Abstract
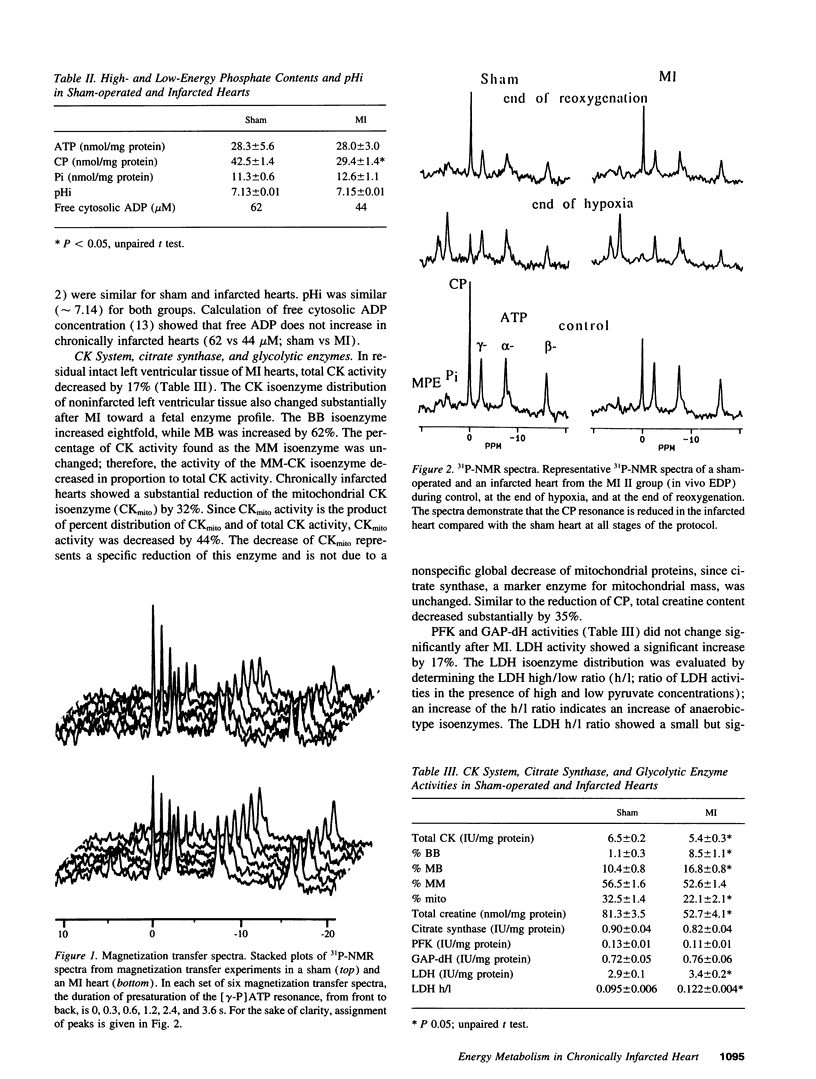
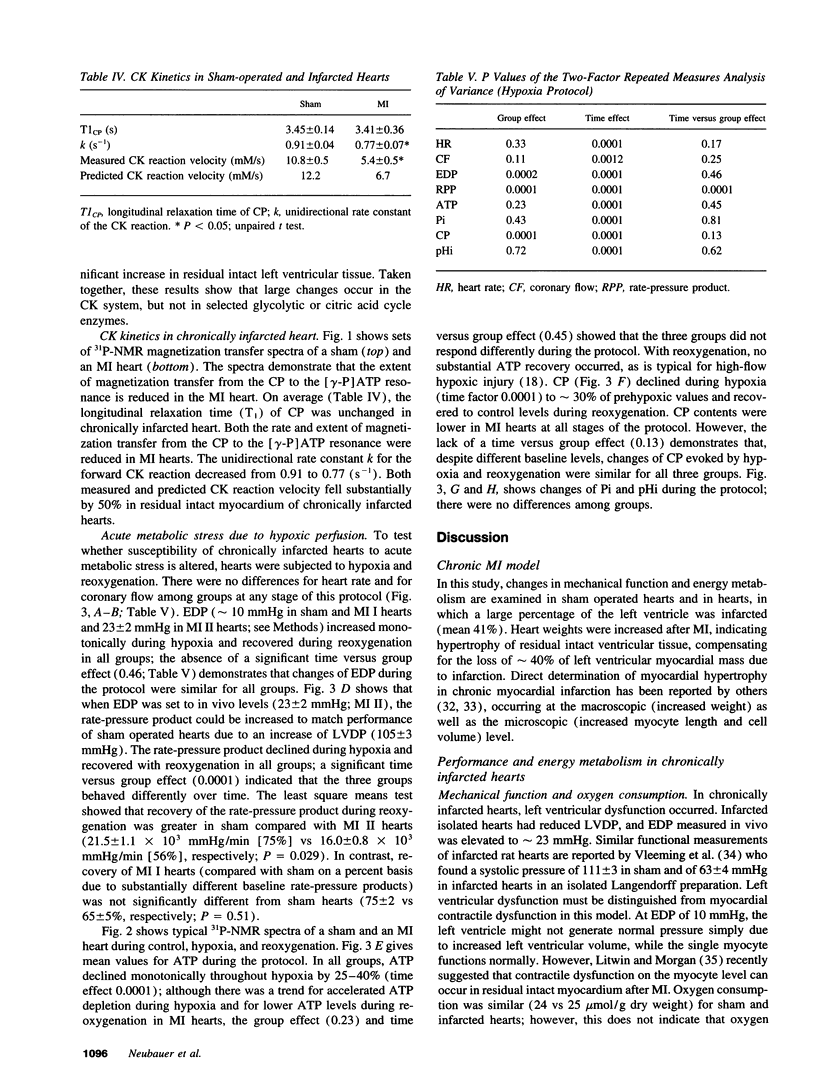
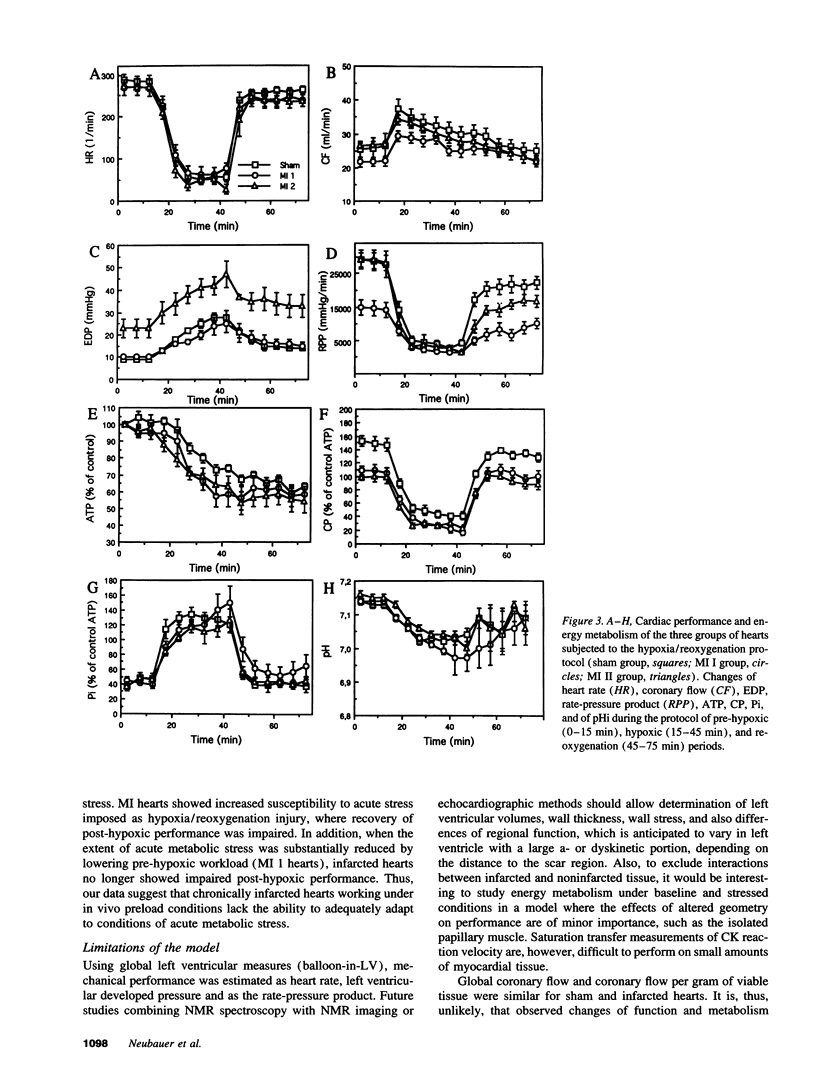
The purpose of this study was to test the hypothesis that energy metabolism is impaired in residual intact myocardium of chronically infarcted rat heart, contributing to contractile dysfunction. Myocardial infarction (MI) was induced in rats by coronary artery ligation. Hearts were isolated 8 wk later and buffer-perfused isovolumically. MI hearts showed reduced left ventricular developed pressure, but oxygen consumption was unchanged. High-energy phosphate contents were measured chemically and by 31P-NMR spectroscopy. In residual intact left ventricular tissue, ATP was unchanged after MI, while creatine phosphate was reduced by 31%. Total creatine kinase (CK) activity was reduced by 17%, the fetal CK isoenzymes BB and MB increased, while the "adult" mitochondrial CK isoenzyme activity decreased by 44%. Total creatine content decreased by 35%. Phosphoryl exchange between ATP and creatine phosphate, measured by 31P-NMR magnetization transfer, fell by 50% in MI hearts. Thus, energy reserve is substantially impaired in residual intact myocardium of chronically infarcted rats. Because phosphoryl exchange was still five times higher than ATP synthesis rates calculated from oxygen consumption, phosphoryl transfer via CK may not limit baseline contractile performance 2 mo after MI. In contrast, when MI hearts were subjected to acute stress (hypoxia), mechanical recovery during reoxygenation was impaired, suggesting that reduced energy reserve contributes to increased susceptibility of MI hearts to acute metabolic stress.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anversa P., Beghi C., Kikkawa Y., Olivetti G. Myocardial infarction in rats. Infarct size, myocyte hypertrophy, and capillary growth. Circ Res. 1986 Jan;58(1):26–37. doi: 10.1161/01.res.58.1.26. [DOI] [PubMed] [Google Scholar]
- Basson C. T., Grace A. M., Roberts R. Enzyme kinetics of a highly purified mitochondrial creatine kinase in comparison with cytosolic forms. Mol Cell Biochem. 1985 Jul;67(2):151–159. doi: 10.1007/BF02370174. [DOI] [PubMed] [Google Scholar]
- Bernstein L. H., Everse J. Determination of the isoenzyme levels of lactate dehydrogenase. Methods Enzymol. 1975;41:47–52. doi: 10.1016/s0076-6879(75)41012-6. [DOI] [PubMed] [Google Scholar]
- Bester A. J., Bajusz E., Lochner A. Effect of ischaemia and infarction on the metabolism and function of the isolated, perfused rat heart. Cardiovasc Res. 1972 May;6(3):284–294. doi: 10.1093/cvr/6.3.284. [DOI] [PubMed] [Google Scholar]
- Bittl J. A., Ingwall J. S. Reaction rates of creatine kinase and ATP synthesis in the isolated rat heart. A 31P NMR magnetization transfer study. J Biol Chem. 1985 Mar 25;260(6):3512–3517. [PubMed] [Google Scholar]
- Fellenius E., Hansen C. A., Mjøs O., Neely J. R. Chronic infarction decreases maximum cardiac work and sensitivity of heart to extracellular calcium. Am J Physiol. 1985 Jul;249(1 Pt 2):H80–H87. doi: 10.1152/ajpheart.1985.249.1.H80. [DOI] [PubMed] [Google Scholar]
- Gaudron P., Blumrich M., Ertl G. Aggravation of left ventricular dilatation and reduction of survival by a calcium channel blocker in rats with chronic myocardial infarction. Am Heart J. 1993 May;125(5 Pt 1):1226–1233. doi: 10.1016/0002-8703(93)90989-m. [DOI] [PubMed] [Google Scholar]
- Hall N., DeLuca M. Electrophoretic separation and quantitation of creatine kinase isozymes. Anal Biochem. 1976 Dec;76(2):561–567. doi: 10.1016/0003-2697(76)90350-x. [DOI] [PubMed] [Google Scholar]
- Ingwall J. S., Atkinson D. E., Clarke K., Fetters J. K. Energetic correlates of cardiac failure: changes in the creatine kinase system in the failing myocardium. Eur Heart J. 1990 Apr;11 (Suppl B):108–115. doi: 10.1093/eurheartj/11.suppl_b.108. [DOI] [PubMed] [Google Scholar]
- Ingwall J. S., Kramer M. F., Fifer M. A., Lorell B. H., Shemin R., Grossman W., Allen P. D. The creatine kinase system in normal and diseased human myocardium. N Engl J Med. 1985 Oct 24;313(17):1050–1054. doi: 10.1056/NEJM198510243131704. [DOI] [PubMed] [Google Scholar]
- Kammermeier H. Microassay of free and total creatine from tissue extracts by combination of chromatographic and fluorometric methods. Anal Biochem. 1973 Dec;56(2):341–345. doi: 10.1016/0003-2697(73)90199-1. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lawson J. W., Veech R. L. Effects of pH and free Mg2+ on the Keq of the creatine kinase reaction and other phosphate hydrolyses and phosphate transfer reactions. J Biol Chem. 1979 Jul 25;254(14):6528–6537. [PubMed] [Google Scholar]
- Litwin S. E., Morgan J. P. Captopril enhances intracellular calcium handling and beta-adrenergic responsiveness of myocardium from rats with postinfarction failure. Circ Res. 1992 Oct;71(4):797–807. doi: 10.1161/01.res.71.4.797. [DOI] [PubMed] [Google Scholar]
- Markiewicz W., Wu S. S., Parmley W. W., Higgins C. B., Sievers R., James T. L., Wikman-Coffelt J., Jasmin G. Evaluation of the hereditary Syrian hamster cardiomyopathy by 31P nuclear magnetic resonance spectroscopy: improvement after acute verapamil therapy. Circ Res. 1986 Dec;59(6):597–604. doi: 10.1161/01.res.59.6.597. [DOI] [PubMed] [Google Scholar]
- McAuliffe J. J., Perry S. B., Brooks E. E., Ingwall J. S. Kinetics of the creatine kinase reaction in neonatal rabbit heart: an empirical analysis of the rate equation. Biochemistry. 1991 Mar 12;30(10):2585–2593. doi: 10.1021/bi00224a004. [DOI] [PubMed] [Google Scholar]
- Meerson F. Z., Javich M. P. Isoenzyme pattern and activity of myocardial creatine phosphokinase under heart adaptation to prolonged overload. Basic Res Cardiol. 1982 Jul-Aug;77(4):349–358. doi: 10.1007/BF02005336. [DOI] [PubMed] [Google Scholar]
- Michel J. B., Lattion A. L., Salzmann J. L., Cerol M. L., Philippe M., Camilleri J. P., Corvol P. Hormonal and cardiac effects of converting enzyme inhibition in rat myocardial infarction. Circ Res. 1988 Apr;62(4):641–650. doi: 10.1161/01.res.62.4.641. [DOI] [PubMed] [Google Scholar]
- Neely J. R., Liebermeister H., Battersby E. J., Morgan H. E. Effect of pressure development on oxygen consumption by isolated rat heart. Am J Physiol. 1967 Apr;212(4):804–814. doi: 10.1152/ajplegacy.1967.212.4.804. [DOI] [PubMed] [Google Scholar]
- Neubauer S., Ertl G., Haas U., Pulzer F., Kochsiek K. Effects of endothelin-1 in isolated perfused rat heart. J Cardiovasc Pharmacol. 1990 Jul;16(1):1–8. doi: 10.1097/00005344-199007000-00001. [DOI] [PubMed] [Google Scholar]
- Neubauer S., Hamman B. L., Perry S. B., Bittl J. A., Ingwall J. S. Velocity of the creatine kinase reaction decreases in postischemic myocardium: a 31P-NMR magnetization transfer study of the isolated ferret heart. Circ Res. 1988 Jul;63(1):1–15. doi: 10.1161/01.res.63.1.1. [DOI] [PubMed] [Google Scholar]
- Neubauer S., Ingwall J. S. Verapamil attenuates ATP depletion during hypoxia: 31P NMR studies of the isolated rat heart. J Mol Cell Cardiol. 1989 Nov;21(11):1163–1178. doi: 10.1016/0022-2828(89)90693-7. [DOI] [PubMed] [Google Scholar]
- Nozawa T., Cheng C. P., Noda T., Little W. C. Relation between left ventricular oxygen consumption and pressure-volume area in conscious dogs. Circulation. 1994 Feb;89(2):810–817. doi: 10.1161/01.cir.89.2.810. [DOI] [PubMed] [Google Scholar]
- Oblinger M. M., Foe L. G., Kwiatkowska D., Kemp R. G. Phosphofructokinase in the rat nervous system: regional differences in activity and characteristics of axonal transport. J Neurosci Res. 1988 Sep;21(1):25–34. doi: 10.1002/jnr.490210105. [DOI] [PubMed] [Google Scholar]
- Pfeffer J. M., Pfeffer M. A., Fletcher P. J., Braunwald E. Progressive ventricular remodeling in rat with myocardial infarction. Am J Physiol. 1991 May;260(5 Pt 2):H1406–H1414. doi: 10.1152/ajpheart.1991.260.5.H1406. [DOI] [PubMed] [Google Scholar]
- Pfeffer J. M., Pfeffer M. A., Fletcher P. J., Braunwald E. Progressive ventricular remodeling in rat with myocardial infarction. Am J Physiol. 1991 May;260(5 Pt 2):H1406–H1414. doi: 10.1152/ajpheart.1991.260.5.H1406. [DOI] [PubMed] [Google Scholar]
- Pfeffer M. A., Pfeffer J. M., Fishbein M. C., Fletcher P. J., Spadaro J., Kloner R. A., Braunwald E. Myocardial infarct size and ventricular function in rats. Circ Res. 1979 Apr;44(4):503–512. doi: 10.1161/01.res.44.4.503. [DOI] [PubMed] [Google Scholar]
- Rosalki S. B. An improved procedure for serum creatine phosphokinase determination. J Lab Clin Med. 1967 Apr;69(4):696–705. [PubMed] [Google Scholar]
- Sanbe A., Tanonaka K., Hanaoka Y., Katoh T., Takeo S. Regional energy metabolism of failing hearts following myocardial infarction. J Mol Cell Cardiol. 1993 Sep;25(9):995–1013. doi: 10.1006/jmcc.1993.1113. [DOI] [PubMed] [Google Scholar]
- Schultheiss H. P., Ullrich G., Schindler M., Schulze K., Strauer B. E. The effect of ACE inhibition on myocardial energy metabolism. Eur Heart J. 1990 Apr;11 (Suppl B):116–122. doi: 10.1093/eurheartj/11.suppl_b.116. [DOI] [PubMed] [Google Scholar]
- Sharkey S. W., Murakami M. M., Smith S. A., Apple F. S. Canine myocardial creatine kinase isoenzymes after chronic coronary artery occlusion. Circulation. 1991 Jul;84(1):333–340. doi: 10.1161/01.cir.84.1.333. [DOI] [PubMed] [Google Scholar]
- Shimamoto N., Goto N., Tanabe M., Imamoto T., Fujiwara S., Hirata M. Myocardial energy metabolism in the hypertrophied hearts of spontaneously hypertensive rats. Basic Res Cardiol. 1982 Jul-Aug;77(4):359–357. doi: 10.1007/BF02005337. [DOI] [PubMed] [Google Scholar]
- Takaoka H., Takeuchi M., Odake M., Hayashi Y., Hata K., Mori M., Yokoyama M. Comparison of hemodynamic determinants for myocardial oxygen consumption under different contractile states in human ventricle. Circulation. 1993 Jan;87(1):59–69. doi: 10.1161/01.cir.87.1.59. [DOI] [PubMed] [Google Scholar]
- Vatner D. E., Ingwall J. S. Effects of moderate pressure overload cardiac hypertrophy on the distribution of creatine kinase isozymes. Proc Soc Exp Biol Med. 1984 Jan;175(1):5–9. doi: 10.3181/00379727-175-1-rc2. [DOI] [PubMed] [Google Scholar]
- Vleeming W., van Rooij H. H., Wemer J., Porsius A. J. Cardiovascular responses to the stereoisomers of dobutamine in isolated rat hearts 48 hours after acute myocardial infarction. J Cardiovasc Pharmacol. 1991 Apr;17(4):634–640. doi: 10.1097/00005344-199104000-00017. [DOI] [PubMed] [Google Scholar]
- Wexler L. F., Lorell B. H., Momomura S., Weinberg E. O., Ingwall J. S., Apstein C. S. Enhanced sensitivity to hypoxia-induced diastolic dysfunction in pressure-overload left ventricular hypertrophy in the rat: role of high-energy phosphate depletion. Circ Res. 1988 Apr;62(4):766–775. doi: 10.1161/01.res.62.4.766. [DOI] [PubMed] [Google Scholar]
- Wolff M. R., de Tombe P. P., Harasawa Y., Burkhoff D., Bier S., Hunter W. C., Gerstenblith G., Kass D. A. Alterations in left ventricular mechanics, energetics, and contractile reserve in experimental heart failure. Circ Res. 1992 Mar;70(3):516–529. doi: 10.1161/01.res.70.3.516. [DOI] [PubMed] [Google Scholar]
- Zimmer H. G., Gerdes A. M., Lortet S., Mall G. Changes in heart function and cardiac cell size in rats with chronic myocardial infarction. J Mol Cell Cardiol. 1990 Nov;22(11):1231–1243. doi: 10.1016/0022-2828(90)90060-f. [DOI] [PubMed] [Google Scholar]
- van Deursen J., Heerschap A., Oerlemans F., Ruitenbeek W., Jap P., ter Laak H., Wieringa B. Skeletal muscles of mice deficient in muscle creatine kinase lack burst activity. Cell. 1993 Aug 27;74(4):621–631. doi: 10.1016/0092-8674(93)90510-w. [DOI] [PubMed] [Google Scholar]



