Abstract
Background
In adults with CKD, protein-energy wasting (PEW) is a risk factor for hospitalizations and death. However, PEW in children with CKD is not well characterized or defined.
Methods
Using data from the Chronic Kidney Disease in Children study, we assessed three alternate definitions of PEW using biochemical parameters, body and muscle mass measurements, and reported appetite as described in adults: a minimal PEW definition (≥2 of the 4 criteria); a standard PEW definition (≥3 of the 4 criteria); and a modified PEW definition (≥3 of the 4 criteria plus a pediatric focused criteria of short stature or poor growth).
Results
Among 528 children (median age 12 years, median glomerular filtration rate (GFR) 45 ml/min|1.73 m2, 39% female and 18% African American), 7 to 20% met the spectrum of definitions for PEW. The unadjusted incidence rates for incident hospitalizations were 1.9, 2.1 and 2.2 times higher for those children classified as PEW using the minimal, standard and modified definitions, respectively (P=0.08, 0.09 and 0.03)). Following adjustment, only the modified PEW definition, which added short stature or poor growth as a criteria, showed modest significance (P=0.06).
Conclusions
The inclusion of PEW criteria based on growth may augment the definition of PEW and improve risk discrimination in children with CKD.
Keywords: cachexia inflammation syndrome, hospitalizations, chronic kidney disease, malnutrition, growth, glomerular filtration rate
Introduction
In adults with chronic kidney disease (CKD), especially end stage renal disease (ESRD), protein-energy wasting is highly prevalent and has been implicated as a risk factor for death and accelerated atherosclerotic cardiovascular disease. Using classic measures of nutritional status, evidence of wasting is present in 18–75% of adult maintenance dialysis patients (1). Protein-energy wasting (PEW) occurring in CKD in adults is characterized by four primary components: biochemical criteria such as low serum albumin or low cholesterol, reduced body mass, reduced muscle mass, and decreased protein intake (2). Although several lines of evidence suggest PEW also exists in the pediatric CKD population, the syndrome is less well characterized in children. Low serum albumin is associated with higher all-cause mortality in children with ESRD (3) as it is with all-cause mortality and cardiovascular disease in adults (4–6). Low body mass as assessed by BMI has been associated with higher mortality in children with ESRD (7), and in adult CKD patients (8–10). In a cross sectional study of children at various stages of CKD by Foster et al, body composition assessed by DEXA demonstrated significant deficits in leg lean mass in children with advanced CKD on dialysis (11).
Though evidence from these studies is suggestive of a syndrome of PEW in childhood CKD similar to that in adults, criteria for diagnosing this syndrome in children have not been evaluated. We therefore sought to assess the clinical components thought to be important for diagnosing PEW and compare the performance of three classifications of PEW in predicting clinical outcomes in a prospective cohort of children with CKD.
Methods
Study participants and design
The Chronic Kidney Disease in Children (CKiD) study has been described previously (12). Briefly children were recruited with mild to moderate CKD (30 to 90 ml/min per 1.73 m2) based on the original Schwartz formula (13–15) from 43 participating pediatric nephrology centers. Eligible subjects were those 1 to 16 years of age who had never been dialyzed or undergone organ transplant. Demographic characteristics and clinical measurements were collected at annual visits. Glomerular filtration rate (GFR) was determined from plasma iohexol disappearance curves at baseline, one year later and every other year thereafter, following methods that have been previously described (16). GFR values unattainable through direct measurement were estimated using the CKiD equation (17;18). Baseline for the current analysis was the second annual visit.
Anthropometry and Tanner Staging
Age- and sex-specific Z-scores (SD scores) for height and weight were calculated using National Center for Health Statistics 2000 Center for Disease Control growth data (19). BMI-for-height-age and sex Z-scores were also calculated, as recommended in the 2008 K/DOQI nutrition guidelines (20); height-age is the age at which the child’s height is at the 50th percentile. The mid-upper arm circumference was taken as the mean of three measurements when available and expressed relative to height-age and sex stratified norms obtained from National Health and Nutrition Examination Survey 2007–2008 sample (21). Skinfold thickness data were not available from the cohort but very few of the patients had clinically significant edema (N=15 [3%] at the second annual visit). Pubertal status was dichotomized into Stage 1 and > Stage 1, with stages defined by Tanner (22).
Biomarker assays
At the study visit where iGFR was measured, an intravenous line or butterfly needle was used to administer 5 ml of iohexol. A second intravenous line was saline locked and used for obtaining blood samples for biomarker measurements. Blood samples were collected at four time points (10, 30, 120, and 300 minutes) following infusion of iohexol (GE Healthcare, Amersham Division, Princeton, NJ) with iohexol concentration determined by high-performance liquid chromatography. Body surface area (BSA) for GFR standardization was determined using the formula of Haycock et al (23). Inflammation was assessed with wide-range C-reactive protein (CRP). Cystatin C was measured at the using a Siemens BN II nephelometer. Serum creatinine (enzymatic) and serum total cholesterol (TC) following an overnight fast were analyzed centrally at the CKiD laboratory at the University of Rochester Medical Center on a Bayer Advia 2400 (Siemens Diagnostics, Tarrytown NY). Assay results were entered by the Central Biochemistry Laboratory into a web-based data management system (Nephron) developed by the Data Coordinating Center.
Defining PEW
Using data from the first two annual visits of the study, indicators for PEW were created based upon the International Society of Renal Nutrition and Metabolism diagnostic criteria (1) with modifications and additions to provide greater applicability to children (24). The criteria for clinical diagnosis of PEW included: 1) Biochemical: TC < 100mg/100ml; serum albumin < 3.8g/100ml, which was approximately the 5th percentile of the data; serum transferrin < 140 mg/dL (25); CRP > 3 mg/L; 2) Reduced body mass: defined by a body mass index (BMI) for height-age less than the 5th percentile at entry into CKiD or a decrease in BMI for height-age and sex percentile greater than or equal to 10% between the first and second annual visits from an initial BMI for height-age and sex percentile of <80th; 3) Reduced muscle mass: mid-upper arm circumference (MUAC) for height-age and sex <5th percentile or a decrease in MUAC for height-age and sex percentile greater than or equal to 10% between the first and second annual visits; 4) Decreased appetite as a surrogate for dietary protein intake: fair, poor or very poor appetite reported over the week prior to the study visit.
We also evaluated the improvement in prediction gained with using a pediatric-specific metric: 5) poor growth defined as either short stature: a height for age and sex percentile< 3% (26) or poor growth velocity: a decrease in height for age and sex percentile greater than or equal to 10% between the first and second annual visits.
Using combinations of the indicators described above, three definitions of PEW were created: a minimal PEW definition requiring any positive test in ≥2 of categories (1) through (4) to be met; a standard PEW definition requiring any positive test in ≥3 of categories (1) through (4); and a modified PEW definition requiring any positive test in ≥3 of categories (1) through (5), such that short stature or poor growth velocity was also included as a separate indicator category.
Statistical Analysis
To avoid a loss of information on those with incomplete data on some indicators, multiple imputation was used to complete missing laboratory and self-report data of PEW indicators integral to classifying participants. Missing values were imputed five times based on the distribution of covariates (Tanner stage, BMI percentile, height percentile, serum creatinine, serum transferrin, cystatin C, hemoglobin, bicarbonate, albumin, urine creatinine, urine protein, LDL, C-reactive protein, appetite score, GFR, mid-upper arm circumference and low birth weight) using a Markov chain Monte Carlo (MCMC) method (27;28) and assuming multivariate normality. Non-fasting lipid measurements were also assumed missing and imputed. Established methods for combining estimates from each imputed dataset were used to appropriately adjust standard errors (27;28). Trends across categories of imputed variables were tested using linear or logistic regression, assuming an ordinal relationship between the independent variable and the categorical dependent variable.
Using longitudinal data on the GFR trajectory of the children following the second annual visit, the annual percentage change in GFR was estimated using a segmented linear mixed effects model with a random intercept and slope. The model estimated the effect on the GFR slope separately for the period from baseline to 2 years and after 2 years to examine both proximate and longer term effects of PEW on CKD progression. In addition to progression of kidney dysfunction, self-reported incident hospitalization events were also examined. Poisson regression was used to assess the association between definitions of PEW (defined at the second annual visit) and the rate of incident hospitalizations within two years after the PEW assessment, with each definition assessed in a separate model. Generalized estimating equations were used to account for repeated reports of hospitalization from a single individual (29). Models were adjusted for age, CKD stage, glomerular diagnosis, urine protein to creatinine ratio > 2, anemia (hemoglobin level < 5th percentile for age and sex), and low birth weight (birth weight <2500 g).
Results
There were 528 children who contributed to the analysis. Of these, data were missing from 61 (12%) children onTC laboratory measurements including non-fasting measurements, 23 (4%) children on albumin measurements, 254 (48%) on serum transferrin, 78 (15%) children on CRP, 67 (13%) children on BMI for height-age and sex percentile at one or both of the first two annual visits, 94 (18%) children on MUAC for height age and sex percentile at one or both of the first two annual visits, 43 (8%) children on appetite information and 87 (16%) children on height for age and sex percentile at one or both of the first two annual visits. The demographic and disease characteristics of the 528 children are shown in Table 1. The overall cohort had a median age of 12 years, a median GFR of 45 ml/min|1.73 m2, were 39% female and 18% African American.
Table 1.
Baseline (CKD annual visit 2) characteristics of the overall cohort and those meeting each of the three PEW definitions. Children meeting each definition were compared to the overall cohort.
| Overall N=528 | Minimal PEW | Standard PEW | Modified PEW | |
|---|---|---|---|---|
| African-American | 18% | 22% | 15% | 23% |
| Female | 39% | 35% | 26% | 35% |
| Pre-Pubertal Status | 48% | 46% | 57% | 51% |
| Glomerular Diagnosis | 21% | 23% | 15% | 22% |
| Age (yr; median [IQR]) | 12 [8, 16] | 12 [8, 16] | 10 [6, 14] | 12 [7, 16] |
| Height for age and sex z-score (median [IQR]) | −0.7 [−1.4, −0.0] | −0.8 [−1.7, −0.1] | −0.7 [−1.7, 0.2] | −1.1 [−2.0, −0.2] |
| Weight for age and sex z-score (median [IQR]) | −0.2 [−0.9, 0.7] | −0.5 [−1.3, 0.3] | −0.8 [−1.6, −0.1] | −0.7 [−1.6, 0.2]* |
| BMI for height-age and sex z-score (median [IQR]) | 0.5 [−0.3, 1.3] | 0.1 [−0.5, 0.9]* | −0.4 [−0.7, −0.1]* | 0.0 [−0.5, 0.8]* |
| Mid Upper Arm Circumference for height-age and sex z-score (median [IQR]) | −0.8 [−3.3, 2.6] | −2.2 [−4.6, −0.5]* | −3.2 [−4.8, −1.4]* | −2.5 [−4.7, −0.7]* |
| Urine Protein to Creatinine Ratio (Upc) | 0.5 [0.2, 1.2] | 0.8 [0.2, 1.8]* | 0.8 [0.2, 1.6] | 0.8 [0.2, 1.8] |
| Upc>2.0 | 14% | 24%* | 18% | 24%* |
| Anemia | 39% | 43% | 32% | 45% |
| Acidosis | 32% | 38% | 28% | 39% |
| Hypertension | 48% | 45% | 41% | 46% |
| Dyslipidemia | 51% | 54% | 62% | 57% |
| Low birth weight | 19% | 25% | 27% | 28% |
| Growth hormone use | 13% | 13% | 13% | 14% |
| ieGFR (median [IQR]) | 45 [33, 58] | 42 [30, 56] | 42 [31, 55] | 43 [30, 56] |
| CKD Stage | ||||
| Stage 1 or 2 | 23% | 24% | 22% | 23% |
| Stage 3a | 26% | 19% | 22% | 20% |
| Stage 3b | 31% | 32% | 30% | 31% |
| Stage 4 or 5 | 20% | 25% | 25% | 26% |
indicates statistically significantly different from the overall cohort using an alpha level of 0.05
Using the data augmented through multiple imputation, we compared the characteristics of those who met each definition of PEW with the overall cohort (Table 1). Children meeting the minimal PEW definition represented 20% (95% CI: 16, 24) of the cohort. They were more likely to have a lower median BMI for height-age and sex z-score (P=0.02), a lower MUAC height-age and sex z-score (P=0.02), a higher urine protein to creatinine ratio (P=0.04). Children meeting the standard PEW definition represented 7% (95% CI: 5, 10)) of the cohort. They were more likely to have a lower median BMI for height-age and sex z-score (P<0.001) and a lower MUAC height-age and sex z-score (P<0.001). Children meeting the modified PEW definition represented 15% (95% CI: 12, 18) of the cohort. They were more likely to have a lower median weight for age and sex z-score (p=0.03), a lower BMI for height-age and sex z-score (P=0.02), a lower MUAC height-age and sex z-score (P=0.02) and a higher percentage of children with a protein to creatinine ratio >2 (p=0.03).
When the prevalence of the various indicators of PEW were examined in the overall cohort and by CKD stage, the data suggested trends towards higher prevalence with lower GFR in hypoalbuminemia (Ptrend=0.04), decreased appetite (Ptrend<0.001), reduced muscle mass (Ptrend=0.20) and reduced body mass (Ptrend=0.16), though only hypoalbuminemia and decreased appetite were significant when the linear trend was tested statistically. (Figure 1). Low transferrin and total cholesterol levels were virtually non-existent in the cohort. The prevalence of high CRP appeared to increase with increasing GFR but was not significant (Ptrend=0.28). There was not a statistically significant increase in the prevalence of PEW with CKD stage regardless of definition. (Figure 2). Notable increases in prevalence were only seen with GFR<30 ml/min and appeared stable in earlier stages of CKD. Collapsing the GFR categories to focus on the threshold of 30 ml/min, the odds of having PEW comparing GFR<30 ml/min to GFR≥30 ml/min were 1.5 (95%CI: 0.7, 2.8) 1.4 (95%CI: 0.5, 3.5) and 1.5 (95% CI: 0.8, 2.9), respectively, by the minimal, standard and modified definitions.
Figure 1.
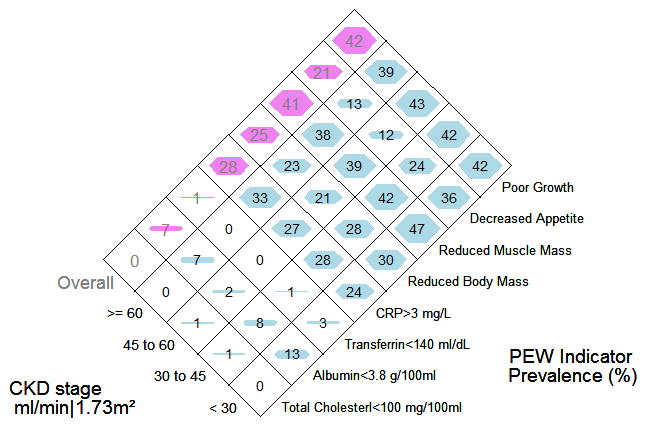
The prevalence of indicators of PEW used to form the three definitions. The prevalence is presented stratified by CKD stage (stage 1or 2, stage 3a, stage 3b and stage 4 or 5) and also overall.
Figure 2.
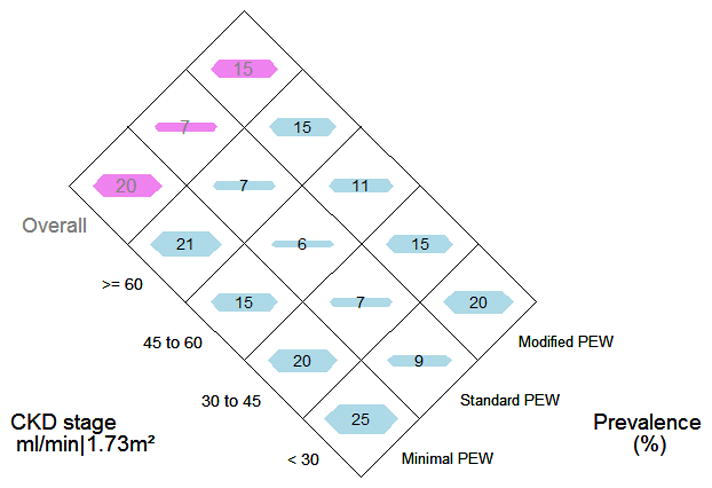
The prevalence of PEW as classified using the three definitions: minimal PEW definition that required at least one test in ≥2 of the 4 original categories; a standard PEW definition that required at least one test in ≥3 of the 4 original categories; and a modified PEW definition that required at least one test in ≥3 of the 5 categories (4 original plus poor growth). The prevalence is presented stratified by CKD stage (stage 1or 2, stage 3a, stage 3b and stage 4 or 5) and also overall.
We were primarily interested in longitudinal risk associations with CKD progression and hospitalization to establish whether any of the definitions of PEW were predictive of clinically important endpoints. First we looked at the annual GFR percentage decline to see if meeting the criteria for one or more of the PEW definitions at baseline (the second annual CKiD visit) was associated with faster kidney function decline. GFR decline was assessed in both the first 2 years after PEW assessment and thereafter, which included data up to 7 years after baseline (V2) with an interquartile range of 1 to 4 years of follow up. From segmented linear mixed models broken at 2 years from baseline, the cohort experienced approximately a 3.5% annual decline in their GFR level and there was no evidence that children meeting any of the PEW definitions experienced greater declines (Table 2). The annual percentage decline was approximately 2.7% per year after 2 years from baseline, suggesting a modest deceleration in decline on average. Again there was no evidence of a difference in the rate of decline among children meeting any of the PEW definitions. Age, CKD stage urine protein to creatinine ratio > 2 and anemia (hemoglobin level < 5th percentile for age and sex) were significantly associated with GFR decline in the multivariate models.
Table 2.
Adjusted estimates of the annual percentage change in GFR from segmented mixed effect models. The effect of meeting a definition of PEW on the GFR slope was assessed for each PEW definition for two periods of time: within 2 years of baseline (CKD 2nd annual visit) and after 2 years from baseline.
| Change in GFR slope (% annual change)1
|
|||
|---|---|---|---|
| Minimal PEW | Standard PEW | Modified PEW | |
| From baseline to 2 years
| |||
| Annual decline in GFR | −3.3% (−5.7%, −0.8%)* | −3.6% (−5.8%, 1.4%)* | −3.5% (−5.8%, 1.2%)* |
| Change in slope for PEW | −1.0% (−7.6%, 6.0%) | 2.3% (7.7%, 13.5%) | 0.0% (5.8%, 7.1%) |
|
| |||
| After 2 years
| |||
| Annual decline in GFR | −2.9% (−4.1%, −1.6%)* | −2.6% (−3.7%, −1.5%)* | −2.7% (−3.9%, −1.6%)* |
| Change in slope for PEW | 1.6% (−1.6%, 4.9%) | 0.0%% (−4.4%, 5.4%) | 1.1%% (−1.1%, 4.5%) |
indicates significant at the α=0.05 level
Adjusted for age, CKD stage, glomerular diagnosis, urine protein to creatinine ratio > 2, anemia (hemoglobin level < 5th percentile for age and sex), and low birth weight (birth weight <2500 g).
Secondly, we examined the incidence rate of hospitalizations during the two years following baseline using Poisson regression analysis. The unadjusted estimated incidence rate ratios were 1.9, 2.1 and 2.2 times higher for those children classified as PEW using the minimal, standard and modified definitions, respectively (P=0.08, 0.09 and 0.03)). Following adjustment, the estimates became to 1.8, 2.1 and 2.0, respectively with the modified PEW definition maintaining modest significance (P=0.06). The estimates are compared in Figure 3. We also examined poor growth as a predictor of hospitalizations and found it to be less predictive in adjusted models than the modified PEW definition, of which it was one component (incidence rate ratio: 1.8; P=0.11). In the multivariate models with PEW, only CKD stage was a significant predictor of hospitalizations in the adjusted models (P=0.05 in all models).
Figure 3.
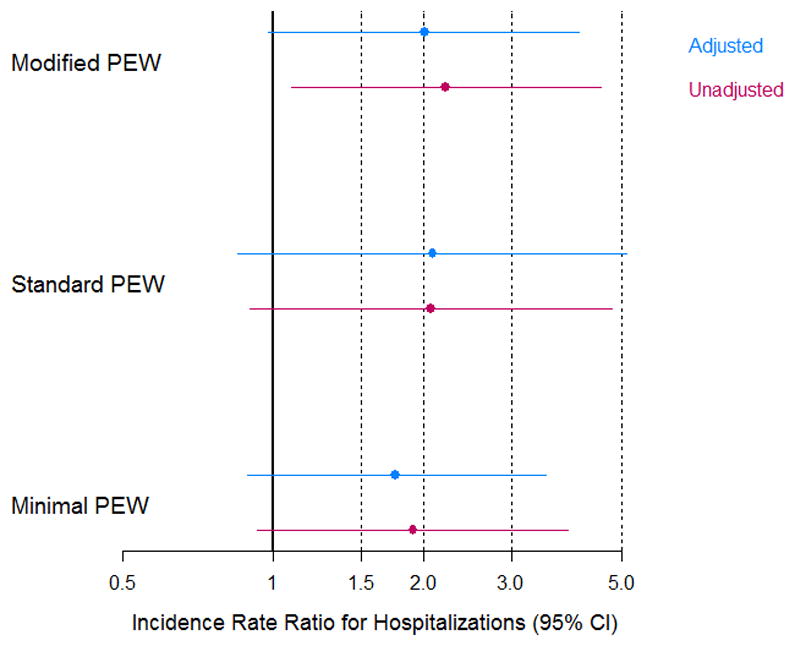
The unadjusted and adjusted incidence rate ratios for hospitalizations within the 2 years from baseline comparing the three PEW definitions.
Discussion
There is a dearth of studies examining the occurrence of PEW in children with CKD. Wasting/cachexia syndrome is very common in end-stage renal disease in adult populations (30 to 75%) and consists of anorexia, increased energy expenditure, decreased protein stores, and loss of weight and muscle mass (30). While this syndrome in its various forms (PEW versus cachexia, which has been considered a more severe state of PEW) has been described for adults and diagnostic criteria have been proposed (1;8), the applicability of these clinical features to children with CKD has not been established. In this study we examined the prevalence of adult diagnostic criteria as well as pediatric focused indicators of PEW in a pediatric CKD cohort, and assessed the degree to which varying definitions using these criteria predict incident hospitalizations and CKD progression. We found that the incidence of PEW ranged from 7% to 20 % in the overall cohort depending upon the definition and an increase in prevalence (corresponding to an odds ratio of 1.5) was suggested in advanced CKD stage 4 and 5, though the increase was not statistically significant. The lack of statistical significance may be the a result of relatively small numbers of children meeting any PEW definition in our cohort. Several of the indicators of PEW tended to increase with decreasing GFR, with hypoalbuminemia and poor appetite having the strongest association. These results may suggest that, of the four laboratory measures assessed, hypoalbuminemia is the mostsensitive indicator of PEW in our pediatric cohort. Both low transferrin and low cholesterol were exceedingly rare. Low transferrin and cholesterol levels may be more valuable indicators in children with more severe disease or those on dialysis. In contrast to what has been reported in adult studies of CKD, high CRP in the CKiD cohort seemed more common in children with milder disease. While PEW is thought to be a process driven by inflammation, a recent report by Foster et al. (11) examining children with more advanced CKD also reported a lack of association between markers of inflammation and wasting as measured by leg lean mass Z-scores, measured by dual-energy X ray absorptiometry (DEXA) scans, except in the most advanced stages of CKD in children. Notably, Foster reported no significant skeletal muscle wasting in children with CKD stage 2–3.
There is no clinical consensus as to the optimal assessment of PEW in children. We used both baseline and longitudinal information to classify participants regarding their muscle mass, body mass and growth status. As multiple prior studies have implicated short stature and poor growth as predictors of adverse outcomes in children with CKD, we included an assessment of growth status in our modified definition of PEW. However, the sensitivity of this poor growth measure could be compromised as we may be including small but non-wasted children. Additionally, by including decreasing BMI percentiles, we may have included overweight children who have intentionally lost body mass. In addition, our lean mass assessment using BMI is imperfect. Both BMI can result in questionable measurements of wasting in CKD, which is ideally defined as diminished lean body mass. Fluid overload, which is common in CKD, confounds BMI and lean/skeletal mass measurements (31). Furthermore, calorie supplementation, which is commonly prescribed in CKD children with weight deficit or linear growth failure, may not correct true lean mass deficits in CKD and may instead increase body weight by increasing fat mass and water content. Rashid et al measured body composition by DEXA scan in growth-retarded children with advanced CKD on energy supplementation, showing that a normal BMI can be associated with reduction in lean mass and reduced BMI can be associated with increased fat mass in these children (32).
The most important test of a PEW definition is the prediction of clinical outcomes. The modified definition of PEW, which included criteria for poor growth specific to a pediatric context was the only definition associated with incident hospitalizations within two years of classification. This finding suggests that growth may be a better standard for diagnosing PEW in children than weight based criteria. Adding poor growth to the definition also increased the prevalence of PEW from 7% to 15%. Indeed, the Society for Cachexia and Wasting Disorders (SCWD), which included participants with diverse backgrounds encompassing many of the diseases states that result in cachexia (such as cancer, human immunodeficiency virus infection, heart failure, CKD, and chronic obstructive pulmonary disease), identified growth failure as the most important clinical feature of cachexia in children (33).
There are limitations to our analysis that should be noted. The CKiD cohort is comprised of children with moderate CKD and thus, the prevalence of PEW was relatively low limiting our ability to look at associations with outcomes and discriminate between PEW definitions. In addition, a direct measurement of GFR was not available at every year by study design and thus estimated GFRs were used in intervening years to supplement iohexol GFR measurements. Estimated GFRs from CKiD’s internally derived equation have shown good agreement with iohexol GFR measurements (18). However, using estimated and directly measured GFR interchangeably could result in bias in estimated GFR decline over time.
In summary, our results suggest diagnosing PEW syndrome in children with CKD requires pediatric specific criteria. The addition of indicators of growth failure or poor growth velocity added to the existing adult criteria improved the prediction of hospitalizations. Therefore, clinicians should consider growth in their assessment of children at risk for PEW.
Acknowledgments
The authors would like to acknowledge the efforts of Derek Ng and Rachel Zack towards the analysis of the data presented in this manuscript. Data in this manuscript were collected by the Chronic Kidney Disease in children prospective cohort study (CKiD) with clinical coordinating centers (Principal Investigators) at Children’s Mercy Hospital and the University of Missouri – Kansas City (Bradley Warady, MD) and Children’s Hospital of Philadelphia (Susan Furth, MD, Ph.D.), data coordinating center at the Johns Hopkins Bloomberg School of Public Health (Alvaro Muñoz, Ph.D.), and the Central Biochemistry Laboratory at the University of Rochester (George J. Schwartz, MD). The CKiD is funded by the National Institute of Diabetes and Digestive and Kidney Diseases, with additional funding from, the National Institute of Child Health and Human Development, and the National Heart, Lung, and Blood Institute (U01 DK82194, U01-DK-66143, U01-DK-66174, and U01-DK-66116). The CKID website is located at http://www.statepi.jhsph.edu/ckid.
Footnotes
Disclosure
No conflict of interest to disclose.
Reference List
- 1.Fouque D, Kalantar-Zadeh K, Kopple J, Cano N, Chauveau P, Cuppari L, Franch H, Guarnieri G, Ikizler TA, Kaysen G, Lindholm B, Massy Z, Mitch W, Pineda E, Stenvinkel P, Trevino-Becerra A, Wanner C. A proposed nomenclature and diagnostic criteria for protein-energy wasting in acute and chronic kidney disease. Kidney Int. 2008;73:391–398. doi: 10.1038/sj.ki.5002585. [DOI] [PubMed] [Google Scholar]
- 2.Kovesdy CP, Kalantar-Zadeh K. Why is protein-energy wasting associated with mortality in chronic kidney disease? Semin Nephrol. 2009;29:3–14. doi: 10.1016/j.semnephrol.2008.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wong CS, Hingorani S, Gillen DL, Sherrard DJ, Watkins SL, Brandt JR, Ball A, Stehman-Breen CO. Hypoalbuminemia and risk of death in pediatric patients with end-stage renal disease. Kidney Int. 2002;61:630–637. doi: 10.1046/j.1523-1755.2002.00169.x. [DOI] [PubMed] [Google Scholar]
- 4.Menon V, Greene T, Wang X, Pereira AA, Marcovina SM, Beck GJ, Kusek JW, Collins AJ, Levey AS, Sarnak MJ. C-reactive protein and albumin as predictors of all-cause and cardiovascular mortality in chronic kidney disease. Kidney Int. 2005;68:766–772. doi: 10.1111/j.1523-1755.2005.00455.x. [DOI] [PubMed] [Google Scholar]
- 5.Muntner P, He J, Astor BC, Folsom AR, Coresh J. Traditional and nontraditional risk factors predict coronary heart disease in chronic kidney disease: results from the atherosclerosis risk in communities study. J Am Soc Nephrol. 2005;16:529–538. doi: 10.1681/ASN.2004080656. [DOI] [PubMed] [Google Scholar]
- 6.Weiner DE, Tighiouart H, Elsayed EF, Griffith JL, Salem DN, Levey AS, Sarnak MJ. The relationship between nontraditional risk factors and outcomes in individuals with stage 3 to 4 CKD. Am J Kidney Dis. 2008;51:212–223. doi: 10.1053/j.ajkd.2007.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wong CS, Gipson DS, Gillen DL, Emerson S, Koepsell T, Sherrard DJ, Watkins SL, Stehman-Breen C. Anthropometric measures and risk of death in children with end-stage renal disease. Am J Kidney Dis. 2000;36:811–819. doi: 10.1053/ajkd.2000.17674. [DOI] [PubMed] [Google Scholar]
- 8.Evans M, Fryzek JP, Elinder CG, Cohen SS, McLaughlin JK, Nyren O, Fored CM. The natural history of chronic renal failure: results from an unselected, population-based, inception cohort in Sweden. Am J Kidney Dis. 2005;46:863–870. doi: 10.1053/j.ajkd.2005.07.040. [DOI] [PubMed] [Google Scholar]
- 9.Kovesdy CP, Anderson JE, Kalantar-Zadeh K. Paradoxical association between body mass index and mortality in men with CKD not yet on dialysis. Am J Kidney Dis. 2007;49:581–591. doi: 10.1053/j.ajkd.2007.02.277. [DOI] [PubMed] [Google Scholar]
- 10.Kwan BC, Murtaugh MA, Beddhu S. Associations of body size with metabolic syndrome and mortality in moderate chronic kidney disease. Clin J Am Soc Nephrol. 2007;2:992–998. doi: 10.2215/CJN.04221206. [DOI] [PubMed] [Google Scholar]
- 11.Foster BJ, Kalkwarf HJ, Shults J, Zemel BS, Wetzsteon RJ, Thayu M, Foerster DL, Leonard MB. Association of chronic kidney disease with muscle deficits in children. J Am Soc Nephrol. 2011;22:377–386. doi: 10.1681/ASN.2010060603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Furth SL, Cole SR, Moxey-Mims M, Kaskel F, Mak R, Schwartz G, Wong C, Muñoz A, Warady BA. Design and methods of the Chronic Kidney Disease in Children (CKiD) prospective cohort study. Clin J Am Soc Nephrol. 2006;1:1006–1015. doi: 10.2215/CJN.01941205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schwartz GJ, Haycock GB, Edelmann CM, Jr, Spitzer A. A simple estimate of glomerular filtration rate in children derived from body length and plasma creatinine. Pediatrics. 1976;58:259–263. [PubMed] [Google Scholar]
- 14.Schwartz GJ, Gauthier B. A simple estimate of glomerular filtration rate in adolescent boys. J Pediatr. 1985;106:522–526. doi: 10.1016/s0022-3476(85)80697-1. [DOI] [PubMed] [Google Scholar]
- 15.Schwartz GJ, Brion LP, Spitzer A. The use of plasma creatinine concentration for estimating glomerular filtration rate in infants, children, and adolescents. Pediatr Clin North Am. 1987;34:571–590. doi: 10.1016/s0031-3955(16)36251-4. [DOI] [PubMed] [Google Scholar]
- 16.Schwartz GJ, Furth S, Cole SR, Warady B, Munoz A. Glomerular filtration rate via plasma iohexol disappearance: pilot study for chronic kidney disease in children. Kidney Int. 2006;69:2070–2077. doi: 10.1038/sj.ki.5000385. [DOI] [PubMed] [Google Scholar]
- 17.Schwartz GJ, Munoz A, Schneider MF, Mak RH, Kaskel F, Warady BA, Furth SL. New equations to estimate GFR in children with CKD. J Am Soc Nephrol. 2009;20:629–637. doi: 10.1681/ASN.2008030287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schwartz GJ, Schneider MF, Maier PS, Moxey-Mims M, Dharnidharka VR, Warady BA, Furth SL, Munoz A. Improved equations estimating GFR in children with chronic kidney disease using an immunonephelometric determination of cystatin C. Kidney Int. 2012;82:445–453. doi: 10.1038/ki.2012.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ogden CL, Kuczmarski RJ, Flegal KM, Mei Z, Guo S, Wei R, Grummer-Strawn LM, Curtin LR, Roche AF, Johnson CL. Centers for Disease Control and Prevention 2000 growth charts for the United States: improvements to the 1977 National Center for Health Statistics version. Pediatrics. 2002;109:45–60. doi: 10.1542/peds.109.1.45. [DOI] [PubMed] [Google Scholar]
- 20.KDOQI Clinical Practice Guideline for Nutrition in Children with CKD. update. Executive summary (2009) Am J Kidney Dis. 2008;53:S11–104. doi: 10.1053/j.ajkd.2008.11.017. [DOI] [PubMed] [Google Scholar]
- 21.Centers for Disease Control and Prevention (CDC) National Center for Health Statistics (NCHS). National Health and Nutrition Examination Survey Data. Hyattsville, MD: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; 2008. http://www.cdc.gov/nchs/nhanes/nhanes2007-2008/nhanes07_08.htm. [Google Scholar]
- 22.Tanner JM. Growth at Adolescence. Oxford: Blackwell Scientific Publication; 1962. [Google Scholar]
- 23.Haycock GB, Schwartz GJ, Wisotsky DH. Geometric method for measuring body surface area: a height-weight formula validated in infants, children, and adults. J Pediatr. 1978;93:62–66. doi: 10.1016/s0022-3476(78)80601-5. [DOI] [PubMed] [Google Scholar]
- 24.Mak RH, Cheung WW, Zhan JY, Shen Q, Foster BJ. Cachexia and protein-energy wasting in children with chronic kidney disease. Pediatr Nephrol. 2012;27:173–181. doi: 10.1007/s00467-011-1765-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kalantar-Zadeh K, Kleiner M, Dunne E, Ahern K, Nelson M, Koslowe R, Luft FC. Total iron-binding capacity-estimated transferrin correlates with the nutritional subjective global assessment in hemodialysis patients. Am J Kidney Dis. 1998;31:263–272. doi: 10.1053/ajkd.1998.v31.pm9469497. [DOI] [PubMed] [Google Scholar]
- 26.Furth SL, Abraham AG, Jerry-Fluker J, Schwartz GJ, Benfield M, Kaskel F, Wong C, Mak RH, Moxey-Mims M, Warady BA. Metabolic abnormalities, cardiovascular disease risk factors, and GFR decline in children with chronic kidney disease. Clin J Am Soc Nephrol. 2011;6:2132–2140. doi: 10.2215/CJN.07100810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Little RJA, Rubin DB. Statistical Analysis with Missing Data. 2. New York: John Wiley & Sons; 2002. [Google Scholar]
- 28.Schafer JL. Analysis of Incomplete Multivariate Data. New York: Chapman and Hill; 1997. [Google Scholar]
- 29.Liang KY, Zeger SL. Longitudinal Data Analysis Using Generalized Linear Models. Biometrika. 1986;73:13–22. [Google Scholar]
- 30.Mak RH, Ikizler AT, Kovesdy CP, Raj DS, Stenvinkel P, Kalantar-Zadeh K. Wasting in chronic kidney disease. J Cachex Sarcopenia Muscle. 2011;2:9–25. doi: 10.1007/s13539-011-0019-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cheung WW, Mak RH. Melanocortin antagonism ameliorates muscle wasting and inflammation in chronic kidney disease. Am J Physiol Renal Physiol. 2012;303:F1315–F1324. doi: 10.1152/ajprenal.00341.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rashid R, Neill E, Smith W, King D, Beattie TJ, Murphy A, Ramage IJ, Maxwell H, Ahmed SF. Body composition and nutritional intake in children with chronic kidney disease. Pediatr Nephrol. 2006;21:1730–1738. doi: 10.1007/s00467-006-0235-y. [DOI] [PubMed] [Google Scholar]
- 33.Evans WJ, Morley JE, Argiles J, Bales C, Baracos V, Guttridge D, Jatoi A, Kalantar-Zadeh K, Lochs H, Mantovani G, Marks D, Mitch WE, Muscaritoli M, Najand A, Ponikowski P, Rossi FF, Schambelan M, Schols A, Schuster M, Thomas D, Wolfe R, Anker SD. Cachexia: a new definition. Clin Nutr. 2008;27:793–799. doi: 10.1016/j.clnu.2008.06.013. [DOI] [PubMed] [Google Scholar]


