Abstract
Platelet Endothelial Cell Adhesion Molecule 1 (PECAM-1) deficient mice in the FVB/n strain exhibit fatal chronic pulmonary fibrotic disease. The illness occurs in the absence of a detectable pro-inflammatory event. PECAM-1 is vital to the stability of vascular permeability, leukocyte extravasation, clotting of platelets, and clearance of apoptotic cells. We show here that the spontaneous development of fibrotic disease in PECAM-1 deficient FVB/n mice is characterized by early loss of vascular integrity in pulmonary capillaries, resulting in spontaneous microbleeds. Hemosiderin-positive macrophages were found in interstitial spaces and bronchoalveolar lavage (BAL) fluid in relatively healthy animals. We also observed a gradually increasing presence of hemosiderin-positive macrophages and fibrin deposition in the advanced stages of disease, corresponding to the accumulation of collagen, IL-10 expression, and myofibroblasts expressing alpha smooth muscle actin (SMA). Together with the growing evidence that pulmonary microbleeds and coagulation play an active part in human pulmonary fibrosis, this data further supports our hypothesis that PECAM-1 expression is necessary for vascular barrier function control and regulation of homeostasis specifically, in the pulmonary environment.
Keywords: interstitial fibrosis, macrophage biology, pulmonary oedema
INTRODUCTION
Platelet Endothelial Cell Adhesion Molecule (PECAM-1, CD31) is a 130 kDa transmembrane adhesion and signaling glycoprotein. It belongs to the Ig superfamily of cell adhesion molecules. It is concentrated at endothelial cell–cell junctions in highly vascularized regions and is expressed by the majority of leukocytes with the exception of activated lymphocytes. PECAM-1 is important to the integrity of the vascular permeability, leukocyte extravasation, control of thrombus activation and contraction, and apoptotic cell/debris clearance (1–4).
Idiopathic Pulmonary Fibrosis (IPF) affects nearly 100,000 Americans. It is progressive and fatal in the absence of a lung transplant (5). PECAM-1 plays a vital role in the regulation of vascular stability, and there is growing evidence that alveolar bleeding and coagulation play essential roles in the development of IPF, especially at initiation phases. The current hypothesis is that the formation of a clot in the lung, followed by activation of protease activated receptors (PAR1 and PAR2) leads to the fibrotic response (6–7). A recent clinical trial using warfarin to treat IPF found that this treatment was detrimental (8). This indicates that coagulation does indeed play a role in the diseases like IPF.
We previously showed that PECAM-1 deficient FVB/n strain mice spontaneously develop a fatal chronic pulmonary disease with some similarities to that seen in patients with IPF, including the absence of a detectable acute inflammatory event and the accumulation of collagen in lesions. We have established pulse oximetry to detect reduced pulmonary function, and found that the earliest occurring disease stage in the PECAM-1 deficient FVBn mice is alveolar collapse, before any actual lesion formation, collagen deposition, and fibrosis occurs (9). Here we show that the early stages of disease process in PECAM-1 deficient FVBn mice are hallmarked by increased numbers of hemosiderin-positive macrophages and fibrin deposition, which increase as the disease progresses. This supports the hypothesis that pulmonary microbleeding and coagulation may play a role in human fibrotic diseases.
MATERIALS AND METHODS
Animals
PECAM-1 deficient and wild type FVB/n mice were housed at Colorado State University Laboratory Animal Resources. Veterinary care and pulse oximetry were done under protocols approved by the Colorado State University Animal Care and Use Committee.
Disease Stages of PECAM-1 Deficient FVB/n Mice
MouseOx system (Starr Life Sciences Corp., Allison Park, PA) was used to non-invasively assess disease state in PECAM-1 deficient FVB/n mice (9), with the disease stages outlined in Table 1.
Table 1.
Disease stages of PECAM-1 deficient FVB/n mice classified by SpO2 readings, using the MouseOx system by StarrLife Sciences. Fig.1 redrawn from previous similar figure appearing in (9).
| Group | SpO2 range (3+ readings) |
Pathology scoring |
|---|---|---|
| Healthy | 89-94 | none |
| Borderline | 88-85 | focal lesions, small, alveolar enlargement |
| Moderate | 85-75 | Larger, numerous lesions, early fibrosis |
| Severe | <75, cyanotic | large lesions and extensive fibrosis |
Prussian Blue Stain for Hemosiderin-Positive Macrophages in Pulmonary Tissue
Mouse lungs were fixed using formalin-free IHC zinc fixative (BD Pharmigen) and put through standard processing. Paraffin-embedded tissue was sectioned on a standard microtome at 7 um and rehydrated to dionized (DI) water. Slides were immersed in equal parts 0.2% hydrochloric acid and 0.2% potassium ferrocyanide solution for 1 hour at room temperature (RT), then counterstained in 0.2% solution of Safranin Orange for 2 minutes, washed in 1% aqueous acetic acid, dehydrated and mounted in synthetic resin. Hemosiderin appears in blue, nuclei in red, and background in pink.
Prussian Blue Stain for Hemosiderin-Positive Macrophages in Bronchoalveolar Lavage (BAL) Fluid
Post-sacrifice, trachea was lavaged with sterile saline via 18-gauge feeding needle attached to a 1-cm3 syringe. 500 ul of BAL fluid from each animal was applied to each slide, centrifuged (Cytospin, Shandon Southern Instruments, Runcorn UK) for 2 minutes at 90×g, and cell pellet obtained. Slides were air-dried, fixed in methanol, and hemosiderin-positive macrophages were detected with Prussian blue stain. At 16× magnification, alveolar macrophages were examined for the number of cells positive for Prussian blue stain, and the total cell number was counted.
Pentachrome Stain for Fibrin Deposition in Pulmonary Tissue
Staining was performed as per the kit protocol (American Master Tech, Lodi, CA), however, due to the porosity of the sections, Verhoeff’s Elastic stain was omitted. Resulting stain shows collagen in greenish-yellow depending on the age of deposition, muscle in red, mucins in blue to green, and fibrin in pale red.
Immunohistochemistry Anti-Smooth Muscle Actin Stain for Myofibroblasts in Pulmonary Tissue
Monoclonal primary anti-smooth muscle actin antibody was used on mouse lung sections as per the Vector Mouse-On-Mouse (M.O.M) staining protocol (VECTOR® M.O.M.™ Immunodetection Kit BASIC). Vector diaminobenzidine was used as substrate as per kit protocol (VECTOR® DAB Substrate Kit for Peroxidase), counterstained with iron hematoxylin QS (Vector® Hematoxylin QS), dehydrated and mounted in synthetic resin. The resulting stain shows myofibroblasts in brown, nuclei in blue.
IL-10
0.3% H202 was used to block endogenous peroxidase, followed by block in 3% bovine serum albumin (BSA) in PBS for 30 min at RT. Slides were incubated overnight at 4°C with a primary antibody for IL-10 (Santa Cruz cat. # 1783), then washed and incubated with the secondary antibody for 1 hour at RT. Specific antibody binding reaction was amplified with Vectastain ABC system (Vector Laboratories, Bulingame, CA) and 3-amino-9-ethylcarbazole (AEC) (Vector Laboratories) was used as substrate, followed by counterstain with Hematoxylin QS (Vector Laboratories), and mounting.
Interleukin-10 (IL-10) ELISA
BAL was collected as previously described. IL-10 was measured from previously frozen mouse bronchoalveolar lavage fluid samples using the eBioscience Mouse IL-10 Ready-SET-Go! ELISA sandwich kit with precoated plates (eBioscience; standard curve range was 2000pg/mL to 0 pg/mL of cytokine, generated by the frozen standard). The plate was incubated for 2 hours at RT, followed by secondary detection antibody for one hour at RT, followed by Avidin-HRP for 30 minutes RT in the dark. Color substrate (TMB) solution was kept on for 12 minutes in dark, stopped with acidic stop solution, and plate was read on a microplate reader (BioRad Model 680) at 450nm.
Tail Bleeding Time Assessment
Tail-bleeding times were assessed by nicking the tail vein at the tip of the tail and then allowing mice to freely move about the cage on clean paper towels, until bleeding stopped without any human assistance or mouse grooming.
Statistics
JMP software (SAS, Cary, NC) was used for comparison between multiple groups of uneven sample size, using Tukey Kramer Honestly Significant Difference (HSD) test as indicated in the figure legends. Bleeding time comparisons between wild type and PECAM-1 deficient mice was compared with the Student's T test.
RESULTS
Presence of Hemosiderin-Positive Alveolar and Interstitial Macrophages Increases in Tissue with the Stage of Disease
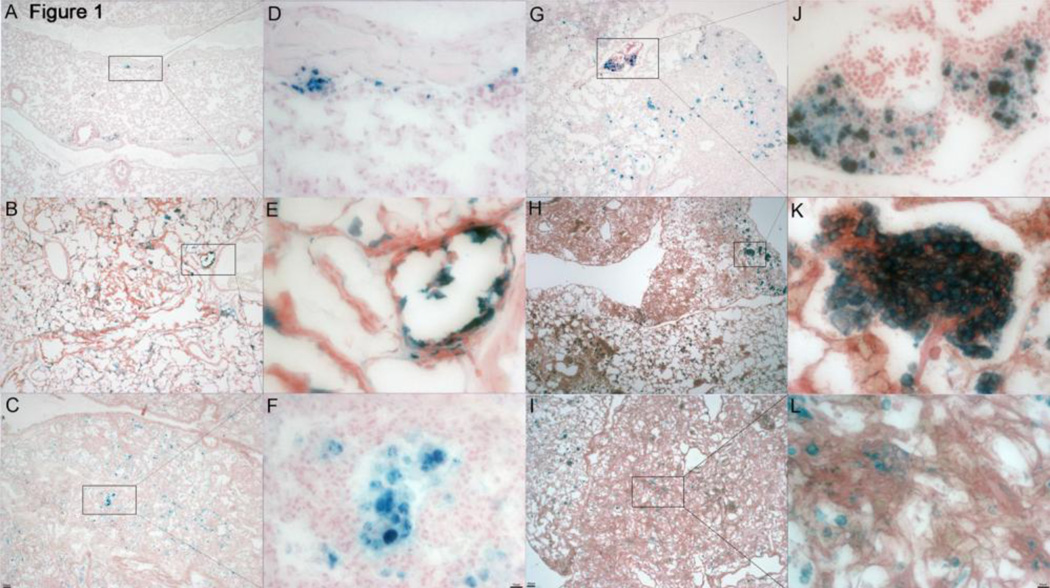
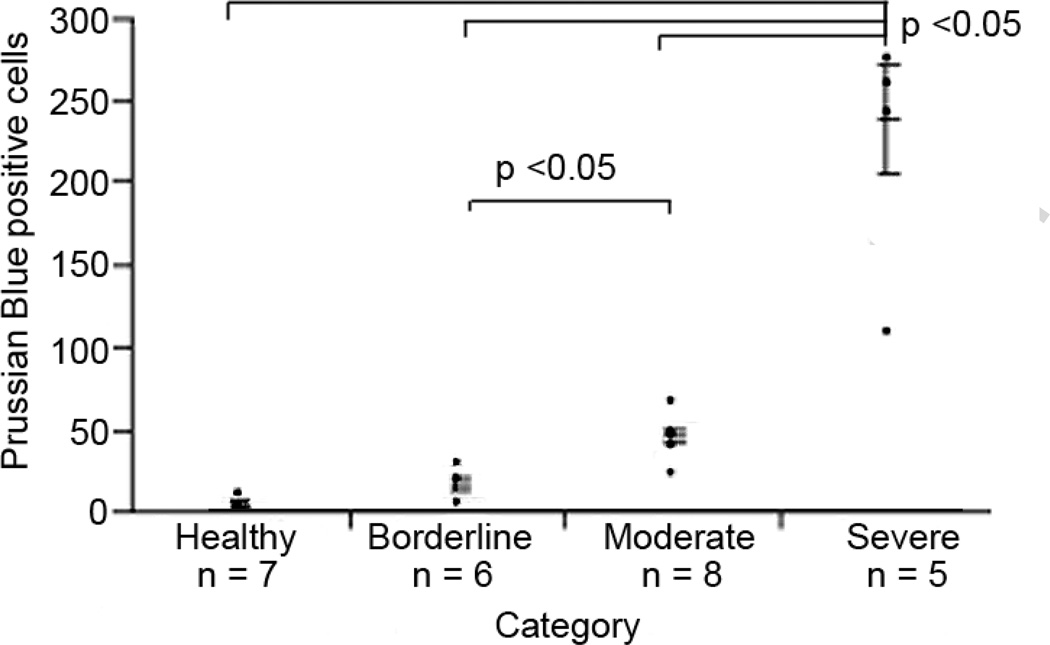
Using a mouse pulse oximetry system to monitor the state of disease in our animals, we classified the state of disease into 4 stages (Table 1; 9). Prussian blue staining confirmed the increased presence of hemosiderin-positive macrophages in sick mice compared to healthy mice (Fig. 1). The number of hemosiderin-positive macrophages in tissue increased with each stage of disease (Fig. 1–2). However, in the highly fibrotic areas of the severe stage the numbers of hemosiderin-positive macrophages became more dispersed, indicating that perhaps microbleed leakage is no longer as intense in those areas (Fig. 1I–L). We have observed the hemosiderinpositive macrophages in the lungs of our animals to be present in the alveoli, as well as in the interstitium. BAL fluid of PECAM-1 deficient FVB/n mice also showed increased presence of alveolar hemosiderin-positive macrophages (Fig. 3).
Figure 1.
Prussian blue stain in healthy and diseased lung. A, D. Healthy PECAM-1 deficient mouse with sporadic iron-positive macrophages (4X and 32X). B, E, C, F. Borderline disease PECAM-1 deficient mice showing an increase in iron positive macrophages around vasculature, lesions, and in the intersitia and alveoli (4X and 32X). G, J. Moderate disease PECAM-1 deficient mouse showing a progressively increasing presence of iron-positive macrophages, with far more extensive regions of fibrosis (4X and 32X). H, K, I, L. Severe disease PECAM-1 deficient mouse, showing a heavy infiltration of iron-positive macrophages. The areas of dense fibrosis appear to be less permeated by these cells than areas of still somewhat retained lung architecture (4X and 32X).
Figure 2.
Graph illustrating the increase of iron-positive macrophages in healthy and diseased lung. Group sizes (n = 4 wt; n = 7 healthy; n = 6 borderline; n = 7 moderate; n = 5 severe). Differences: what groups are different from what groups. p < 0.05 Tukey Kramer Honestly Significant Difference (HSD) test. Error bars indicate standard error.
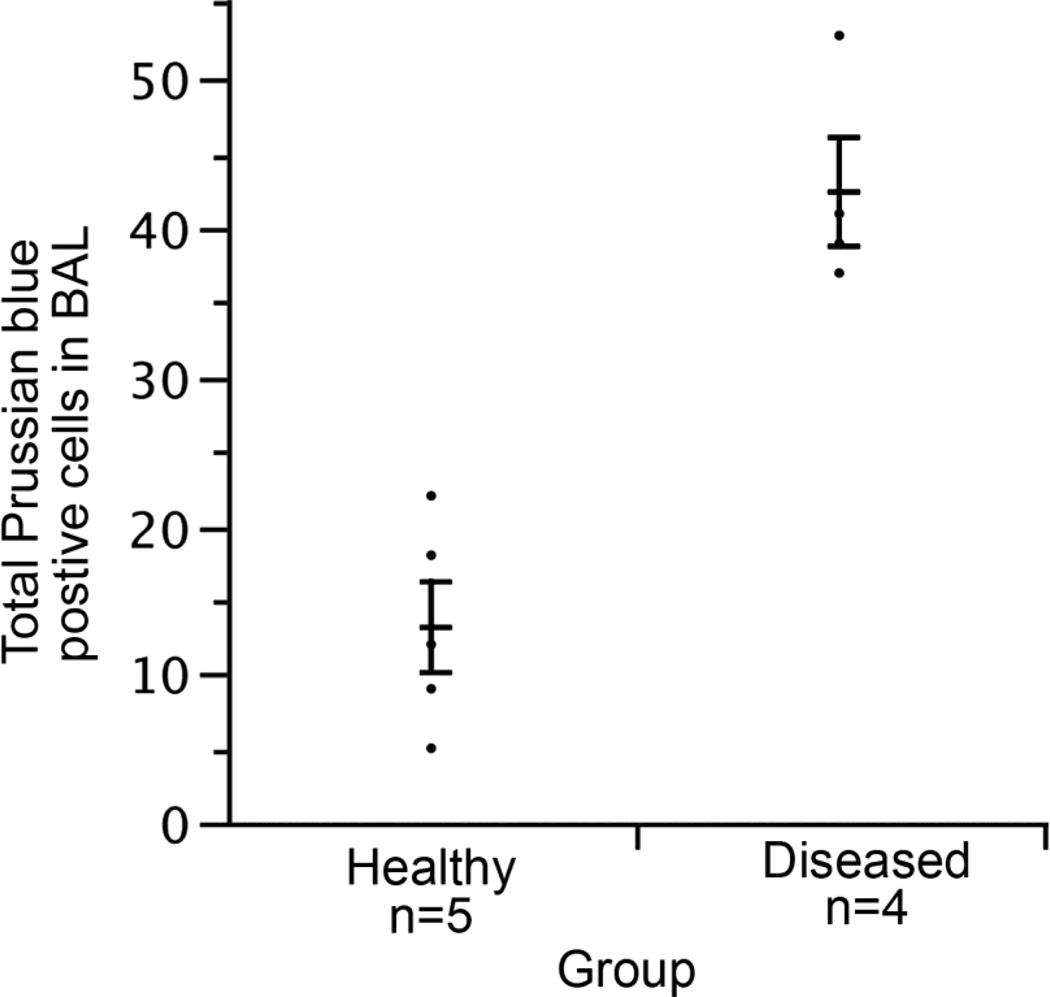
Figure 3.
Quantification of Prussian blue stain of bronchoalveolar lavage in healthy and diseased lung shows an increase in the presence of iron-positive macrophages with the progression of disease. Group sizes (n = 17 wt; n = 5 healthy; n = 5 moderate; n = 6 unknowns). Due to limited numbers of PECAM-1 deficient mice, we used most for histological analysis and did not do broncho-aleveolar lavages (BAL) in order to maintain lung architecture. We did have frozen BAL samples from earlier studies before we had blood oximetry data, and we performed cytospins on five healthy and four diseased samples (confirmed by pathology), stained them with Prussian blue, and looked for hemosiderin-positive macrophages and red blood cells. We were unable to find red blood cells probably due to the freezing and thawing of the samples but did note significantly higher numbers of hemosiderin-positive macrophages in BAL from diseased mice (p <0.05, Tukey Kramer HSD). Error bars indicate standard error.
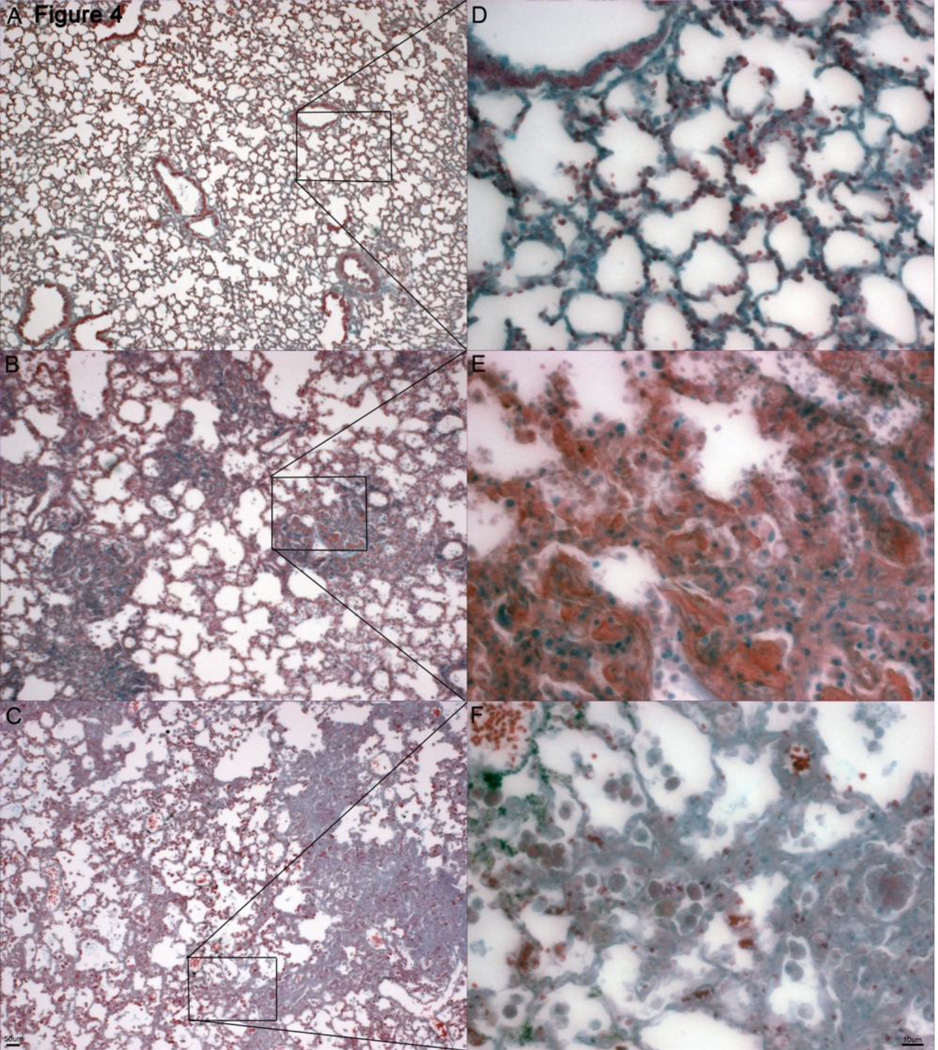
Fibrin Deposition increases in Pulmonary Tissue of PECAM-1 Deficient FVBn Mice with Increasing Disease Stage
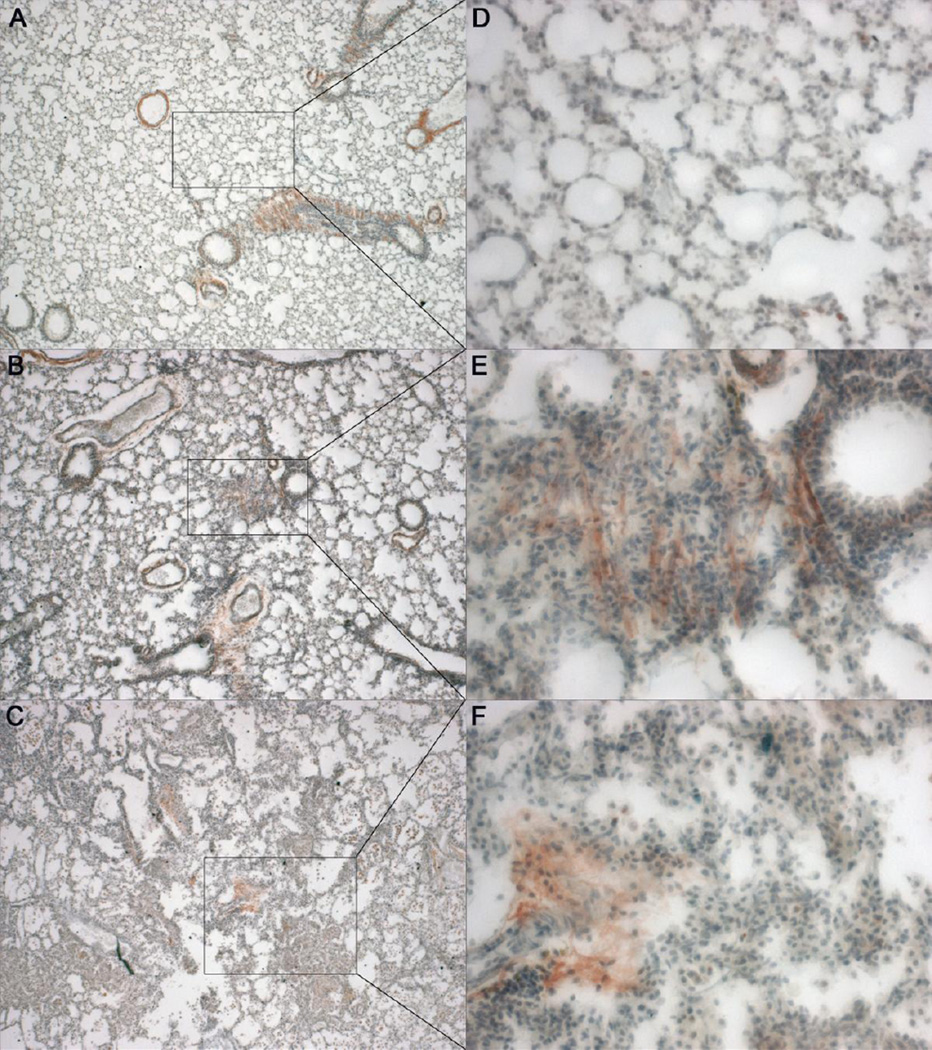
Pentachrome stain was used to detect deposition of fibrin as the disease progressed (Fig. 4). Fibrin stains as light, transparent pink in the healthy mice, intense pinkish-red in the borderline moderate animal, and diffuse pink in the severe animal, probably due to macrophage clearance of the fibrin. Fibrin deposition was observed to increase as disease pathology progressed, but in the severe, highly fibrotic areas it was not as intense, which may be due to the macrophage clearance of fibrin deposits, however significant hyaline membrane (fibrin polymers) formation were not found. That may be due to the fact that mice are euthanized for humane care considerations once they fall below 65% blood oxygen saturation, which does not allow enough time for the membranes to form. Another possibility is that where hyaline membranes are a typical presence in acute lung injury, the injury in PECAM-1 deficient animals consists of chronic and slow microbleeds, which may account for a different response.
Figure 4.
Pentachrome stain for fibrin deposition in healthy and diseased lung. A, D. Healthy PECAM-1 deficient mouse with very minimal to no fibrin deposition (pinkish-red stain)) around alveoli and interstitia (4X and 32X). B, E. Borderline PECAM-1 deficient mouse showing an increase in fibrin deposition in the fibrotic lesions (pinkred stain) (4X and 32X). C, F. Severe PECAM-1 deficient mouse showing further fibrin deposition (pink-red stain), which appears to be less intense in the areas of dense fibrosis and more pervasive in the areas of still somewhat retained lung architecture.
Presence of Myofibroblasts in Pulmonary Tissue of PECAM-1 Deficient FVBn Mice
In order to determine the phenotypic characteristics of the interstitial fibroblasts, antibody against α-Smooth Muscle Actin (SMA) was used to detect the presence of myofibroblasts, and has shown a strongly increased presence of myofibroblasts in areas of fibrotic lesion formation (Fig. 5). Animals in the moderate disease stage were stained for α-SMA and show developing lesions with the characteristic swarming “swimming fish” pattern of myofibroblasts, clearly visible in brown. The presence of fibroblasts expressing smooth muscle actin is indicative of type I collagen secretion, an increase in the deposition of extracellular matrix and an increase in the contractile function of the pulmonary parenchyma, all of which contribute to the development of fibrotic foci (10). We've previously shown the deposition of collagen in lesions of these mice (9).
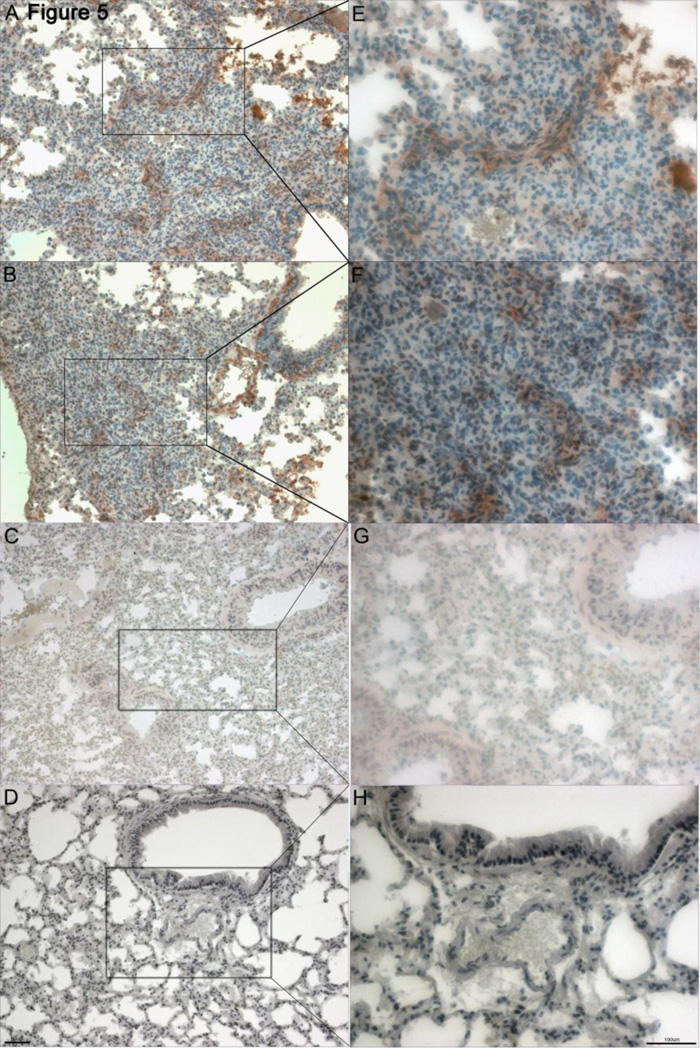
Figure 5.
Anti-smooth muscle actin stain in diseased PECAM-1 deficient mouse lung and wild type FVB/n lung. A, E, B, F. Moderate-severe PECAM-1 deficient mouse lung showing a heavy presence of myofibroblasts (brown stain) in a characteristic swarming swimming fish pattern around areas of collagen deposition (10X and 20X). C, G. Wild type FVB/n mouse was used as control. Some background staining is present in light brown around the interstitia and vasculature (10X and 20X). D, H. Tissue from a borderline PECAM-1 deficient mouse was used as a negative control due to shortage of tissue on the moderate-severe blocks (10X and 20X).
IL-10 Expression increases with fibrosis in PECAM-1 deficient mice
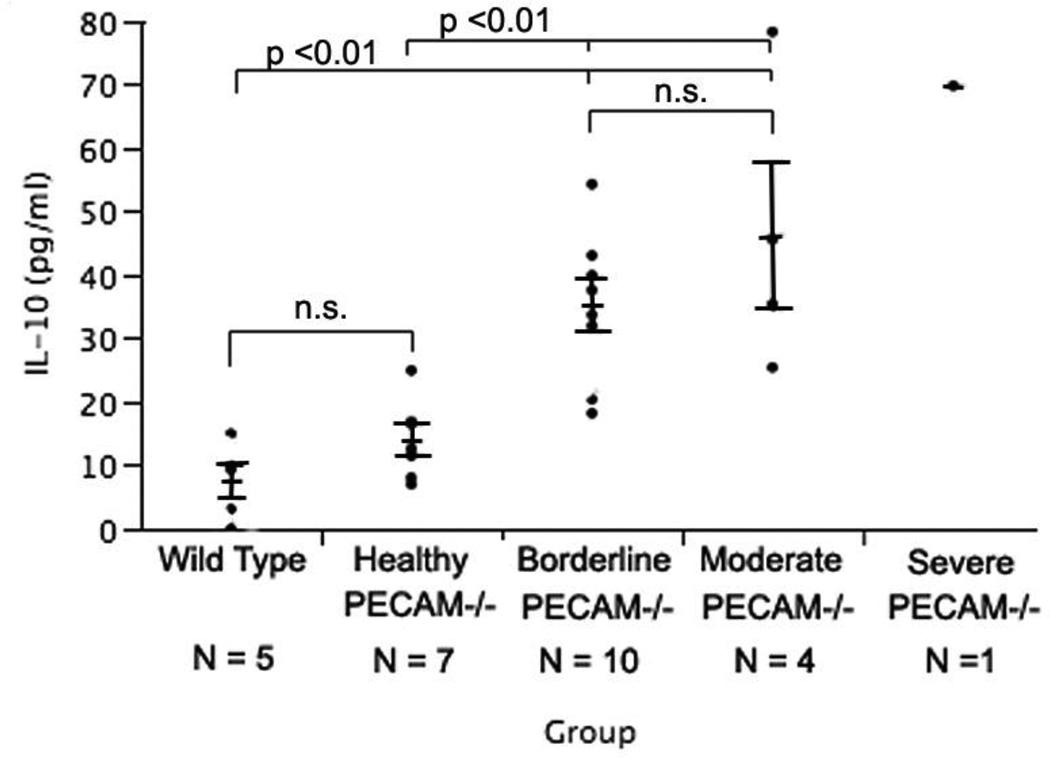
IL-10 is reported to be an anti-inflammatory cytokine and new evidence is coming forward that in some cases it may actually play a pro-fibrotic role (11). We expected the levels of IL-10 to increase with the disease progression in our animals. Both Wild type and Healthy PECAM-1 deficient mice showed significant difference from Borderline PECAM-1 deficient mice (Fig. 6). WT and Healthy PECAM-1 deficient mice did not differ significantly from each other. Borderline and Moderate PECAM-1 deficient mice also did not differ significantly from each other. Only one mouse classified as Severe was available for analysis and included for comparison. Qualitative evaluation of IL-10 expression per disease stage by immunohistochemistry (Fig. 7) indicated that IL-10 was present in greater amount in fibrotic lung tissue and increased as disease stage progressed. IL-10 was detected mainly around and in the fibrotic lesions, supporting the assumption that erythrocytosis and consequent iron release by macrophages, together with the hypoxic environment, may be contributing greatly to increased expression of IL-10. We did see IL-10 expression around major airways in both control and diseased lungs, possibly expressed in the airway smooth muscle or airway fibroblast populations (12–13).
Figure 6.
IL-10 concentrations from lung lysates of both Wild type and Healthy PECAM−/− mice was significantly different from Borderline PECAM−/− and Moderate PECAM−/− mice (p 0.01, Tukey-Kramer HSD). Wild type mice were not significantly different from healthy PECAM−/− mice. Borderline PECAM−/− mice were not significantly different from Moderate PECAM−/− mice. Unfortunately, only one mouse could be classified as Severe but it's data is shown here for comparison.
Figure 7.
IHC for IL-10 expression in healthy and diseased lung. A, D. Healthy PECAM-1 deficient mouse with minor IL-10 expression deposition (reddish-brown color) around alveoli and interstitia (4X and 20X). B, E. Borderline-Moderate PECAM-1 deficient mouse showing an increase in IL-10 expression in the fibrotic lesions (reddish-brown color) (4X and 20X). C, F. Moderate-Severe PECAM-1 deficient mouse showing further fincrease in IL-10 expression (reddish-brown color), visible more intensely around developing lesions and more diffuse in areas of existing dense fibrosis.
PECAM-1 Deficient Mice in the FVB/n Background Have Increased Bleeding Times
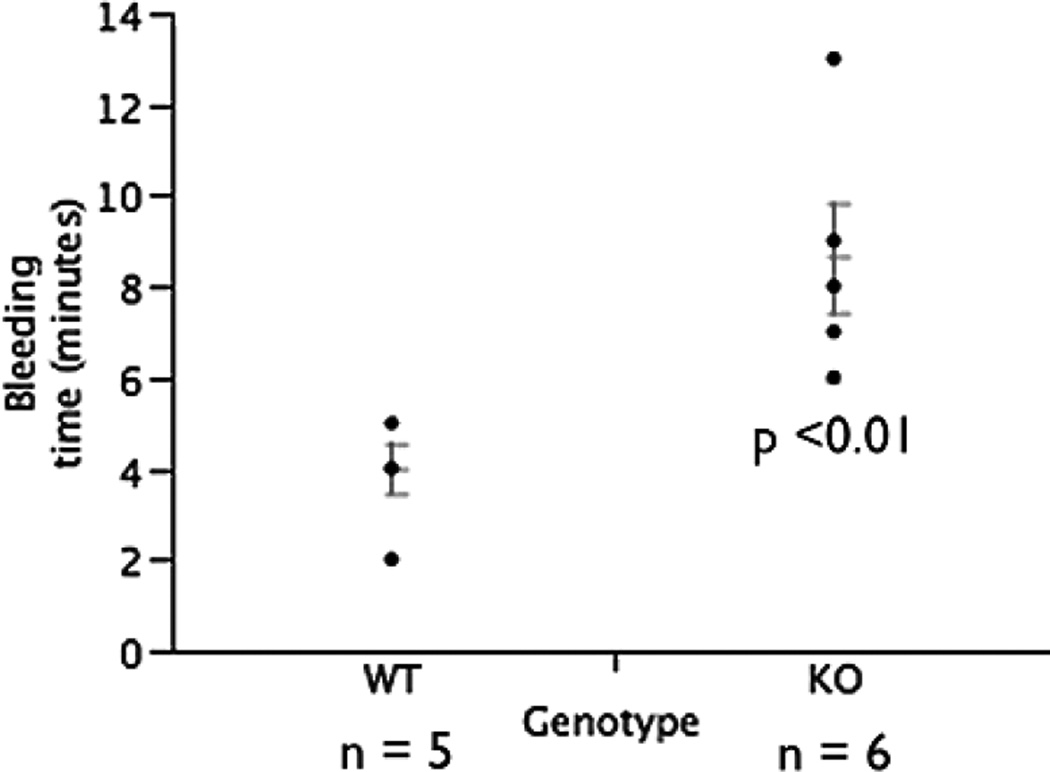
PECAM-1 deficient FVB/n mice demonstrated significantly increased bleeding times. Average bleeding time of wild type mice was approximately 4 minutes (Fig. 8). In PECAM-1 deficient mice, the average bleeding time was 8.6 minutes. This result agrees with Mahooti et al, who found that this is due to the overall vascular weakness due to missing endothelial PECAM-1 and not PECAM-1 on platelets s in the PECAM-1 deficient mice in bone marrow chimeras (14).
Figure 8.
Increased bleeding times in PECAM-1 deficient FVB/n compared to wild type control FVB/n mice. N = X wild type mice, N = Y PECAM deficient mice. p < 0.01 Student's T test.
DISCUSSION
Our current working hypothesis is that PECAM-1 dependent mechanisms, regulated by PECAM on platelets and/or endothelial cells, are essential for controlling vascular integrity in the lung. These mechanisms may include cell-cell communication, thrombus activation, and endothelial cell survival. There is increasing evidence that vascular microinjury and coagulation are instrumental in the development of pulmonary fibrotic disease. Magro et al. has shown the role of vascular microinjury by anti-endothelial cell antibodies, extravasated red blood cells in alveoli and interstitia, and hemosiderin-positive alveolar and interstitial macrophages in IPF (15). In 2012, Noth et al. assessed the role clotting plays in IPF by showing in a large clinical trial that anti-coagulant therapies exacerbated the process in IPF patients (8). Several studies show that components of the pro-coagulant and anti-coagulant pathways, such as Tissue Factor upregulation, decreased anticoagulant Protein C, and thrombin generation are instrumental in fibrotic events. The current coagulation hypothesis in human interstitial lung disease is that the formation of a clot in the lung, followed by activation of protease activated receptors (PAR1 and PAR2), leads to the fibrotic response (6). One of the immediate consequences of pulmonary microbleeds is the deposition of fibrin in the interstitia and alveoli. While a normal, uninjured lung has an environment that is antithrombotic and pro-fibrinolytic, a chronic, repetitive injury appears to shift the balance towards procoagulant and anti-fibrinolytic events.
Numerous studies have demonstrated that the stability of the endothelial cell junctions in the blood vessels in PECAM deficient mice is far easier to disrupt when these animals are subjected to physiological stress (17–18). PECAM-1 is important for vascularization and alveolarization. Anti-PECAM-1 antibody treatment of rat pups inhibited endothelial cell (EC) migration but not proliferation or survival in vitro, thereby disrupting the development of normal alveolar septae without reducing the actual EC content. Studies in PECAM-deficient C57 mice showed impaired alveolarization in spite of unaffected EC content, proliferation, or survival, while EC migration was reduced. Taken together, these studies provide evidence that it is the inhibition of EC function rather than loss of ECs that inhibits alveolarization in PECAM-1 deficient C57 mice (19). In 2005, two studies demonstrated, that when challenged with LPS, the blood vessels of PECAM-1-deficient mice showed increased permeability and a much increased loss of blood volume, with the resulting lethal drop in blood pressure (18, 20). Privratsky et al. found that unlike PECAM-1-deficient endothelial cells, PECAM-1 competent endothelial cells show increased barrier stability and quickly regain barrier integrity after challenging them with a thrombin-induced disruption to the monolayer (17). The prolonged tail-vein bleeding times in PECAM-1 deficient mice points towards the function of the vascular endothelium, rather then circulating platelets (14), although we are examining the role PECAM-1 in the functioning of platelets as well.
In contrast to diseases like Diffuse Alveolar Damage or Idiopathic Pulmonary Hemosiderosis, we see a relatively mild bleeding and alveolar collapse that eventually manifests into a chronic fibrosis, although both Diffuse Alveolar Damage or Idiopathic Pulmonary Hemosiderosis do involve fibrosis. Additionally, one of the hallmarks of our model is the relative absence of a pro-inflammatory cell infiltrate (such as neutrophils), and indeed our animals exhibit an anti-inflammatory profile. Based on other murine models of pulmonary fibrosis (21) our a priori hypothesis for this study was that inappropriate inflammation played a major role in disease initiation. One of the inflammatory cell types commonly present in an inflammatory response are macrophages, such as the classically activated pro-inflammatory M1 type and various subtypes of the alternatively activated pro-repair M2 type. M2 macrophages express arginase-1 (Arg-1), Fizz1, chitinase-3-like-3 (Ym1), and mannose receptor C-type lectin-1. A subtype of regulatory macrophages is marked by the high production of IL-10 and low IL-12, and their immunosuppressive qualities (22–23). We previously found high numbers of Ym1 positive M2 macrophages (24). In this current study, we found increased IL-10 levels, both in BAL and in the tissue of these animals. Extravasated red blood cells and iron can also polarize macrophages towards a M2 or repair phenotype (25). This likely contributes to the accumulation of Ym1- postitive alternatively activated macrophages that were found in our first examination of the fibrotic disease in PECAM-1 deficient mice (24). Interestingly, corticosteroids polarize macrophages towards the M2 phenotype, which might explain why these drugs have been found ineffective against fibrotic disease (26).
Hemosiderin is an iron-storage complex composed of ferritin, denatured ferritin and other material, located exclusively within cells. Hemosiderin is often found in macrophages after a hemorrhage. After blood exits a compromised blood vessel, the red blood cells die, releasing their hemoglobin. Macrophages phagocytose the hemoglobin and degrade it into hemosiderin and billiverdin (27). In an inflammatory response, pro-inflammatory M1 macrophages take up and use the iron bacteriostatically. Alternatively activated M2 macrophages exhibit high numbers of scavenger receptors (CD163). These CD163 receptors play a dual role: they let the M2 macrophages take up iron very rapidly, and they allow the M2 macrophages to maintain a ferritin-low/ferroportinhigh phenotype that allows the M2 macrophages to donate iron to their immediate surroundings (25). Additionally, the M2 macrophages make anti-inflammatory mediators through the heme-oxygenase-dependent heme catabolism. Finally, the M2 phenotype is suspected of providing iron to fibroblasts for collagen synthesis, thus contributing to inappropriate tissue repair in the context of microbleeds (25). We found that iron-positive macrophages increased as disease progressed, however, we then observed a slightly lessened but continued presence in the severe disease areas of dense fibrosis (Fig. 1). This would indicate that the microbleed leakage is no longer as intense in areas of dense fibrosis.
CONCLUSIONS
We conclude that this data supports the notion that PECAM-1-mediated adhesion and/or signaling contributes appreciably to vascular integrity and maintenance of a stable vascular permeability barrier, especially following disrupting stimuli, and that the absence of PECAM-1 is leading to increased vascular permeability and subsequent pulmonary microbleeds in these mice. Although the DeLisser study demonstrated transiently-delayed alveolarization in the PECAM-1 deficient C57 mice (19), we have not yet performed these studies in FVB/n PECAM-1 deficient mice partially due to breeding difficulties with this strain. We find that heterozygous breeding yields on average 10% FVB/n PECAM-1 deficient pups rather than the expected 25%, indicating that there may be potential embryonic lethality at vascularization and/or alveolarization stages phases of development. These questions will be the focus of future studies.
HIGHLIGHTS.
PECAM-1 deficient FVB/n mouse model does not require stimulus such as bleomycin, and shows spontaneous disease initiation, which closer models human fibrotic pulmonary disease.
generation of spontaneous disease in PECAM-1 deficient mice suggests that PECAM-1 is an important regulator of vascular barrier function.
pulmonary microbleeds and coagulation have been increasingly implicated in human pulmonary fibrotic diseases.
extravasated red blood cells in alveoli and interstitia, and hemosiderin-positive alveolar and interstitial macrophages have been shown in IPF cases previously (15).
extravasated red blood cells and iron have previously been shown to polarize macrophages towards a M2 or repair phenotype
ACKNOWLEDGEMENTS
The authors want to thank Bianca Junge, Sheryl Carter, and Elisa French (Colorado State University Animal Facilities) for expert animal care, Dr. Anne Lenaerts and Dr. Gavin Ryan for kindly allowing the use of histology instruments and reagents, and Jamie S. Schenkel for critical reading of the manuscript.
FUNDING
This study was supported by National Institutes of Health R37 HL064774 (W.A.M.), a graduate student training fellowship from the United States Department of Agriculture Food and Nutrition Service Animal Biosecurity Pathology Training Grant, and a Student Experiential Learning grant funded by the College of Veterinary Medicine and Biomedical Sciences, Colorado State University.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Ethics Statement
All animal experimental protocols approved by the Colorado State University Animal Care and Use Committee, under guidelines issued by the United States National Institutes of Health and in concordance with the Declaration of Helsinki.
License for Publication
The Corresponding Author has the right to grant on behalf of all authors and does grant on behalf of all authors, an exclusive license (or non exclusive for government employees) on a worldwide basis to the Elsevier Publishing Group Ltd and its Licensees to permit this article (if accepted) to be published in Experimental and Molecular Pathology editions and any other Elsevier products.
COMPETING INTEREST STATEMENTS
Competing Interests: None to declare.
Author contributions:
Marta Lishnevsky and Alan R. Schenkel conceived and designed the experiments.
Marta Lishnevsky, Lena C. Young, David M. Higgins, John M. Gilchrist, and Steven J. Woods performed the experiments.
Mercedes Gonzalez-Juarrero kindly donated the IL-10 primary antibody.
Marta Lishnevsky and Alan R. Schenkel analyzed the data.
Marta Lishnevsky and Alan R. Schenkel wrote the manuscript.
Steven D. Groshong and Randall J. Basaraba kindly contributed their expertise in pathology.
Todd A. Bass kindly contributed reagents/materials, and expertise in histology.
William A. Muller kindly contributed the FVB/n PECAM-1 deficient mice for the research.
Contributor Information
Marta Lishnevsky, Department of Microbiology, Immunology, and Pathology Colorado State University 1682 Campus Delivery, Fort Collins, CO 80523 United States, Tel: (720) 938-8234, lishnevM@gmail.com.
Lena C. Young, Department of Microbiology, Immunology, and Pathology Colorado State University Fort Collins, Colorado United States
Steven J. Woods, Department of Microbiology, Immunology, and Pathology Colorado State University Fort Collins, Colorado United States
Steven D. Groshong, Division of Pathology, National Jewish Health, Denver, Colorado United States
Randall J. Basaraba, Department of Microbiology, Immunology, and Pathology Colorado State University Fort Collins, Colorado United States
John M. Gilchrist, Department of Physiology, School of Medicine, John Hopkins University, Baltimore, Maryland United States
David M. Higgins, School of Medicine, University of Colorado Health Sciences Center, Denver, Colorado, United States
Mercedes Gonzalez-Juarrero, Department of Microbiology, Immunology, and Pathology Colorado State University Fort Collins, Colorado United States.
Todd A. Bass, Histology Core Services, Veterinary Teaching Hospital, Colorado State University, Fort Collins, Colorado United States
William A. Muller, Department of Pathology, Northwestern University, Chicago, Illinois
Alan R. Schenkel, Department of Microbiology, Immunology, and Pathology Colorado State University Fort Collins, Colorado United States
REFERENCES
- 1.Muller WA, Weigl SA, Deng X, et al. PECAM-1 is required for transendothelial migration of leukocytes. J Exp Med. 1993;178:449–460. doi: 10.1084/jem.178.2.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Privratsky JR, Newman DK, Newman PJ. PECAM-1: conflicts of interest in inflammation. Life Sci. 2010;87(3–4):69–82. doi: 10.1016/j.lfs.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bogen S, Pak J, Garifallou M, et al. Monoclonal antibody to murine PECAM-1 [CD31] blocks acute inflammation in vivo. J. Exp. Med. 1994;179:1059–1064. doi: 10.1084/jem.179.3.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ming Z, Hu Y, Xiang J, et al. Lyn and PECAM-1 function as interdependent inhibitors of platelet aggregation. Blood. 2011;117:3903–3906. doi: 10.1182/blood-2010-09-304816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boon K, Bailey NW, Yang J, et al. Molecular Phenotypes Distinguish Patients with Relatively Stable from Progressive Idiopathic Pulmonary Fibrosis (IPF) [cited 2012 December 2];PLoS ONE. 2009 4(4):e5134. doi: 10.1371/journal.pone.0005134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chambers RC. Procoagulant signaling mechanisms in lung inflammation and fibrosis: novel opportunities for pharmacological intervention? Br. J. Pharmacol. 2008;153(Suppl 1):367–378. doi: 10.1038/sj.bjp.0707603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ruppert C, Markart P, Wygrecka M, et al. Role of coagulation and fibrinolysis in lung and renal fibrosis. Hamostaseologie. 2008;28:30–36. [PubMed] [Google Scholar]
- 8.Noth I, Anstrom KJ, Calvert SB. A Placebo-Controlled Randomized Trial of Warfarin in Idiopathic Pulmonary Fibrosis. Am. J. Respir. Crit. Care Med. 2012;186:88–95. doi: 10.1164/rccm.201202-0314OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Early MA, Lishnevsky M, Gilchrist JM. Non-invasive diagnosis of early pulmonary disease in PECAM-deficient mice using infrared pulse oximetry. Exp. Mol. Pathol. 2009;87(2):152–158. doi: 10.1016/j.yexmp.2009.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bogatkevich GS, Tourkina E, Silver RM. Thrombin differentiates normal lung fibroblasts to a myofibroblast phenotype via the proteolytically activated receptor-1 and a protein kinase C-dependent pathway. J. Biol. Chem. 2001;276:45184–45192. doi: 10.1074/jbc.M106441200. [DOI] [PubMed] [Google Scholar]
- 11.Barbarin V, Xing Z, Delos M, et al. Pulmonary overexpression of Il-10 augments lung fibrosis and Th2 responses induced by silica particles. AJP-Lung Physiol. 2005;288(5):L841–L848. doi: 10.1152/ajplung.00329.2004. [DOI] [PubMed] [Google Scholar]
- 12.Singh SR, Hall IP. Airway myofibroblasts and their relationship with airway myocytes and fibroblasts. Proc. Am. Thorac. Soc. 2008;5(1):127–132. doi: 10.1513/pats.200706-070VS. [DOI] [PubMed] [Google Scholar]
- 13.Ina K, Kusugami K, Kawano Y, et al. Intestinal fibroblast-derived IL-10 increases survival of mucosal T-cells by inhibiting growth factor deprovation and Fas-mediated apoptosis. J. Immunol. 2005;175(3):2000–2009. doi: 10.4049/jimmunol.175.3.2000. [DOI] [PubMed] [Google Scholar]
- 14.Mahooti S, Graesser D, Patil S, et al. PECAM-1 (CD31) expression modulates bleeding time in vivo. Am. J. Pathol. 2000;157:75–81. doi: 10.1016/S0002-9440(10)64519-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Magro CM, Waldman WJ, Knight DA. Idiopathic pulmonary fibrosis related to endothelial injury and antiendothelial cell antibodies. Hum. Immunol. 2006;67:284–297. doi: 10.1016/j.humimm.2006.02.026. [DOI] [PubMed] [Google Scholar]
- 16.Idell S. Coagulation, fibrinolysis, and fibrin deposition in acute lung injury. Crit. Care Med. 2003;31(4 Suppl):S213–S220. doi: 10.1097/01.CCM.0000057846.21303.AB. [DOI] [PubMed] [Google Scholar]
- 17.Privratsky JR, Paddock CM, Florey O, et al. Relative contribution of PECAM-1 adhesion and signaling to the maintenance of vascular integrity. J. Cell. Sci. 2011;124:1477–1485. doi: 10.1242/jcs.082271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maas M, Stapleton M, Bergom C, et al. Endothelial cell PECAM-1 confers protection against endotoxic shock. Am. J. Physiol. Heart Circ. Physiol. 2005;288(1):H159–H164. doi: 10.1152/ajpheart.00500.2004. [DOI] [PubMed] [Google Scholar]
- 19.DeLisser HM, Helmke BP, Cao G, et al. Loss of PECAM-1 function impairs alveolarization. J. Biol. Chem. 2006;28:8724–8731. doi: 10.1074/jbc.M511798200. [DOI] [PubMed] [Google Scholar]
- 20.Carrithers M, Tandon S, Canosa S, et al. Enhanced susceptibility to endotoxic shock and impaired STAT3 signaling in CD31-deficient mice. Am. J. Pathol. 2005;166:185–196. doi: 10.1016/S0002-9440(10)62243-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moore BB, Hogaboam CM. Murine models of pulmonary fibrosis. Lung Cell. Mol. Phys. 2007;294:L152–L160. doi: 10.1152/ajplung.00313.2007. [DOI] [PubMed] [Google Scholar]
- 22.Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat. Rev. Immunol. 2008;8:958–969. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gordon S, Martinez FO. Alternative activation of macrophages: mechanism and functions. Immunity. 2010;32:593–604. doi: 10.1016/j.immuni.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 24.Schenkel AR, Chew TW, Chlipala E, et al. Different susceptabilities of PECAM-deficient mice to spontaneous idiopathic pneumonitis. Exp. Mol. Pathol. 2006;81(1):23–30. doi: 10.1016/j.yexmp.2005.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gaetano C, Locati M, Mantovani A. Control of iron homeostasis as a key component of macrophage polarization. Haemotologica. 2010;95(11):1801–1803. doi: 10.3324/haematol.2010.030239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.John M, Lim S, Seybold J, et al. Inhaled corticosteroids increase interleukin-10 but reduce macrophage inflammatory protein 1-alpha, granulocyte-macrophages colony-stimulating factor, and interferon-gamma release from alveolar macrophages in asthma. Am. J. Respir. Crit. Care Med. 1998;157:256–262. doi: 10.1164/ajrccm.157.1.9703079. [DOI] [PubMed] [Google Scholar]
- 27.Bowen R. Ferritins and Hemosiderin. 2001 http://www.vivo.colostate.edu/hbooks/molecules/ferritin.html. [Google Scholar]