Abstract
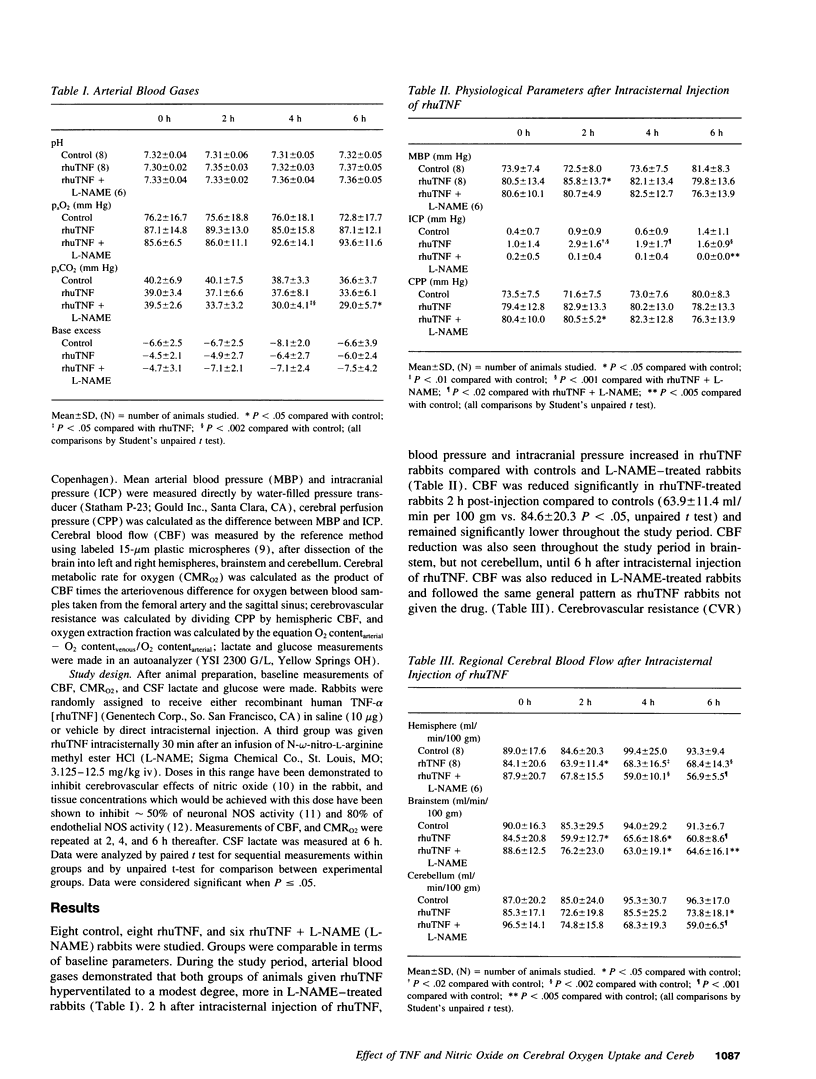
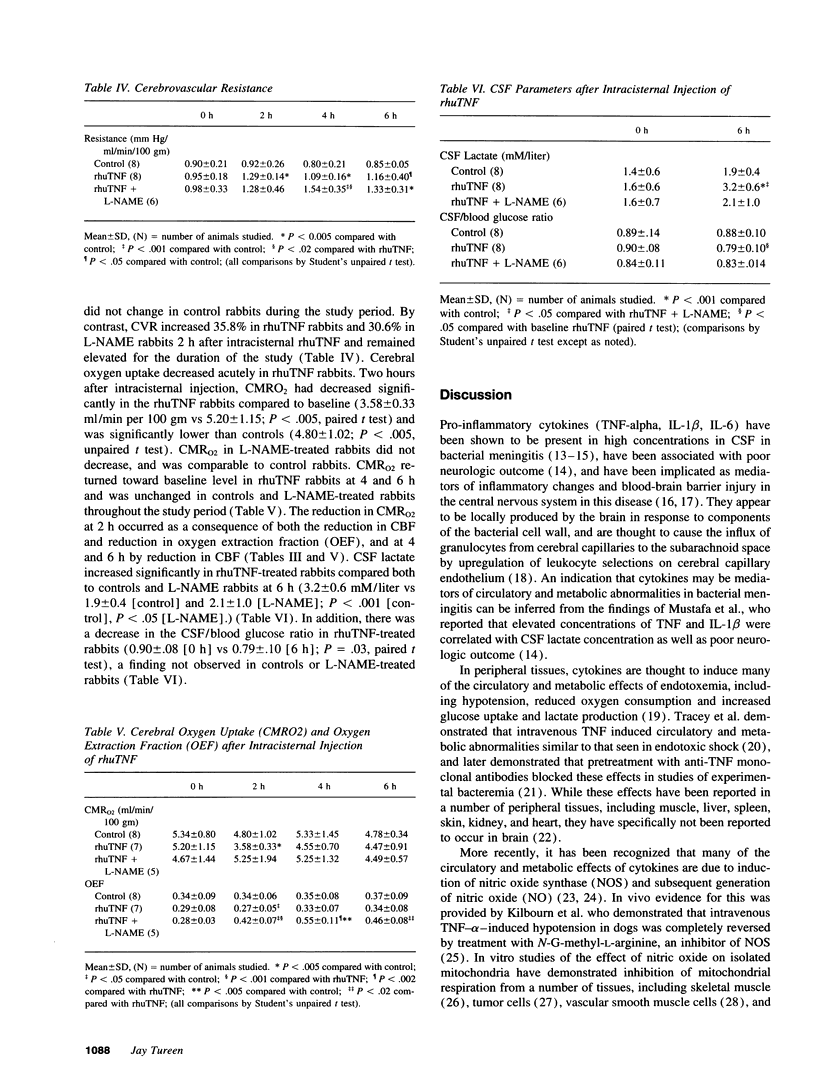
Among the important pathophysiologic alterations in the brain in bacterial meningitis are abnormalities of cerebral circulation and metabolism; however, the precise mechanisms by which these disturbances occur are not completely delineated. It has been recently recognized that cytokines are produced by tissues in the central nervous system in meningitis and play a critical role in the host inflammatory response. Because these mediators are involved in circulatory and metabolic disturbances in other tissues in sepsis, we investigated the role of tumor necrosis factor-alpha in the central nervous system in a rabbit model. We found that injection of recombinant human TNF into the cisterna magna in the rabbit led to an acute reduction in cerebral oxygen uptake and a more prolonged reduction in cerebral blood flow. This was accompanied by an increase in intracranial pressure and an increase in cerebrospinal fluid lactate. Reduction in oxygen uptake and increases in intracranial pressure and CSF lactate were blocked by pretreatment with L-NAME, an inhibitor of nitric oxide synthase. Reduction in cerebral blood flow was not affected by L-NAME treatment and was due to increased cerebrovascular resistance and reduced oxygen demand. These results suggest that TNF may be a critical mediator of changes in cerebral circulation and metabolism and that some of these changes occur via the nitric oxide pathway.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brook I., Bricknell K. S., Overturf G. D., Finegold S. M. Measurement of lactic acid in cerebrospinal fluid of patients with infections of the central nervous system. J Infect Dis. 1978 Apr;137(4):384–390. doi: 10.1093/infdis/137.4.384. [DOI] [PubMed] [Google Scholar]
- Busse R., Mülsch A. Induction of nitric oxide synthase by cytokines in vascular smooth muscle cells. FEBS Lett. 1990 Nov 26;275(1-2):87–90. doi: 10.1016/0014-5793(90)81445-t. [DOI] [PubMed] [Google Scholar]
- Cleeter M. W., Cooper J. M., Darley-Usmar V. M., Moncada S., Schapira A. H. Reversible inhibition of cytochrome c oxidase, the terminal enzyme of the mitochondrial respiratory chain, by nitric oxide. Implications for neurodegenerative diseases. FEBS Lett. 1994 May 23;345(1):50–54. doi: 10.1016/0014-5793(94)00424-2. [DOI] [PubMed] [Google Scholar]
- Faraci F. M., Heistad D. D. Does basal production of nitric oxide contribute to regulation of brain-fluid balance? Am J Physiol. 1992 Feb;262(2 Pt 2):H340–H344. doi: 10.1152/ajpheart.1992.262.2.H340. [DOI] [PubMed] [Google Scholar]
- Geng Y., Hansson G. K., Holme E. Interferon-gamma and tumor necrosis factor synergize to induce nitric oxide production and inhibit mitochondrial respiration in vascular smooth muscle cells. Circ Res. 1992 Nov;71(5):1268–1276. doi: 10.1161/01.res.71.5.1268. [DOI] [PubMed] [Google Scholar]
- Grubb R. L., Jr, Raichle M. E., Eichling J. O., Ter-Pogossian M. M. The effects of changes in PaCO2 on cerebral blood volume, blood flow, and vascular mean transit time. Stroke. 1974 Sep-Oct;5(5):630–639. doi: 10.1161/01.str.5.5.630. [DOI] [PubMed] [Google Scholar]
- Guerra-Romero L., Täuber M. G., Fournier M. A., Tureen J. H. Lactate and glucose concentrations in brain interstitial fluid, cerebrospinal fluid, and serum during experimental pneumococcal meningitis. J Infect Dis. 1992 Sep;166(3):546–550. doi: 10.1093/infdis/166.3.546. [DOI] [PubMed] [Google Scholar]
- Haberl R. L., Anneser F., Ködel U., Pfister H. W. Is nitric oxide involved as a mediator of cerebrovascular changes in the early phase of experimental pneumococcal meningitis? Neurol Res. 1994 Apr;16(2):108–112. doi: 10.1080/01616412.1994.11740205. [DOI] [PubMed] [Google Scholar]
- Heymann M. A., Payne B. D., Hoffman J. I., Rudolph A. M. Blood flow measurements with radionuclide-labeled particles. Prog Cardiovasc Dis. 1977 Jul-Aug;20(1):55–79. doi: 10.1016/s0033-0620(77)80005-4. [DOI] [PubMed] [Google Scholar]
- Hochwald G. M., Malhan C., Brown J. Effect of hypercapnia on CSF turnover and blood-CSF barrier to protein. Arch Neurol. 1973 Mar;28(3):150–155. doi: 10.1001/archneur.1973.00490210030002. [DOI] [PubMed] [Google Scholar]
- Iadecola C., Xu X., Zhang F., Hu J., el-Fakahany E. E. Prolonged inhibition of brain nitric oxide synthase by short-term systemic administration of nitro-L-arginine methyl ester. Neurochem Res. 1994 Apr;19(4):501–505. doi: 10.1007/BF00967330. [DOI] [PubMed] [Google Scholar]
- Kilbourn R. G., Gross S. S., Jubran A., Adams J., Griffith O. W., Levi R., Lodato R. F. NG-methyl-L-arginine inhibits tumor necrosis factor-induced hypotension: implications for the involvement of nitric oxide. Proc Natl Acad Sci U S A. 1990 May;87(9):3629–3632. doi: 10.1073/pnas.87.9.3629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsden P. A., Brenner B. M. Transcriptional regulation of the endothelin-1 gene by TNF-alpha. Am J Physiol. 1992 Apr;262(4 Pt 1):C854–C861. doi: 10.1152/ajpcell.1992.262.4.C854. [DOI] [PubMed] [Google Scholar]
- Megyeri P., Abrahám C. S., Temesvári P., Kovács J., Vas T., Speer C. P. Recombinant human tumor necrosis factor alpha constricts pial arterioles and increases blood-brain barrier permeability in newborn piglets. Neurosci Lett. 1992 Dec 14;148(1-2):137–140. doi: 10.1016/0304-3940(92)90823-p. [DOI] [PubMed] [Google Scholar]
- Mustafa M. M., Lebel M. H., Ramilo O., Olsen K. D., Reisch J. S., Beutler B., McCracken G. H., Jr Correlation of interleukin-1 beta and cachectin concentrations in cerebrospinal fluid and outcome from bacterial meningitis. J Pediatr. 1989 Aug;115(2):208–213. doi: 10.1016/s0022-3476(89)80067-8. [DOI] [PubMed] [Google Scholar]
- Mustafa M. M., Ramilo O., Olsen K. D., Franklin P. S., Hansen E. J., Beutler B., McCracken G. H., Jr Tumor necrosis factor in mediating experimental Haemophilus influenzae type B meningitis. J Clin Invest. 1989 Oct;84(4):1253–1259. doi: 10.1172/JCI114292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustafa M. M., Ramilo O., Sáez-Llorens X., Mertsola J., Magness R. R., McCracken G. H., Jr Prostaglandins E2 and I2, interleukin 1-beta, and tumor necrosis factor in cerebrospinal fluid in infants and children with bacterial meningitis. Pediatr Infect Dis J. 1989 Dec;8(12):921–922. doi: 10.1097/00006454-198912000-00045. [DOI] [PubMed] [Google Scholar]
- Mészáros K., Lang C. H., Bagby G. J., Spitzer J. J. Tumor necrosis factor increases in vivo glucose utilization of macrophage-rich tissues. Biochem Biophys Res Commun. 1987 Nov 30;149(1):1–6. doi: 10.1016/0006-291x(87)91596-8. [DOI] [PubMed] [Google Scholar]
- Natanson C., Eichenholz P. W., Danner R. L., Eichacker P. Q., Hoffman W. D., Kuo G. C., Banks S. M., MacVittie T. J., Parrillo J. E. Endotoxin and tumor necrosis factor challenges in dogs simulate the cardiovascular profile of human septic shock. J Exp Med. 1989 Mar 1;169(3):823–832. doi: 10.1084/jem.169.3.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson C., Lindvall-Axelsson M., Owman C. Simultaneous and continuous measurement of choroid plexus blood flow and cerebrospinal fluid production: effects of vasoactive intestinal polypeptide. J Cereb Blood Flow Metab. 1991 Sep;11(5):861–867. doi: 10.1038/jcbfm.1991.146. [DOI] [PubMed] [Google Scholar]
- Nussler A. K., Di Silvio M., Billiar T. R., Hoffman R. A., Geller D. A., Selby R., Madariaga J., Simmons R. L. Stimulation of the nitric oxide synthase pathway in human hepatocytes by cytokines and endotoxin. J Exp Med. 1992 Jul 1;176(1):261–264. doi: 10.1084/jem.176.1.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PETERSDORF R. G., SWARNER D. M., GARCIA M. Studies on the pathogenesis of meningitis. III. Relationship of phagocytosis to the fall in cerebrospinal fluid sugar in experimental pneumococcal meningitis. J Lab Clin Med. 1963 May;61:745–754. [PubMed] [Google Scholar]
- Paulson O. B., Brodersen P., Hansen E. L., Kristensen H. S. Regional cerebral blood flow, cerebral metabolic rate of oxygen, and cerebrospinal fluid acid-base variables in patients with acute meningitis and with acute encephalitis. Acta Med Scand. 1974 Sep;196(3):191–198. doi: 10.1111/j.0954-6820.1974.tb00994.x. [DOI] [PubMed] [Google Scholar]
- Pfister H. W., Koedel U., Haberl R. L., Dirnagl U., Feiden W., Ruckdeschel G., Einhäupl K. M. Microvascular changes during the early phase of experimental bacterial meningitis. J Cereb Blood Flow Metab. 1990 Nov;10(6):914–922. doi: 10.1038/jcbfm.1990.148. [DOI] [PubMed] [Google Scholar]
- Quagliarello V. J., Wispelwey B., Long W. J., Jr, Scheld W. M. Recombinant human interleukin-1 induces meningitis and blood-brain barrier injury in the rat. Characterization and comparison with tumor necrosis factor. J Clin Invest. 1991 Apr;87(4):1360–1366. doi: 10.1172/JCI115140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quagliarello V., Scheld W. M. Bacterial meningitis: pathogenesis, pathophysiology, and progress. N Engl J Med. 1992 Sep 17;327(12):864–872. doi: 10.1056/NEJM199209173271208. [DOI] [PubMed] [Google Scholar]
- Radi R., Rodriguez M., Castro L., Telleri R. Inhibition of mitochondrial electron transport by peroxynitrite. Arch Biochem Biophys. 1994 Jan;308(1):89–95. doi: 10.1006/abbi.1994.1013. [DOI] [PubMed] [Google Scholar]
- Rees D. D., Palmer R. M., Schulz R., Hodson H. F., Moncada S. Characterization of three inhibitors of endothelial nitric oxide synthase in vitro and in vivo. Br J Pharmacol. 1990 Nov;101(3):746–752. doi: 10.1111/j.1476-5381.1990.tb14151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saukkonen K., Sande S., Cioffe C., Wolpe S., Sherry B., Cerami A., Tuomanen E. The role of cytokines in the generation of inflammation and tissue damage in experimental gram-positive meningitis. J Exp Med. 1990 Feb 1;171(2):439–448. doi: 10.1084/jem.171.2.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schalk K. A., Faraci F. M., Williams J. L., VanOrden D., Heistad D. D. Effect of atriopeptin on production of cerebrospinal fluid. J Cereb Blood Flow Metab. 1992 Jul;12(4):691–696. doi: 10.1038/jcbfm.1992.94. [DOI] [PubMed] [Google Scholar]
- Scheld W. M., Dacey R. G., Winn H. R., Welsh J. E., Jane J. A., Sande M. A. Cerebrospinal fluid outflow resistance in rabbits with experimental meningitis. Alterations with penicillin and methylprednisolone. J Clin Invest. 1980 Aug;66(2):243–253. doi: 10.1172/JCI109850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadler J., Billiar T. R., Curran R. D., Stuehr D. J., Ochoa J. B., Simmons R. L. Effect of exogenous and endogenous nitric oxide on mitochondrial respiration of rat hepatocytes. Am J Physiol. 1991 May;260(5 Pt 1):C910–C916. doi: 10.1152/ajpcell.1991.260.5.C910. [DOI] [PubMed] [Google Scholar]
- Tracey K. J., Beutler B., Lowry S. F., Merryweather J., Wolpe S., Milsark I. W., Hariri R. J., Fahey T. J., 3rd, Zentella A., Albert J. D. Shock and tissue injury induced by recombinant human cachectin. Science. 1986 Oct 24;234(4775):470–474. doi: 10.1126/science.3764421. [DOI] [PubMed] [Google Scholar]
- Tracey K. J., Fong Y., Hesse D. G., Manogue K. R., Lee A. T., Kuo G. C., Lowry S. F., Cerami A. Anti-cachectin/TNF monoclonal antibodies prevent septic shock during lethal bacteraemia. Nature. 1987 Dec 17;330(6149):662–664. doi: 10.1038/330662a0. [DOI] [PubMed] [Google Scholar]
- Tucker S. D., Auzenne E. J., Sivaramakrishnan M. R. Inhibition of tumor cell mitochondrial respiration by macrophage cytotoxic mediators distinct from interferon-gamma. J Leukoc Biol. 1993 Feb;53(2):138–143. doi: 10.1002/jlb.53.2.138. [DOI] [PubMed] [Google Scholar]
- Tureen J. H., Dworkin R. J., Kennedy S. L., Sachdeva M., Sande M. A. Loss of cerebrovascular autoregulation in experimental meningitis in rabbits. J Clin Invest. 1990 Feb;85(2):577–581. doi: 10.1172/JCI114475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tureen J. H., Täuber M. G., Sande M. A. Effect of hydration status on cerebral blood flow and cerebrospinal fluid lactic acidosis in rabbits with experimental meningitis. J Clin Invest. 1992 Mar;89(3):947–953. doi: 10.1172/JCI115676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vemulapalli S., Chiu P. J., Rivelli M., Foster C. J., Sybertz E. J. Modulation of circulating endothelin levels in hypertension and endotoxemia in rats. J Cardiovasc Pharmacol. 1991 Dec;18(6):895–903. doi: 10.1097/00005344-199112000-00017. [DOI] [PubMed] [Google Scholar]
- WILLIAMS R. D. ALTERATIONS IN THE GLUCOSE TRANSPORT MECHANISM IN PATIENTS WITH COMPLICATIONS OF BACTERIAL MENINGITIS. Pediatrics. 1964 Oct;34:491–502. [PubMed] [Google Scholar]
- Waage A., Halstensen A., Shalaby R., Brandtzaeg P., Kierulf P., Espevik T. Local production of tumor necrosis factor alpha, interleukin 1, and interleukin 6 in meningococcal meningitis. Relation to the inflammatory response. J Exp Med. 1989 Dec 1;170(6):1859–1867. doi: 10.1084/jem.170.6.1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh N., Sandler S. Interleukin-1 beta induces nitric oxide production and inhibits the activity of aconitase without decreasing glucose oxidation rates in isolated mouse pancreatic islets. Biochem Biophys Res Commun. 1992 Jan 15;182(1):333–340. doi: 10.1016/s0006-291x(05)80149-4. [DOI] [PubMed] [Google Scholar]



