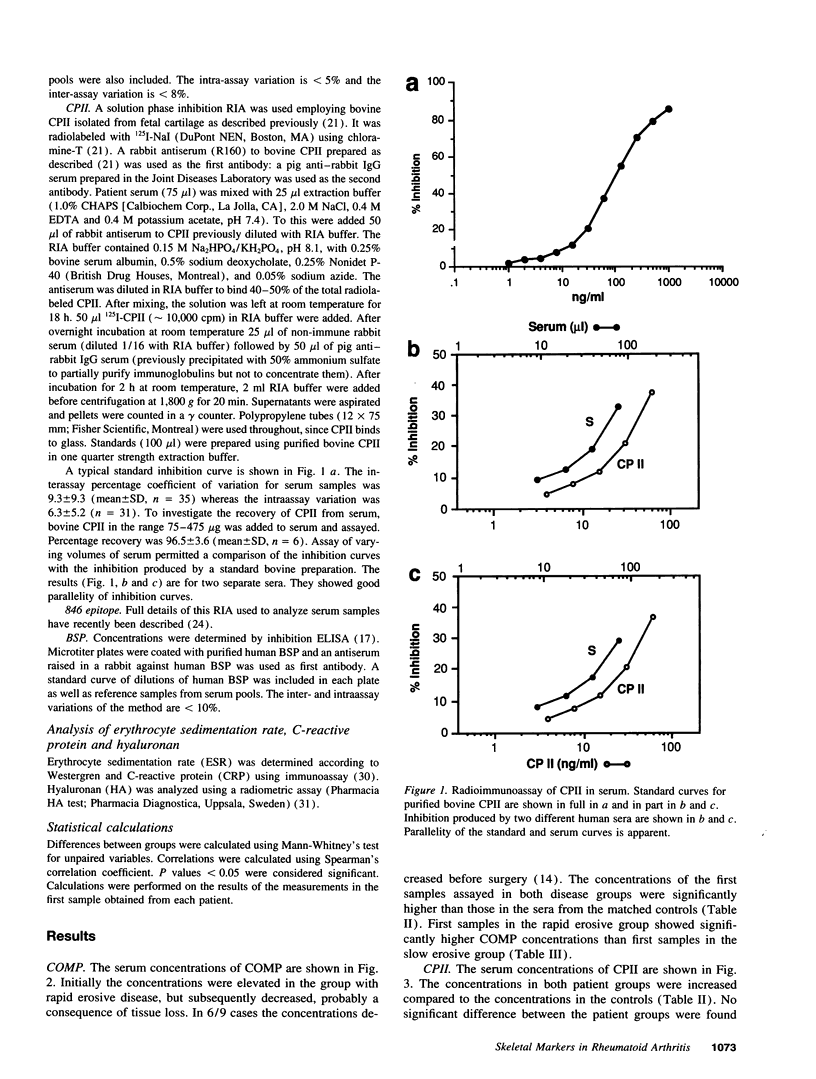
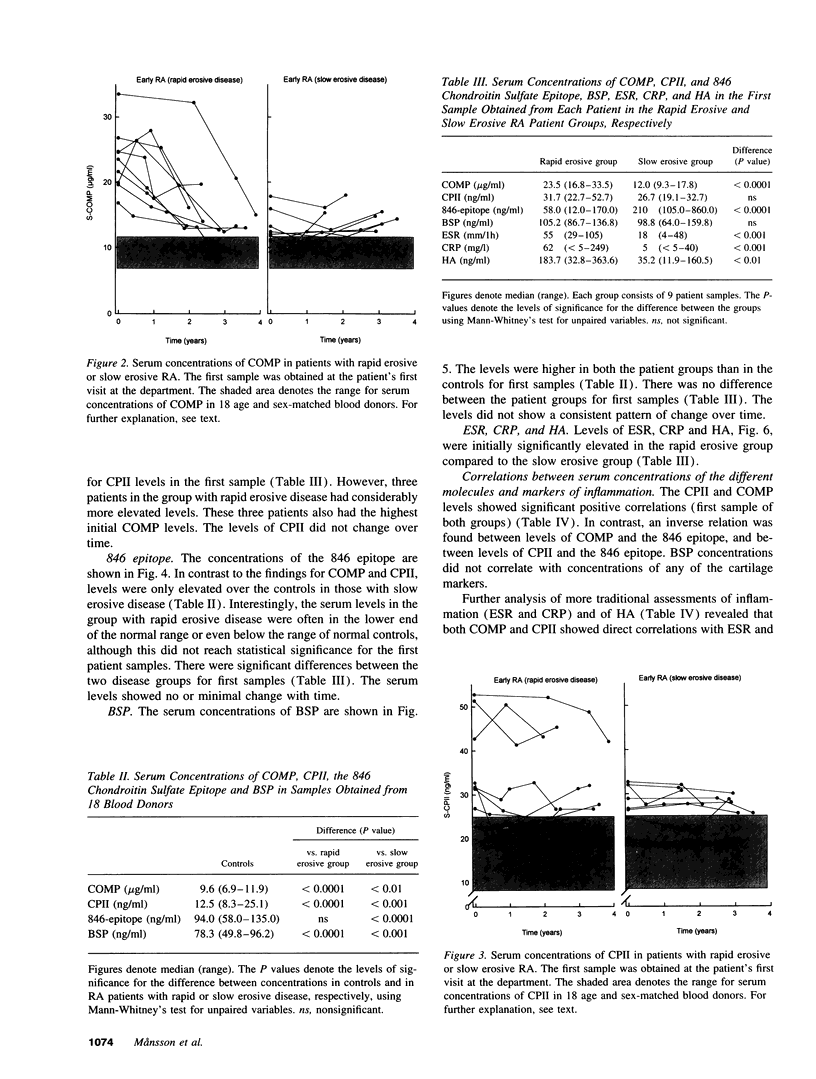
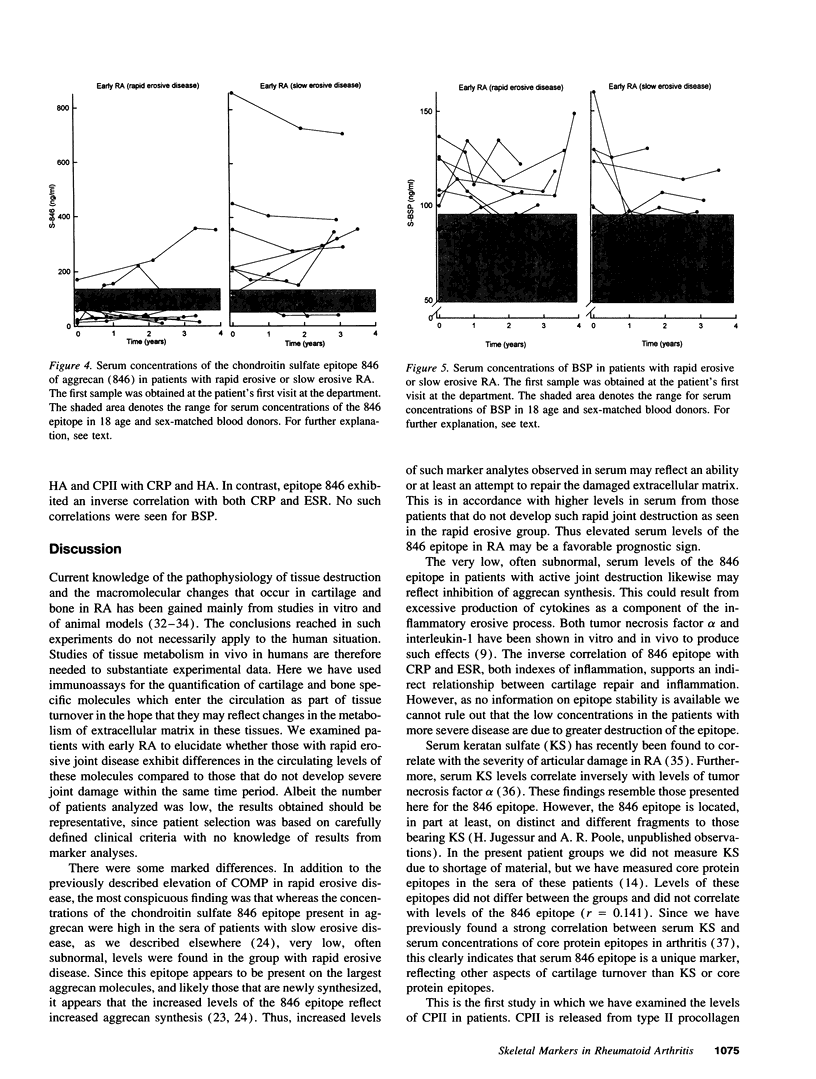
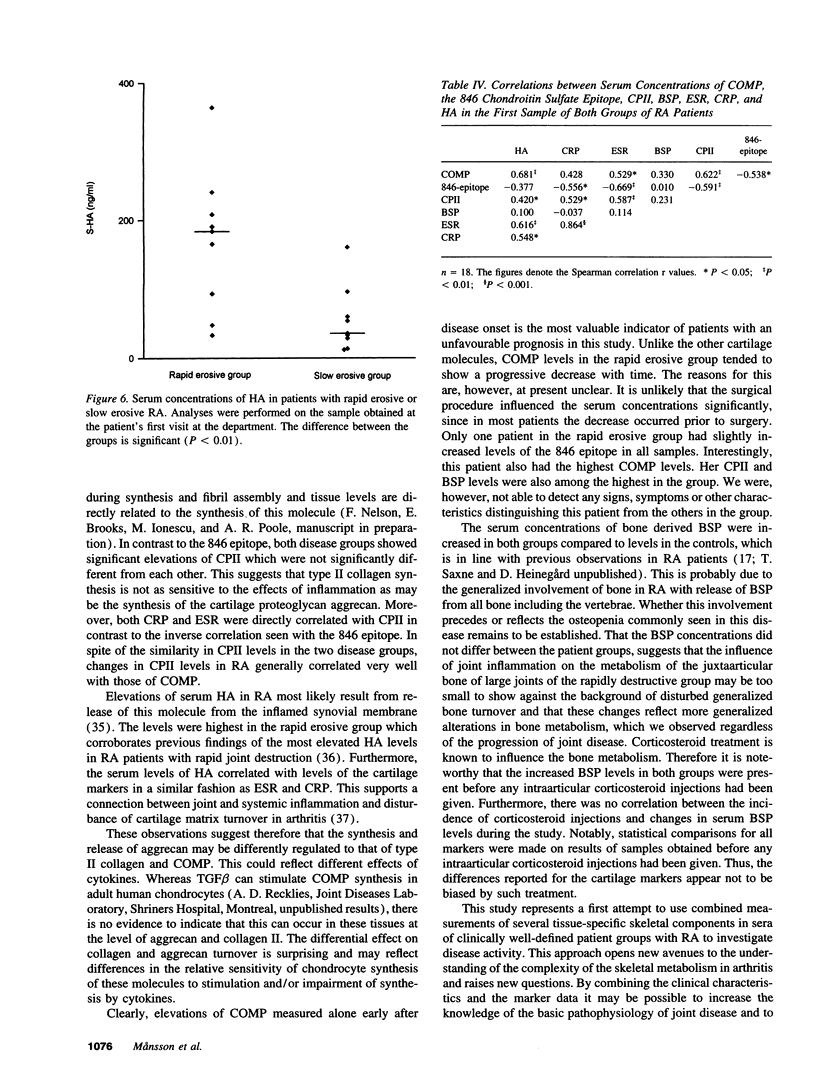
Abstract
Serum concentrations of specific cartilage and bone molecules reflecting tissue turnover were measured in two well-defined patient groups with early rheumatoid arthritis with distinctly different disease outcome to see if early differences in their levels are prognostic of the rate of joint destruction. Compared with a matched normal population, increased concentrations of cartilage oligomeric matrix protein (COMP) were found in all patients who developed rapid hip joint destruction. In contrast, levels of a putative marker of cartilage aggrecan synthesis, the chondroitin sulfate epitope 846, were increased only in patients with slow joint destruction. Levels of bone sialoprotein (BSP) were increased in both groups, as were levels of the C-propeptide of type II procollagen (CPII), a marker of collagen II synthesis. The increased concentrations of the 846 epitope in patients with slow joint destruction suggest increased aggrecan synthesis. The low levels of the 846 epitope in patients with rapid joint destruction, concomitant with elevated levels of CPII, suggest a selective increase in collagen synthesis. The elevated BSP levels indicate an increased bone turnover in both groups. Thus elevated serum levels of COMP may indicate an unfavorable prognosis for rapid joint destruction, whereas elevated 846 epitope indicates a more favorable prognosis.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnett F. C., Edworthy S. M., Bloch D. A., McShane D. J., Fries J. F., Cooper N. S., Healey L. A., Kaplan S. R., Liang M. H., Luthra H. S. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988 Mar;31(3):315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- Brandt R., Hedlöf E., Asman I., Bucht A., Tengblad A. A convenient radiometric assay for hyaluronan. Acta Otolaryngol Suppl. 1987;442:31–35. doi: 10.3109/00016488709102835. [DOI] [PubMed] [Google Scholar]
- Choi H. U., Tang L. H., Johnson T. L., Pal S., Rosenberg L. C., Reiner A., Poole A. R. Isolation and characterization of a 35,000 molecular weight subunit fetal cartilage matrix protein. J Biol Chem. 1983 Jan 10;258(1):655–661. [PubMed] [Google Scholar]
- Eberhardt K. B., Rydgren L. C., Pettersson H., Wollheim F. A. Early rheumatoid arthritis--onset, course, and outcome over 2 years. Rheumatol Int. 1990;10(4):135–142. doi: 10.1007/BF02274837. [DOI] [PubMed] [Google Scholar]
- Forslind K., Eberhardt K., Jonsson A., Saxne T. Increased serum concentrations of cartilage oligomeric matrix protein. A prognostic marker in early rheumatoid arthritis. Br J Rheumatol. 1992 Sep;31(9):593–598. doi: 10.1093/rheumatology/31.9.593. [DOI] [PubMed] [Google Scholar]
- Glant T. T., Mikecz K., Roughley P. J., Buzás E., Poole A. R. Age-related changes in protein-related epitopes of human articular-cartilage proteoglycans. Biochem J. 1986 May 15;236(1):71–75. doi: 10.1042/bj2360071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haraoui B., Thonar E. J., Martel-Pelletier J., Goulet J. R., Raynauld J. P., Ouellet M., Pelletier J. P. Serum keratan sulfate levels in rheumatoid arthritis: inverse correlation with radiographic staging. J Rheumatol. 1994 May;21(5):813–817. [PubMed] [Google Scholar]
- Harris E. D., Jr Rheumatoid arthritis. Pathophysiology and implications for therapy. N Engl J Med. 1990 May 3;322(18):1277–1289. doi: 10.1056/NEJM199005033221805. [DOI] [PubMed] [Google Scholar]
- Hedbom E., Antonsson P., Hjerpe A., Aeschlimann D., Paulsson M., Rosa-Pimentel E., Sommarin Y., Wendel M., Oldberg A., Heinegård D. Cartilage matrix proteins. An acidic oligomeric protein (COMP) detected only in cartilage. J Biol Chem. 1992 Mar 25;267(9):6132–6136. [PubMed] [Google Scholar]
- Hedin P. J., Weitoft T., Hedin H., Engström-Laurent A., Saxne T. Serum concentrations of hyaluronan and proteoglycan in joint disease. Lack of association. J Rheumatol. 1991 Oct;18(10):1601–1605. [PubMed] [Google Scholar]
- Heinegård D., Oldberg A. Structure and biology of cartilage and bone matrix noncollagenous macromolecules. FASEB J. 1989 Jul;3(9):2042–2051. doi: 10.1096/fasebj.3.9.2663581. [DOI] [PubMed] [Google Scholar]
- Heinegård D., Saxne T. Molecular markers of processes in cartilage in joint disease. Br J Rheumatol. 1991;30 (Suppl 1):21–24. [PubMed] [Google Scholar]
- Hinek A., Poole A. R. The influence of vitamin D metabolites on the calcification of cartilage matrix and the C-propeptide of type II collagen (chondrocalcin). J Bone Miner Res. 1988 Aug;3(4):421–429. doi: 10.1002/jbmr.5650030409. [DOI] [PubMed] [Google Scholar]
- Hunter G. K., Goldberg H. A. Nucleation of hydroxyapatite by bone sialoprotein. Proc Natl Acad Sci U S A. 1993 Sep 15;90(18):8562–8565. doi: 10.1073/pnas.90.18.8562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen A., Dale K., Eek M. Radiographic evaluation of rheumatoid arthritis and related conditions by standard reference films. Acta Radiol Diagn (Stockh) 1977 Jul;18(4):481–491. doi: 10.1177/028418517701800415. [DOI] [PubMed] [Google Scholar]
- Laurell C. B. Electroimmuno assay. Scand J Clin Lab Invest Suppl. 1972;124:21–37. doi: 10.3109/00365517209102748. [DOI] [PubMed] [Google Scholar]
- Manicourt D. H., Triki R., Fukuda K., Devogelaer J. P., Nagant de Deuxchaisnes C., Thonar E. J. Levels of circulating tumor necrosis factor alpha and interleukin-6 in patients with rheumatoid arthritis. Relationship to serum levels of hyaluronan and antigenic keratan sulfate. Arthritis Rheum. 1993 Apr;36(4):490–499. doi: 10.1002/art.1780360409. [DOI] [PubMed] [Google Scholar]
- Mankin H. J., Lippiello L. The glycosaminoglycans of normal and arthritic cartilage. J Clin Invest. 1971 Aug;50(8):1712–1719. doi: 10.1172/JCI106660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mörgelin M., Heinegård D., Engel J., Paulsson M. Electron microscopy of native cartilage oligomeric matrix protein purified from the Swarm rat chondrosarcoma reveals a five-armed structure. J Biol Chem. 1992 Mar 25;267(9):6137–6141. [PubMed] [Google Scholar]
- Oldberg A., Antonsson P., Lindblom K., Heinegård D. COMP (cartilage oligomeric matrix protein) is structurally related to the thrombospondins. J Biol Chem. 1992 Nov 5;267(31):22346–22350. [PubMed] [Google Scholar]
- Paimela L., Heiskanen A., Kurki P., Helve T., Leirisalo-Repo M. Serum hyaluronate level as a predictor of radiologic progression in early rheumatoid arthritis. Arthritis Rheum. 1991 Jul;34(7):815–821. doi: 10.1002/art.1780340706. [DOI] [PubMed] [Google Scholar]
- Poole A. R. Immunochemical markers of joint inflammation, skeletal damage and repair: where are we now? Ann Rheum Dis. 1994 Jan;53(1):3–5. doi: 10.1136/ard.53.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole A. R., Ionescu M., Swan A., Dieppe P. A. Changes in cartilage metabolism in arthritis are reflected by altered serum and synovial fluid levels of the cartilage proteoglycan aggrecan. Implications for pathogenesis. J Clin Invest. 1994 Jul;94(1):25–33. doi: 10.1172/JCI117314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole A. R., Witter J., Roberts N., Piccolo F., Brandt R., Paquin J., Baron M. Inflammation and cartilage metabolism in rheumatoid arthritis. Studies of the blood markers hyaluronic acid, orosomucoid, and keratan sulfate. Arthritis Rheum. 1990 Jun;33(6):790–799. doi: 10.1002/art.1780330605. [DOI] [PubMed] [Google Scholar]
- Rizkalla G., Reiner A., Bogoch E., Poole A. R. Studies of the articular cartilage proteoglycan aggrecan in health and osteoarthritis. Evidence for molecular heterogeneity and extensive molecular changes in disease. J Clin Invest. 1992 Dec;90(6):2268–2277. doi: 10.1172/JCI116113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxne T., Heinegård D. Cartilage oligomeric matrix protein: a novel marker of cartilage turnover detectable in synovial fluid and blood. Br J Rheumatol. 1992 Sep;31(9):583–591. doi: 10.1093/rheumatology/31.9.583. [DOI] [PubMed] [Google Scholar]
- Thompson R. C., Jr, Oegema T. R., Jr Metabolic activity of articular cartilage in osteoarthritis. An in vitro study. J Bone Joint Surg Am. 1979 Apr;61(3):407–416. [PubMed] [Google Scholar]
- Thonar E. J., Shinmei M., Lohmander L. S. Body fluid markers of cartilage changes in osteoarthritis. Rheum Dis Clin North Am. 1993 Aug;19(3):635–657. [PubMed] [Google Scholar]
- Wollheim F. A., Pettersson H., Saxne T., Sjöblom K. G. Radiographic assessment in relation to clinical and biochemical variables in rheumatoid arthritis. Scand J Rheumatol. 1988;17(6):445–453. doi: 10.3109/03009748809098805. [DOI] [PubMed] [Google Scholar]
- van der Heijde D. M., van Riel P. L., van Leeuwen M. A., van 't Hof M. A., van Rijswijk M. H., van de Putte L. B. Prognostic factors for radiographic damage and physical disability in early rheumatoid arthritis. A prospective follow-up study of 147 patients. Br J Rheumatol. 1992 Aug;31(8):519–525. doi: 10.1093/rheumatology/31.8.519. [DOI] [PubMed] [Google Scholar]
- van der Heijde D. M., van Riel P. L., van Rijswijk M. H., van de Putte L. B. Influence of prognostic features on the final outcome in rheumatoid arthritis: a review of the literature. Semin Arthritis Rheum. 1988 May;17(4):284–292. doi: 10.1016/0049-0172(88)90013-3. [DOI] [PubMed] [Google Scholar]


