Abstract
Gastrulation is comprised of a series of cell polarization and directional cell migration events that establish the physical body plan of the embryo. One of the major ligand-based pathways that has emerged to play crucial roles in the regulation of gastrulation is the non-canonical Wnt signaling pathway. This aspect of Wnt signaling is comprised of a number of signaling branches that are subsequently integrated for the regulation of changes to the actin cytoskeleton during cell polarization and cell migration during vertebrate gastrulation. The Rho family of small GTPases are activated and required for non-canonical Wnt signaling during gastrulation, and in this chapter, we describe biochemical assays for the detection of Wnt-mediated activation of Rho, Rac, and Cdc42 in both mammalian cells and Xenopus embryo explants.
Keywords: Non-canonical Wnt signaling, Rac, Rho, Cdc42, Gastrulation, Xenopus
1. Introduction
Ligand-based signaling pathways play central roles during development of the early embryo and intensive efforts are directed towards elucidating their mechanism of function. The Wnt signaling pathway is an evolutionarily conserved pathway that regulates critical aspects of cell fate determination, cell polarity, cell migration, neural patterning and organogenesis during embryonic development (1). This large family of secreted glycoproteins binds to the seven-pass transmembrane receptor Frizzled (Fz) in conjunction with other co-receptors to regulate a plethora of cellular processes (2). Upon the binding of Wnt protein to its receptor complex, the Wnt signal is transduced to the cytoplasmic protein Dishevelled (Dsh/Dvl) that focuses signaling into two basic branches of Wnt signaling, namely the canonical (β-catenin dependent) and non-canonical (β-catenin independent) pathways. Although Wnt signaling via the canonical β-catenin pathway has been most intensively studied in cell fate determination, cell proliferation and human cancer (1, 3), Wnt activation of the Rho family of GTPases via the non-canonical pathway has recently received increased attention (Fig. 1). In addition to the activation of β-catenin and the small GTPases, Wnt proteins can also stimulate less-defined non-canonical signaling pathways such as the Wnt/Ca2+, Wnt/Rap, Wnt/ROR, Wnt/PKA, Wnt/aPKC, Wnt/Ryk, and Wnt/mTOR pathways (4).
Fig. 1. A schematic representation of actin cytoskeletal regulation via non-canonical Wnt signaling.
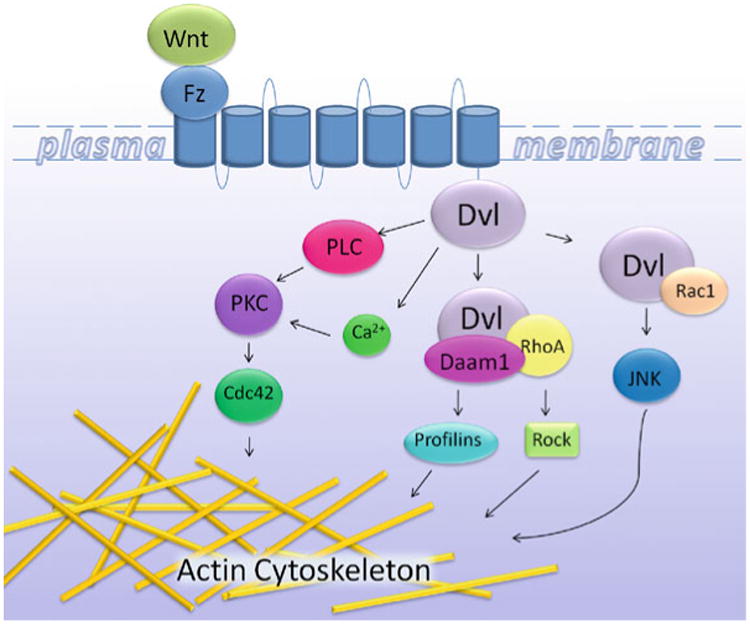
Non-canonical Wnt signaling is defined as Wnt/Frizzled (Fz) initiated signaling independent of β-catenin transcriptional function. The Wnt signal is transduced through the Fz receptor, activating the assembly of different Dvl/effector complexes, ultimately leading to the activation of different pathways regulating the actin cytoskeleton and cell adhesion. A hallmark of non-canonical Wnt signaling is the activation of the small GTPases Rho and Rac. In turn, this activates Rho Kinase (Rock) and Jun N-terminal kinase (JNK), leading to the modulation of the cytoskeleton for cell movement and polarity. For the Wnt/Ca2+ pathway, Wnt signaling via Fz mediates the activation of G-proteins to activate PLC, PKC, and Cdc42 for roles in actin dynamics.
The Wnt signaling pathway was first identified in Drosophila melanogaster, and was later shown to be conserved from nematodes to mammals (5). The canonical pathway was the first branch of Wnt signaling to be identified and remains the most extensively characterized (6). The hallmark of the canonical Wnt pathway is the accumulation and translocation of β-catenin into the nucleus for the regulation of gene transcription (7). The non-canonical pathway functions independently of β-catenin-mediated transcription and regulates actin cytoskeleton organization through the activation of the small GTPases Rho, Rac, and Cdc42. The non-canonical pathway or planar cell polarity (PCP) pathway can be divided into several different branches of which different downstream targets are activated (8) (Fig. 1). It is important to note that crosstalk between the canonical and non-canonical pathways exist as the Rac GTPase was recently shown to modulate canonical signaling pointing to levels of signaling complexity that remain to be unraveled (9).
During early embryogenesis, canonical Wnt signaling plays a pivotal role in cell fate determination, while the non-canonical Wnt pathway is essential for cell polarity and migration during vertebrate gastrulation. During gastrulation, mesodermal cells intercalate along the mediolateral axis, resulting in mediolateral narrowing (convergence) and anteroposterior elongation (extension), ultimately resulting in the highly dynamic process of convergent extension (10, 11). The driving force for convergent extension comes from polarized cell protrusions (lamellipodia), stabilization of these protrusions and migration in the dorsal marginal zone of embryo driven by organized cytoskeletal changes (12, 13). The non-canonical pathway was shown to regulate these convergent extension movements and this pathway shares some common components of the PCP pathway in Drosophila (14).
In the non-canonical pathway, Wnts bind to the receptor complex and induce activation of Dvl (15). Activated Dvl is then able to transduce signaling into pathways that lead to the activation of the small GTPases Rho, Rac, and Cdc42 (Fig. 1). The small GTPase Cdc42 may also be activated via the Fz receptor independent of Dvl (16). The Rho family of small GTPases acts as molecular switches that cycle between an active (GTP-bound) and an inactive (GDP-bound) conformation under the control of guanine nucleotide exchange factors (GEFs) and GTPase-activating proteins (GAPs) (6, 17, 18). Rho, Rac, and Cdc42 are responsible for the regulation, assembly, and organization of the actin cytoskeleton in eukaryotic cells. Rho controls the assembly of actin/myosin filaments to generate contractile forces, while Rac and Cdc42 promote actin polymerization at the cell periphery to generate protrusive forces, in the form of lamellipodia and filopodia, respectively (19). The first pathway of activation of the small GTPases in non-canonical Wnt signaling involves Daam1, a protein that binds to the PDZ domain of Dvl. Activation of Rho GTPase leads to the activation of the Rho-associated kinase (ROCK) and myosin, which leads to modification of the actin cytoskeleton and cytoskeletal rearrangement (20– 22). During the activation of Rho via Daam1-mediated signaling, the activation of at least one Rho GEF has been identified thus far, WGEF (23), though other GEFs and GAPs remain unknown. The second pathway requires the DEP domain of Dvl and activates Rac GTPase, which in turn stimulates Jun N-terminal Kinase (JNK) activity, independent of Daam1 (20, 24). Wnt/Fz signaling also activates another branch of the non-canonical Wnt pathway called the Wnt/Ca2+ pathway. In this case, Wnt/Fz stimulation leads to the release of intracellular Ca2+ through trimeric G proteins. Ca2+ can then activate protein kinase C (PKC), which regulates cell–cell contact during convergent extension via activation of Cdc42 (6, 25) (Fig. 1).
It is accepted that the Frizzled (Fz) family of serpentine transmembrane receptors are cell surface receptors for Wnt proteins (26). There are 19 Wnt genes and 10 Fz genes in both the mouse and human genomes (27). In addition to Fz, several other families of cell surface receptors, including LDL receptor-related proteins 5 and 6 (LRP5/6) (28), Ryk (29), and proteoglycans (30, 31), are also important for Wnt signal transduction, highlighting the complexity and versatility of the Wnt signaling system. While LRP5/6 were identified as co-receptors for Fz proteins and required specifically for the Wnt/β-catenin pathway (28), a recent study has implicated LRP6 in regulating convergent extension movements during gastrulation (32). Additionally, proteoglycans appear to be involved in extracellular Wnt ligand transport and distribution, thus potentially affect many Wnt pathways (33). Attempts have been made to classify Wnt molecules into either the canonical subfamily (Wnt-1, Wnt-3a, and Wnt-8) or the non-canonical subfamily (Wnt-4, Wnt-5a, and Wnt-11) (6). However, accumulating evidence suggests that such a classification may be oversimplified, and many Wnt proteins, including Wnt-1, Wnt-3a, Wnt-5a, and Wnt-11, can activate multiple pathways in different experimental contexts, likely depending upon receptor complements (including Fz and other receptors) and other cofactors that these Wnt proteins may interact with (20, 22, 34–37). Despite these caveats, it is now well established that activation of the Rho family of GTPases by Wnt/Fz signaling is critical for vertebrate development (10, 11, 38).
Unlike the PCP pathway in Drosophila in which the Wnt ligand has not yet been identified, the major ligand regulating convergent extension in Xenopus is believed to be Wnt-11 with corresponding receptor as Fz7 (14). It has been reported that Rho and Rac cooperate with Wnt/Fz to promote different important aspects of the process of convergent extension. Wnt-11 activation of RhoA and Rac can be demonstrated in dorsal embryo explants (Figs. 2 and 3), in which interfering with Wnt-11 or Fz function prevents RhoA and Rac activation (20, 39). Rho activation was blocked after expression of dominant-negative Wnt-11, an extracellular fragment of Fz7, or an inhibitor of Dvl. Conversely, overexpression of Wnt-11 or Xenopus Fz7 (Xfz7) in the embryo's ventral region, which is not involved in CE movements and does not express Wnt-11 or Xfz7, is sufficient to activate RhoA and Rac (20, 39). Wnt/Fz activation of Rho and Rac has also been observed in several commonly used mammalian cell lines (20, 34, 39). In these experiments (Fig. 2), transfection of Wnt-1 or Wnt-3a cDNA, or of certain (but not all) Fz cDNAs, or treatment with Wnt-1 or Wnt-3a conditioned medium, results in RhoA and Rac activation (20, 34, 39). These observations in vertebrate embryos and mammalian cells followed and paralleled earlier genetic studies in Drosophila, which demonstrated that Fz function in PCP relies on RhoA and Rac gene function (40). Thus both genetically and biochemically, Wnt/Fz signaling to RhoA and Rac is a highly evolutionarily conserved function. Likewise, there are some reports that demonstrated Cdc42 plays an important role in convergent extension (6). The expression of either dominant-negative or wild-type Cdc42, in either the animal pole or marginal zone of four-cell embryos, inhibited convergent extension movements (16). Activation of Cdc42 by Wnt-11 is dependent on Gβγ (subunits of heterotrimeric G proteins) acting through protein kinase C (PKC) (41).
Fig. 2. A schematic overview of the Rho, Rac, and Cdc42 assays.
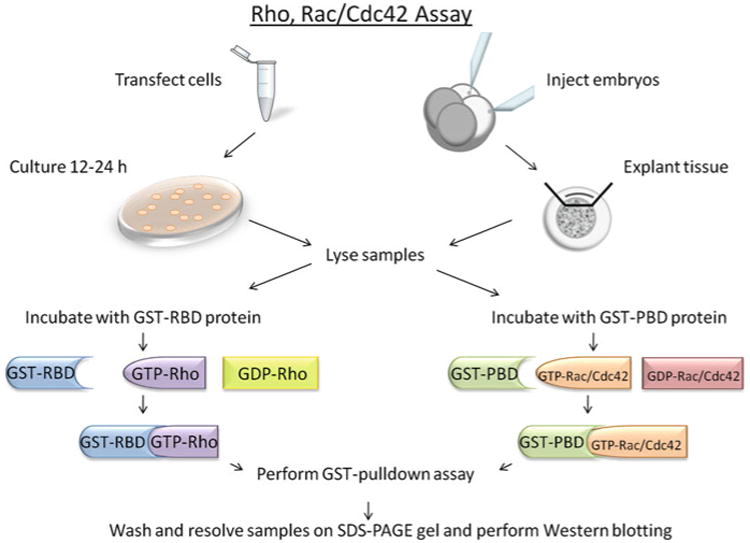
For in vitro experiments, cells are transfected and cultured for 12–24 h and are subsequently lysed for binding studies. For in vivo experiments, Xenopus embryos are injected with RNA at Stage 3 and explants are removed at Stage 10.5 to be lysed and incubated with GST-proteins. Samples are then incubated with either GST-RBD or PBD proteins and a GST-pulldown assay is performed. Samples are then subject to SDS-PAGE and Western blotting.
Fig. 3. Rho, Rac, and Cdc42 assays in Xenopus.
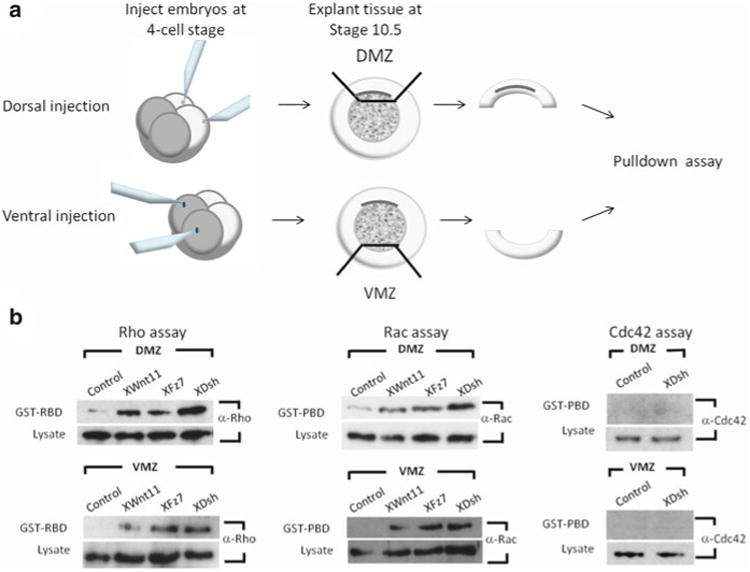
(a) Embryos are injected at the four-cell stage into either the two dorsal or two ventral cells. Embryos are then allowed to develop until stage 10.5 for explants. At this point, either the dorsal marginal zone (DMZ) or ventral marginal zone (VMZ) is cut and subjected to Rho or Rac and Cdc42 pulldown assays. (b) Examples of Rho, Rac, and Cdc42 assays performed with the DMZs and VMZs of Xenopus embryos. Rho, Rac, and Cdc42 activation detected via GST-RBD or GST-PBD pulldown samples show Rho and Rac, but not Cdc42, are activated preferentially in the DMZ. Endogenous Rho, Rac, and Cdc42 levels are shown in the lysate samples.
In this chapter, we describe biochemical assays used to investigate the activation of Rho, Rac, and Cdc421 GTPases in mammalian culture cells and in the Xenopus embryo. These assays utilize a gluthathione S-transferase (GST)-pulldown strategy using fusion proteins that specifically bind to the activated/GTP-bound forms of Rho, Rac, and Cdc42. For Rho assays, a Rho-binding fragment of the Rho-effector Rhotekin is fused to GST and termed GST-RBD (42) (Fig. 2). For the Rac and Cdc42 assays, the Rac and Cdc42 binding fragment of p21 (PAK) is fused to GST and termed GST-PBD (43, 44) (Fig. 2). The GST-RBD and GST-PBD fusion proteins are produced in bacterial cells, purified and incubated with cell lysates derived from either mammalian cells or Xenopus embryo explants (Fig. 2). GST-RBD and GST-PBD bind specifically to the GTP-bound forms of Rho, Rac, or Cdc42, respectively, which are precipitated using Glutathione-agarose beads and detected by conventional immunoblotting (Figs. 2 and 3). The following protocols provide an efficient method to study the activation of the small GTPases Rho, Rac, and Cdc42 both in vitro and in vivo.
2. Materials
2.1. Bacteria
BL21 bacterial cells
1M IPTG
LB-ampicillin plates: 1% Bacto tryptone, 0.5% yeast extract, 1% NaCl, 100 mg/ml ampicillin, 1.5% Bacto Agar
LB-ampicillin growth media: 1% Bacto tryptone, 0.5% yeast extract, 1% NaCl, 100 microgram/ml ampicillin
1× PBS: 1.54 mM KH2 PO4, 155.17 mM NaCl, 2.71 mM Na2 HPO4·7H2 O (pH 7.2)
Glutathione sepharose beads
1× PBS/10 mM DTT/1% TritonX 100
Protease inhibitor cocktail
50 mg/ml lysozyme; 10% Triton-X 100
1 M MgCl2
10 mg/ml DNase1
2.2. Mammalian Cells
HEK293T cells
10% fetal bovine serum (FBS) in Dulbecco's Modifi ed Eagle Medium (DMEM) supplemented with penicillin and streptomycin
Ca2+ phosphate
1× Trypsin
1× PBS
2.3. Embryos
Xenopus laevis embryos
10× MMR: 1 M NaCl, 20 mM KCl, 20 mM CaCl2, 10 mM MgCl2, 50 mM Hepes, pH to 7.6
3% Ficoll-0.5× MMR
1% BSA/0.5× MMR
2.4. GST-PRD and GST-PBD Binding Assay Buffers
Rho Lysis Buffer: 50 mM Tris–HCl pH 7.2, 500 mM NaCl, 1% Triton-X 100, 0.5% Sodium deoxycholic acid, 0.1% SDS, 10 mM MgCl2 and 1 × protease inhibitors (added fresh each time).
Rho Wash Buffer: 50 mM Tris–HCl pH 7.2, 1% Triton-X 100, 150 mM NaCl, 10 mM MgCl2, and 1 × protease inhibitors.
Rac and Cdc42 Lysis Buffer: 50 mM Tris, pH 7.5, 200 mM NaCl, 2% NP40, 10% glycerol, 10 mM MgCl2, and 1 × protease inhibitors.
Rac and Cdc42 Wash Buffer: 25 mM Tris, pH 7.5, 40 mM NaCl, 1% NP40, 30 mM MgCl2, and 1 × protease inhibitors.
2.5. Western Blotting
12% SDS-PAGE gel
Running Buffer: 25 mM Tris, 192 mM Glycine, 0.1% SDS
Transfer Buffer: 25 mM Tris, 192 mM Glycine, 20% Methanol
1× PBST: 1 × PBS, 0.5% Tween-20
2× sample buffer; 125 mM Tris–HCl (pH 6.8), 10% 2-mercapto- ethanol, 4% SDS, 20% Glycerol
5% Non-fat dry milk
Rho monoclonal and polyclonal antibodies (Santa Cruz)
Rac/Cdc42 monoclonal antibodies (Transduction Labs)
SuperSignal PicoWest ECL (Pierce)
3. Methods
3.1. Rho, Rac, and Cdc42 Assays
3.1.1. Preparation of Recombinant GST-RBD Protein
Grow an overnight culture of a single colony of BL21 bacterial cells containing the GST-PBD plasmid in 20 ml of LB-amp (100 μg/ml) at 30°C.
Dilute the culture into 1 l of LB-amp (100 μg/ml) and grow at 30°C until the optical density at 600 nm is 1.0. This takes 5–7 h depending on the starting optical density.
Induce the bacterial culture with 1 ml of 1 M IPTG and incubate for 3–4 h at 30°C.
Aliquot the bacteria into 50 ml Falcon tubes and spin at 1,500 × g for 10 min to pellet the bacteria (see Notes 1 and 2). Discard supernatant and flash freeze the pellets in liquid nitrogen.
Store the pellets at −80°C. The pellets are stable for up to 1 year.
3.1.2. Preparation of Recombinant GST-PBD Fusion Protein
Grow an overnight culture of a single colony of BL21 bacterial cell containing the GST-PBD plasmid in 20 ml LB-amp (100 μg/ml) at 30°C.
Dilute the culture into 1 l of LB-amp (100 μg/ml) and grow at 30°C until the optical density at 600 nm is 1.0. This takes 5–7 h depending on starting optical density.
Induce the bacterial culture with 1 ml of 1M IPTG and incubate for 3–4 h at 30°C.
Lyse the cells in 1 × PBS with protease inhibitors using either sonication or the French press method.
Pellet the lysate by spinning at 16,000 × g for 15 min and isolate the supernatant.
Aliquot the supernatant into 1.5 ml eppendorf tubes and flash freeze in liquid nitrogen (see Notes 1 and 2).
Store at −80°C.
3.1.3. Extraction of GST-RBD and GST-PBD
GST-RBD
Prepare the Glutathione Sepharose Beads by swelling approximately 100 μl with 1 × PBS/10 mM DTT/1% Triton-X 100 for at least 30 min on ice, then wash three times with 500 μl of 1 × PBS/10 mM DTT/1% Triton-X 100. After the final wash, the beads can be stored on ice as a 1 × slurry. Do not spin beads higher than 800 × g during the pelleting and washing stages since this will damage the beads. Also prepare two tubes of beads, for you will have 2 ml of lysate (see Note 3).
Thaw one aliquot of frozen GST-RBD pellet on ice and resus-pend in 2 ml 1 × PBS.
Add 20 μl 1 M DTT, 20 μl Protease inhibitor cocktail (Boehringer-Mannheim) and 40 μl of 50 mg/ml lysozyme.
Vortex briefly to mix well and incubate on ice for 30 min.
Add 225 μl 10% Triton-X 100, 22.5 μl 1 M MgCl2 and 22.5 μl of 10 mg/ml DNase1.
Vortex briefly to mix well and incubate on ice for 30 min.
Spin at 16,000 × g at 4°C for 2 min, and add 1 ml supernatant to each of the two tubes of the pre-swollen beads.
Incubate on a Nutator at 4°C for 45 min (do not exceed 1 h).
Spin and wash beads three times with 500 μl of 1 × PBS/10 mM DTT/1% Triton-X 100.
After the final wash, store on ice in 1 × slurry with the final volume approximately 500 μl.
GST-PBD
Prepare the Glutathione Sepharose Beads by swelling approximately 100 μl with 1 × PBS/10 mM DTT/1% Triton-X 100 for at least 30 min on ice. After this, wash three times with 500 μl of 1 × PBS/10 mM DTT/1% Triton-X 100 and after the final wash store on ice as a 1 × slurry. Do not spin beads higher than 800 × g during the pelleting and washing stages in order to not damage the beads. Prepare two tubes of beads for the 1 ml of bacterial lysate (see Note 3).
Thaw one aliquot of frozen bacterial supernatant on ice.
Add 500 μl supernatant to each of the two tubes of the pre-swollen beads.
Incubate on a Nutator at 4°C for 45 min and do not exceed 1 h.
Spin and wash beads three times with 500 μl of 1 × PBS/10 mM DTT/1% Triton-X 100. After the final wash, store on ice in 1 × slurry with the final volume approximately 500 μl.
3.1.4. Sample Preparations for Pulldown Assays
Mammalian Cells
Rho Assay:
Mammalian HEK293T cells are cultured in 6-well plates (30 mm) in 10% fetal bovine serum and DMEM media supplemented penicillin/streptomycin until 60% confluency.
1 μg of cDNA is transfected into the cells using standard Ca2+-Phosphate method.
Media is changed 12 h post-transfection and cells are incubated for 12–24 h.
Rac and Cdc42 Assay:
Mammalian HEK293T cells are cultured in 6-well plates (60 mm) in 10% fetal bovine serum and DMEM media supplemented penicillin/streptomycin until 60% confluency. At this point, the cells are changed into 0.5% fetal bovine serum and DMEM media supplemented penicillin/streptomycin. This step helps to reduce the basal level of activated Rac and Cdc42.
Six hours after media is changed to 0.5% sera, 1 μg of cDNA is transfected into the cells using standard Ca2+-Phosphate method.
Media is changed 12 h post-transfection and cells are incubated for an additional 12–24 h in 0.5% fetal bovine serum and DMEM media supplemented penicillin/streptomycin.
Xenopus Embryos and Explants:
Xenopus embryos are injected at the four-cell stage into the two dorsal cells (for dorsal marginal zone [DMZ] explants) or into the two ventral cells (for ventral marginal zone [VMZ] explants) in 3%Ficoll/0.5 × MMR.
Two hours after injections, embryos are changed into 0.1 × MMR and cultured to Stage 10.5.
The vitelline membranes are removed from the embryo and the DMZ or VMZ is explanted using forceps. Explants are pooled and stored on ice until they are lysed. All embryos are dissected on agarose-coated culture dishes in a solution of 0.5 × MMR/1%BSA. Xenopus embryos and explants are handled as described elsewhere (45).
3.1.5. GST-RBD and GST-PBD Binding Assay
Lyse the cells in 500 μl of Rho or Rac lysis buffer and to each sample add 10 μl of 10 mg/ml DNase1 solution. Incubate on ice for 10 min and then spin samples at 16,000 × g at 4°C. For Xenopus explants, we typically use 50 explants (DMZ or VMZ) for each sample.
Remove 25 μl of supernatant and add 25 μl of 2 × sample buffer, heat at 90°C for 5 min and store. This is your whole cell lysate for control Western blotting.
Remove the remaining 475 μl of supernatant and add to tubes containing approximately 50 μl of GST-beads coupled to the RBD or to PBD.
Incubate on a Nutator at 4°C for 1 h and wash three times with Rho or Rac wash buffer, accordingly. After final wash, resuspend in 50 μl of 2 × sample buffer and heat at 90°C for 5 min.
Perform Western blot analysis.
3.2. Western Blot Analysis
Resolve samples on a 12% SDS-PAGE gel and run until the Bromophenol dye is approximately 1 inch from the bottom of the gel.
Transfer to nitrocellulose and block for 1 h with 5% non-fat dry milk for 1 h at room temperature.
Wash twice for 5 min with 1 × PBST.
Incubate with the primary antibody (Rho monoclonal, Santa Cruz for mammalian cell extracts or Rho polyclonal, Santa Cruz, for Xenopus explant extracts, Rac or Cdc42 monoclonal, Transduction Labs for both mammalian cell and Xenopus explant extracts) at a 1/500 dilution for 1 h at room temperature or overnight at 4°C.
Wash once for 15 min and four times for 5 min with 1 × PBST.
Incubate with secondary antibody at 1/5,000 solution for 1 h at room temperature.
Wash once for 15 min and four times for 5 min with 1 × PBST.
Perform ECL reaction. We typically use SuperSignal PicoWest (Pierce).
The endogenous Rho, Rac, or Cdc42 is detectable within 1 min in total lysates or 5 min in the GST-RBD/PBD pulldown samples for mammalian cell extracts. For Xenopus explant extracts, the endogenous Rho, Rac, and Cdc42 are detectable within 3–5 min in lysates or 10 min in the GST-RBD/PBD pulldown samples.
4. Notes
For each preparation of GST-RBD or GST-PBD bacteria, use one aliquot and follow the protocol to extract the fusion protein and resolve a fraction of the sample on a 12% SDS-PAGE gel and Coomassie Blue stain. GST-RBD runs at approximately 35 kD and GST-PBD approximately 37 kD. If there is extensive degradation, redo the protein preparation.
Each 50-ml bacterial aliquot of GST-RBD yield approximately 200–300 μg of fusion protein and each is enough for ten samples for the GST-RBD assay. Each 1-ml aliquot of GST-PBD yields approximately 150–250 μg of protein and each is enough for ten samples for the GST-PBD assay.
Always keep samples on ice whenever possible and do not exceed any of the incubation times.
References
- 1.Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol. 2004;20:781–810. doi: 10.1146/annurev.cellbio.20.010403.113126. [DOI] [PubMed] [Google Scholar]
- 2.Komiya Y, Habas R. Wnt signal transduction pathways. Organogenesis. 2008;4(2):68–75. doi: 10.4161/org.4.2.5851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Giles RH, van Es JH, Clevers H. Caught up in a Wnt storm: Wnt signaling in cancer. Biochim Biophys Acta. 2003;1653(1):1–24. doi: 10.1016/s0304-419x(03)00005-2. [DOI] [PubMed] [Google Scholar]
- 4.Semenov MV, et al. SnapShot: Noncanonical Wnt Signaling Pathways. Cell. 2007;131(7):1378. doi: 10.1016/j.cell.2007.12.011. [DOI] [PubMed] [Google Scholar]
- 5.Funato Y, et al. Nucleoredoxin regulates the Wnt/planar cell polarity pathway in Xenopus. Genes Cells. 2008;13(9):965–75. doi: 10.1111/j.1365-2443.2008.01220.x. [DOI] [PubMed] [Google Scholar]
- 6.Schlessinger K, Hall A, Tolwinski N. Wnt signaling pathways meet Rho GTPases. Genes Dev. 2009;23(3):265–77. doi: 10.1101/gad.1760809. [DOI] [PubMed] [Google Scholar]
- 7.Fuerer C, Nusse R, Ten Berge D. Wnt signalling in development and disease. Max Delbruck Center for Molecular Medicine meeting on Wnt signaling in Development and Disease. EMBO Rep. 2008;9(2):134–8. doi: 10.1038/sj.embor.7401159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Simons M, Mlodzik M. Planar cell polarity signaling: from fly development to human disease. Annu Rev Genet. 2008;42:517–40. doi: 10.1146/annurev.genet.42.110807.091432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu X, et al. Rac1 activation controls nuclear localization of beta-catenin during canonical Wnt signaling. Cell. 2008;133(2):340–53. doi: 10.1016/j.cell.2008.01.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Keller R. Shaping the vertebrate body plan by polarized embryonic cell movements. Science. 2002;298(5600):1950–4. doi: 10.1126/science.1079478. [DOI] [PubMed] [Google Scholar]
- 11.Wallingford JB, Fraser SE, Harland RM. Convergent extension: the molecular control of polarized cell movement during embryonic development. Dev Cell. 2002;2(6):695–706. doi: 10.1016/s1534-5807(02)00197-1. [DOI] [PubMed] [Google Scholar]
- 12.Roszko I, Sawada A, Solnica-Krezel L. Regulation of convergence and extension movements during vertebrate gastrulation by the Wnt/PCP pathway. Semin Cell Dev Biol. 2009;20(8):986–97. doi: 10.1016/j.semcdb.2009.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wallingford JB. Planar cell polarity, ciliogenesis and neural tube defects. Hum Mol Genet. 2006;15 Spec No 2:R227–34. doi: 10.1093/hmg/ddl216. [DOI] [PubMed] [Google Scholar]
- 14.Modarresi R, et al. WNT/beta-catenin signaling is involved in regulation of osteoclast differentiation by human immunodeficiency virus protease inhibitor ritonavir: relationship to human immunodeficiency virus-linked bone mineral loss. Am J Pathol. 2009;174(1):123–35. doi: 10.2353/ajpath.2009.080484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wallingford JB, Habas R. The developmental biology of Dishevelled: an enigmatic protein governing cell fate and cell polarity. Development. 2005;132(20):4421–36. doi: 10.1242/dev.02068. [DOI] [PubMed] [Google Scholar]
- 16.Choi SC, Han JK. Xenopus Cdc42 regulates convergent extension movements during gastrulation through Wnt/Ca2+ signaling pathway. Dev Biol. 2002;244(2):342–57. doi: 10.1006/dbio.2002.0602. [DOI] [PubMed] [Google Scholar]
- 17.Raftopoulou M, et al. Regulation of cell migration by the C2 domain of the tumor suppressor PTEN. Science. 2004;303(5661):1179–81. doi: 10.1126/science.1092089. [DOI] [PubMed] [Google Scholar]
- 18.Raftopoulou M, Hall A. Cell migration: Rho GTPases lead the way. Dev Biol. 2004;265(1):23–32. doi: 10.1016/j.ydbio.2003.06.003. [DOI] [PubMed] [Google Scholar]
- 19.Jaffe AB, Hall A. Rho GTPases: biochemistry and biology. Annu Rev Cell Dev Biol. 2005;21:247–69. doi: 10.1146/annurev.cellbio.21.020604.150721. [DOI] [PubMed] [Google Scholar]
- 20.Habas R, Dawid IB, He X. Coactivation of Rac and Rho by Wnt/Frizzled signaling is required for vertebrate gastrulation. Genes Dev. 2003;17(2):295–309. doi: 10.1101/gad.1022203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jessen JR, et al. Zebrafish trilobite identifies new roles for Strabismus in gastrulation and neuronal movements. Nat Cell Biol. 2002;4(8):610–5. doi: 10.1038/ncb828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kishida S, Yamamoto H, Kikuchi A. Wnt-3a and Dvl induce neurite retraction by activating Rho-associated kinase. Mol Cell Biol. 2004;24(10):4487–501. doi: 10.1128/MCB.24.10.4487-4501.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tanegashima K, Zhao H, Dawid IB. WGEF activates Rho in the Wnt-PCP pathway and controls convergent extension in Xenopus gastrulation. Embo J. 2008;27(4):606–17. doi: 10.1038/emboj.2008.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Simons M, et al. Electrochemical cues regulate assembly of the Frizzled/Dishevelled complex at the plasma membrane during planar epithelial polarization. Nat Cell Biol. 2009;11(3):286–94. doi: 10.1038/ncb1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kohn AD, Moon RT. Wnt and calcium signaling: beta-catenin-independent pathways. Cell Calcium. 2005;38(3–4):439–46. doi: 10.1016/j.ceca.2005.06.022. [DOI] [PubMed] [Google Scholar]
- 26.Huang HC, Klein PS. The Frizzled family: receptors for multiple signal transduction pathways. Genome Biol. 2004;5(7):234. doi: 10.1186/gb-2004-5-7-234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Willert J, et al. A transcriptional response to Wnt protein in human embryonic carcinoma cells. BMC Dev Biol. 2002;2:8. doi: 10.1186/1471-213x-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.He X, et al. LDL receptor-related proteins 5 and 6 in Wnt/beta-catenin signaling: arrows point the way. Development. 2004;131(8):1663–77. doi: 10.1242/dev.01117. [DOI] [PubMed] [Google Scholar]
- 29.Lu W, et al. Mammalian Ryk is a Wnt coreceptor required for stimulation of neurite outgrowth. Cell. 2004;119(1):97–108. doi: 10.1016/j.cell.2004.09.019. [DOI] [PubMed] [Google Scholar]
- 30.Ohkawara B, et al. Role of glypican 4 in the regulation of convergent extension movements during gastrulation in Xenopus laevis. Development. 2003;130(10):2129–38. doi: 10.1242/dev.00435. [DOI] [PubMed] [Google Scholar]
- 31.Topczewski J, et al. The zebrafish glypican knypek controls cell polarity during gastrulation movements of convergent extension. Dev Cell. 2001;1(2):251–64. doi: 10.1016/s1534-5807(01)00005-3. [DOI] [PubMed] [Google Scholar]
- 32.Tahinci E, et al. Lrp6 is required for convergent extension during Xenopus gastrulation. Development. 2007;134(22):4095–106. doi: 10.1242/dev.010272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lin X. Functions of heparan sulfate proteoglycans in cell signaling during development. Development. 2004;131(24):6009–21. doi: 10.1242/dev.01522. [DOI] [PubMed] [Google Scholar]
- 34.Endo Y, et al. Wnt-3a-dependent cell motility involves RhoA activation and is specifically regulated by dishevelled-2. J Biol Chem. 2005;280(1):777–86. doi: 10.1074/jbc.M406391200. [DOI] [PubMed] [Google Scholar]
- 35.He X, et al. A member of the Frizzled protein family mediating axis induction by Wnt-5A. Science. 1997;275(5306):1652–4. doi: 10.1126/science.275.5306.1652. [DOI] [PubMed] [Google Scholar]
- 36.Qiang YW, et al. Wnt signaling in B-cell neoplasia. Oncogene. 2003;22(10):1536–45. doi: 10.1038/sj.onc.1206239. [DOI] [PubMed] [Google Scholar]
- 37.Tao Q, et al. A novel G protein-coupled receptor, related to GPR4, is required for assembly of the cortical actin skeleton in early Xenopus embryos. Development. 2005;132(12):2825–36. doi: 10.1242/dev.01866. [DOI] [PubMed] [Google Scholar]
- 38.Veeman MT, et al. Zebrafish prickle, a modulator of noncanonical Wnt/Fz signaling, regulates gastrulation movements. Curr Biol. 2003;13(8):680–5. doi: 10.1016/s0960-9822(03)00240-9. [DOI] [PubMed] [Google Scholar]
- 39.Habas R, Kato Y, He X. Wnt/Frizzled activation of Rho regulates vertebrate gastrulation and requires a novel Formin homology protein Daam1. Cell. 2001;107(7):843–54. doi: 10.1016/s0092-8674(01)00614-6. [DOI] [PubMed] [Google Scholar]
- 40.Mlodzik M. Planar cell polarization: do the same mechanisms regulate Drosophila tissue polarity and vertebrate gastrulation? Trends Genet. 2002;18(11):564–71. doi: 10.1016/s0168-9525(02)02770-1. [DOI] [PubMed] [Google Scholar]
- 41.Penzo-Mendez A, et al. Activation of Gbetagamma signaling downstream of Wnt-11/Xfz7 regulates Cdc42 activity during Xenopus gastrulation. Dev Biol. 2003;257(2):302–14. doi: 10.1016/s0012-1606(03)00067-8. [DOI] [PubMed] [Google Scholar]
- 42.Ren XD, Kiosses WB, Schwartz MA. Regulation of the small GTP-binding protein Rho by cell adhesion and the cytoskeleton. Embo J. 1999;18(3):578–85. doi: 10.1093/emboj/18.3.578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Akasaki T, Koga H, Sumimoto H. Phosphoinositide 3-kinase-dependent and -independent activation of the small GTPase Rac2 in human neutrophils. J Biol Chem. 1999;274(25):18055–9. doi: 10.1074/jbc.274.25.18055. [DOI] [PubMed] [Google Scholar]
- 44.Benard V, Bohl BP, Bokoch GM. Characterization of rac and cdc42 activation in chemoattractant-stimulated human neutrophils using a novel assay for active GTPases. J Biol Chem. 1999;274(19):13198–204. doi: 10.1074/jbc.274.19.13198. [DOI] [PubMed] [Google Scholar]
- 45.Sive HL, Grainger RM, Harland RM. A laboratory manual. Cold Spring Harbor, NY: Cold Spring Harbor Press; 2000. Early Development of Xenopus laevis. [Google Scholar]


