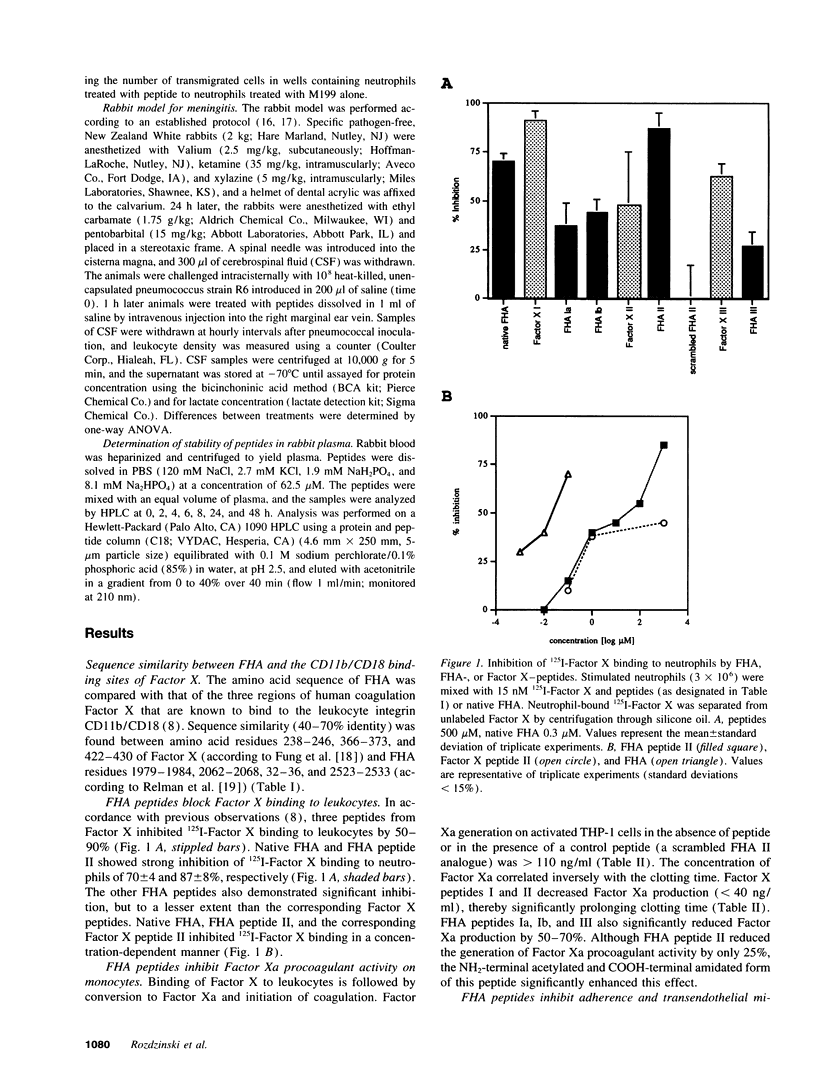
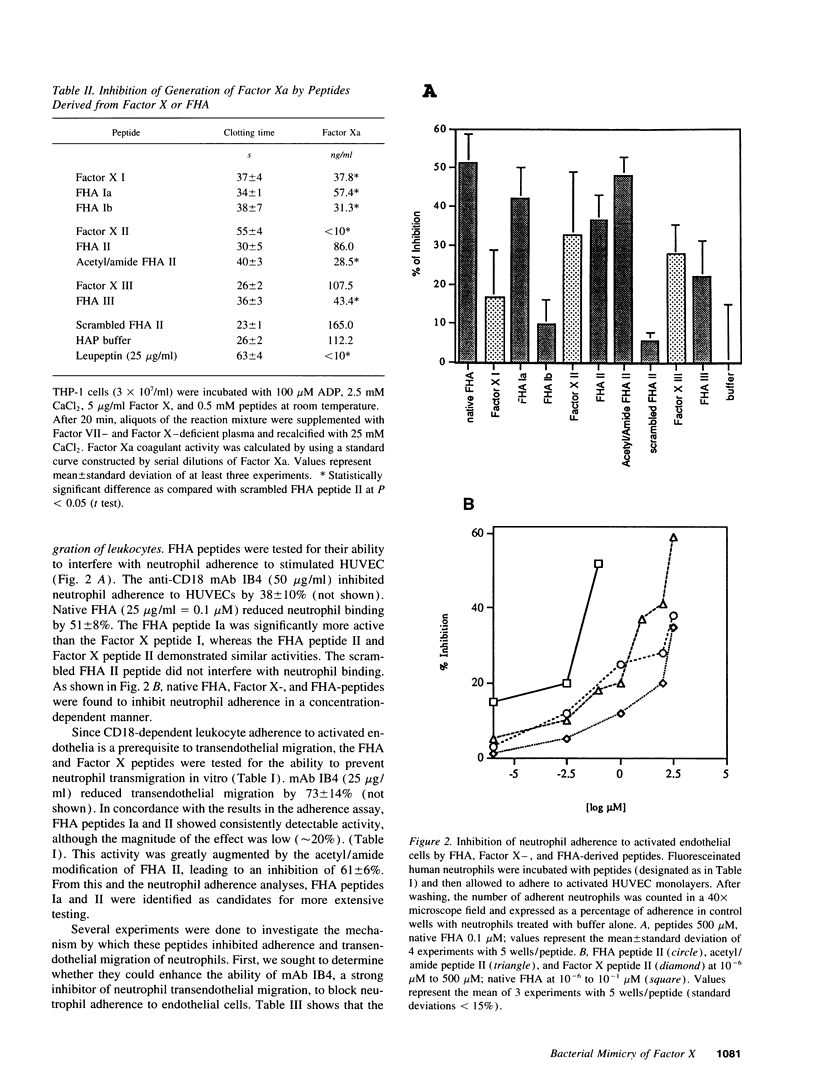
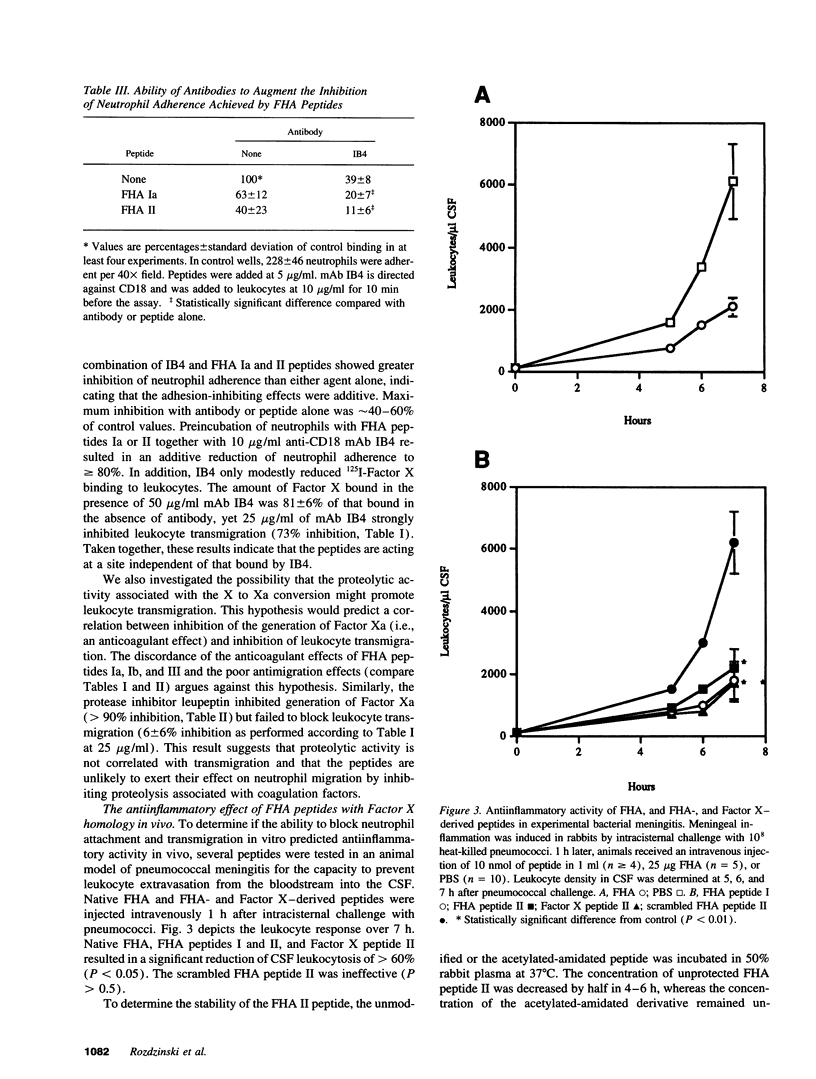
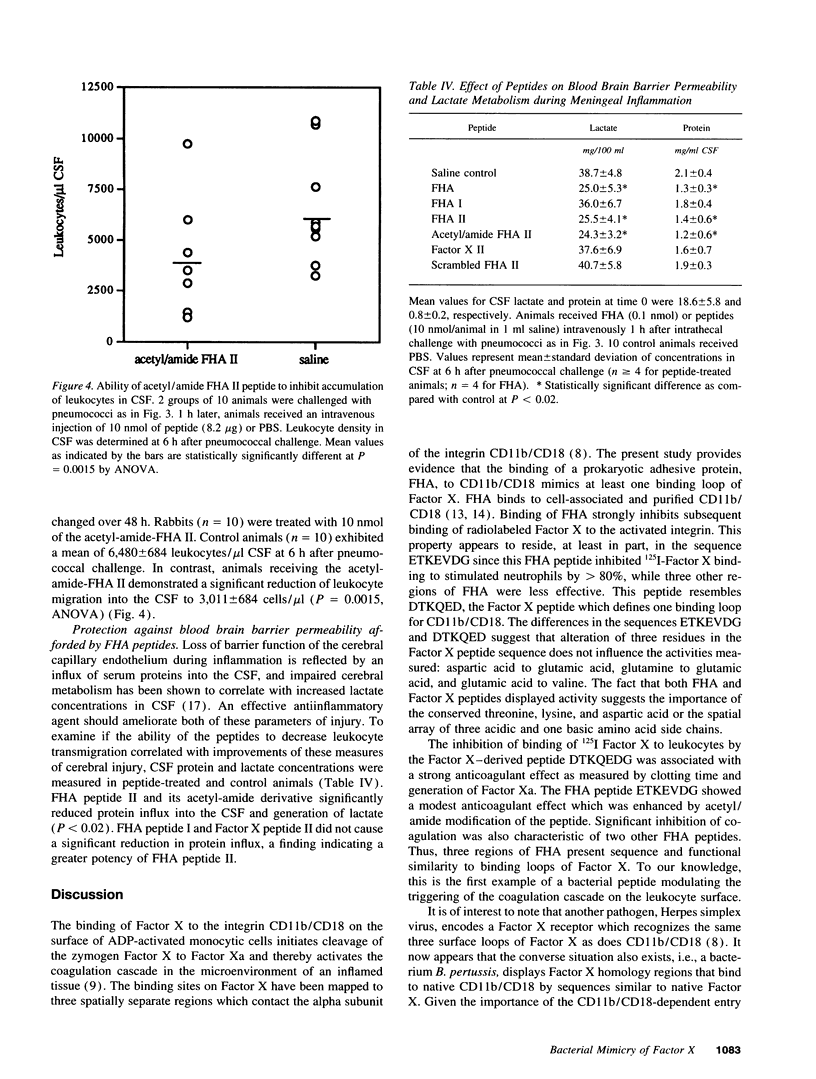
Abstract
Factor X (factor ten) of the coagulation cascade binds to the integrin CD11b/CD18 during inflammation, initiating procoagulant activity on the surface of leukocytes (Altieri, D.C., O.R. Etingin, D.S. Fair, T.K. Brunk, J.E. Geltosky, D.P. Hajjar, and T. S. Edgington. 1991. Science [Wash.DC]. 254:1200-1202). Filamentous hemagglutinin (FHA), an adhesin of Bordetella pertussis also binds to the CD11b/CD18 integrin (Relman D., E. Tuomanen, S. Falkow, D.T. Golenbock, K. Saukkonen, and S.D. Wright. 1990. Cell. 61:1375-1382). FHA and the CD11b/CD18 binding loops of Factor X share amino acid sequence similarity. FHA peptides similar to Factor X binding loops inhibited 125I-Factor X binding to human neutrophils and prolonged clotting time. In addition, ETKEVDG and its Factor X analogue prevented transendothelial migration of leukocytes in vitro and reduced leukocytosis and blood brain barrier disruption in vivo. Interference with leukocyte migration by a coagulation-based peptide suggests a novel strategy for antiinflammatory therapy.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altieri D. C., Agbanyo F. R., Plescia J., Ginsberg M. H., Edgington T. S., Plow E. F. A unique recognition site mediates the interaction of fibrinogen with the leukocyte integrin Mac-1 (CD11b/CD18). J Biol Chem. 1990 Jul 25;265(21):12119–12122. [PubMed] [Google Scholar]
- Altieri D. C., Edgington T. S. The saturable high affinity association of factor X to ADP-stimulated monocytes defines a novel function of the Mac-1 receptor. J Biol Chem. 1988 May 25;263(15):7007–7015. [PubMed] [Google Scholar]
- Altieri D. C., Etingin O. R., Fair D. S., Brunck T. K., Geltosky J. E., Hajjar D. P., Edgington T. S. Structurally homologous ligand binding of integrin Mac-1 and viral glycoprotein C receptors. Science. 1991 Nov 22;254(5035):1200–1202. doi: 10.1126/science.1957171. [DOI] [PubMed] [Google Scholar]
- Altieri D. C., Morrissey J. H., Edgington T. S. Adhesive receptor Mac-1 coordinates the activation of factor X on stimulated cells of monocytic and myeloid differentiation: an alternative initiation of the coagulation protease cascade. Proc Natl Acad Sci U S A. 1988 Oct;85(20):7462–7466. doi: 10.1073/pnas.85.20.7462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dacey R. G., Sande M. A. Effect of probenecid on cerebrospinal fluid concentrations of penicillin and cephalosporin derivatives. Antimicrob Agents Chemother. 1974 Oct;6(4):437–441. doi: 10.1128/aac.6.4.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond M. S., Staunton D. E., de Fougerolles A. R., Stacker S. A., Garcia-Aguilar J., Hibbs M. L., Springer T. A. ICAM-1 (CD54): a counter-receptor for Mac-1 (CD11b/CD18). J Cell Biol. 1990 Dec;111(6 Pt 2):3129–3139. doi: 10.1083/jcb.111.6.3129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung M. R., Hay C. W., MacGillivray R. T. Characterization of an almost full-length cDNA coding for human blood coagulation factor X. Proc Natl Acad Sci U S A. 1985 Jun;82(11):3591–3595. doi: 10.1073/pnas.82.11.3591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlan J. M., Killen P. D., Senecal F. M., Schwartz B. R., Yee E. K., Taylor R. F., Beatty P. G., Price T. H., Ochs H. D. The role of neutrophil membrane glycoprotein GP-150 in neutrophil adherence to endothelium in vitro. Blood. 1985 Jul;66(1):167–178. [PubMed] [Google Scholar]
- Hoepelman A. I., Tuomanen E. I. Consequences of microbial attachment: directing host cell functions with adhesins. Infect Immun. 1992 May;60(5):1729–1733. doi: 10.1128/iai.60.5.1729-1733.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence M. B., Springer T. A. Leukocytes roll on a selectin at physiologic flow rates: distinction from and prerequisite for adhesion through integrins. Cell. 1991 May 31;65(5):859–873. doi: 10.1016/0092-8674(91)90393-d. [DOI] [PubMed] [Google Scholar]
- Lo S. K., Lee S., Ramos R. A., Lobb R., Rosa M., Chi-Rosso G., Wright S. D. Endothelial-leukocyte adhesion molecule 1 stimulates the adhesive activity of leukocyte integrin CR3 (CD11b/CD18, Mac-1, alpha m beta 2) on human neutrophils. J Exp Med. 1991 Jun 1;173(6):1493–1500. doi: 10.1084/jem.173.6.1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miletich J. P., Jackson C. M., Majerus P. W. Properties of the factor Xa binding site on human platelets. J Biol Chem. 1978 Oct 10;253(19):6908–6916. [PubMed] [Google Scholar]
- Muller W. A., Weigl S. A. Monocyte-selective transendothelial migration: dissection of the binding and transmigration phases by an in vitro assay. J Exp Med. 1992 Sep 1;176(3):819–828. doi: 10.1084/jem.176.3.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odio C. M., Faingezicht I., Paris M., Nassar M., Baltodano A., Rogers J., Sáez-Llorens X., Olsen K. D., McCracken G. H., Jr The beneficial effects of early dexamethasone administration in infants and children with bacterial meningitis. N Engl J Med. 1991 May 30;324(22):1525–1531. doi: 10.1056/NEJM199105303242201. [DOI] [PubMed] [Google Scholar]
- Quagliarello V., Scheld W. M. Bacterial meningitis: pathogenesis, pathophysiology, and progress. N Engl J Med. 1992 Sep 17;327(12):864–872. doi: 10.1056/NEJM199209173271208. [DOI] [PubMed] [Google Scholar]
- Relman D. A., Domenighini M., Tuomanen E., Rappuoli R., Falkow S. Filamentous hemagglutinin of Bordetella pertussis: nucleotide sequence and crucial role in adherence. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2637–2641. doi: 10.1073/pnas.86.8.2637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Relman D., Tuomanen E., Falkow S., Golenbock D. T., Saukkonen K., Wright S. D. Recognition of a bacterial adhesion by an integrin: macrophage CR3 (alpha M beta 2, CD11b/CD18) binds filamentous hemagglutinin of Bordetella pertussis. Cell. 1990 Jun 29;61(7):1375–1382. doi: 10.1016/0092-8674(90)90701-f. [DOI] [PubMed] [Google Scholar]
- Saukkonen K., Cabellos C., Burroughs M., Prasad S., Tuomanen E. Integrin-mediated localization of Bordetella pertussis within macrophages: role in pulmonary colonization. J Exp Med. 1991 May 1;173(5):1143–1149. doi: 10.1084/jem.173.5.1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuomanen E. I., Saukkonen K., Sande S., Cioffe C., Wright S. D. Reduction of inflammation, tissue damage, and mortality in bacterial meningitis in rabbits treated with monoclonal antibodies against adhesion-promoting receptors of leukocytes. J Exp Med. 1989 Sep 1;170(3):959–969. doi: 10.1084/jem.170.3.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuomanen E., Liu H., Hengstler B., Zak O., Tomasz A. The induction of meningeal inflammation by components of the pneumococcal cell wall. J Infect Dis. 1985 May;151(5):859–868. doi: 10.1093/infdis/151.5.859. [DOI] [PubMed] [Google Scholar]
- Tuomanen E., Weiss A. Characterization of two adhesins of Bordetella pertussis for human ciliated respiratory-epithelial cells. J Infect Dis. 1985 Jul;152(1):118–125. doi: 10.1093/infdis/152.1.118. [DOI] [PubMed] [Google Scholar]
- Van Strijp J. A., Russell D. G., Tuomanen E., Brown E. J., Wright S. D. Ligand specificity of purified complement receptor type three (CD11b/CD18, alpha m beta 2, Mac-1). Indirect effects of an Arg-Gly-Asp (RGD) sequence. J Immunol. 1993 Sep 15;151(6):3324–3336. [PubMed] [Google Scholar]
- Wright S. D., Rao P. E., Van Voorhis W. C., Craigmyle L. S., Iida K., Talle M. A., Westberg E. F., Goldstein G., Silverstein S. C. Identification of the C3bi receptor of human monocytes and macrophages by using monoclonal antibodies. Proc Natl Acad Sci U S A. 1983 Sep;80(18):5699–5703. doi: 10.1073/pnas.80.18.5699. [DOI] [PMC free article] [PubMed] [Google Scholar]


