. 2004;89:386–389. doi: 10.1136/adc.2002.017186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oto T, Rosenfeldt F, Rowland M, Pick A, Rabinov M, Preovolos A, et al. Extracorporeal membrane oxygenation after lung transplantation: evolving technique improves outcomes. Ann Thorac Surg. 2004;78:1230–1235. doi: 10.1016/j.athoracsur.2004.03.095. [DOI] [PubMed] [Google Scholar]
- 25.Goldstein B, Giroir B, Randolph A for the International Consensus Conference on Pediatric Sepsis. International pediatric sepsis consensus conference: definitions for sepsis and organ dysfunction in pediatrics. Pediatr Crit Care Med. 2005;6:2–8. doi: 10.1097/01.PCC.0000149131.72248.E6. [DOI] [PubMed] [Google Scholar]