Abstract
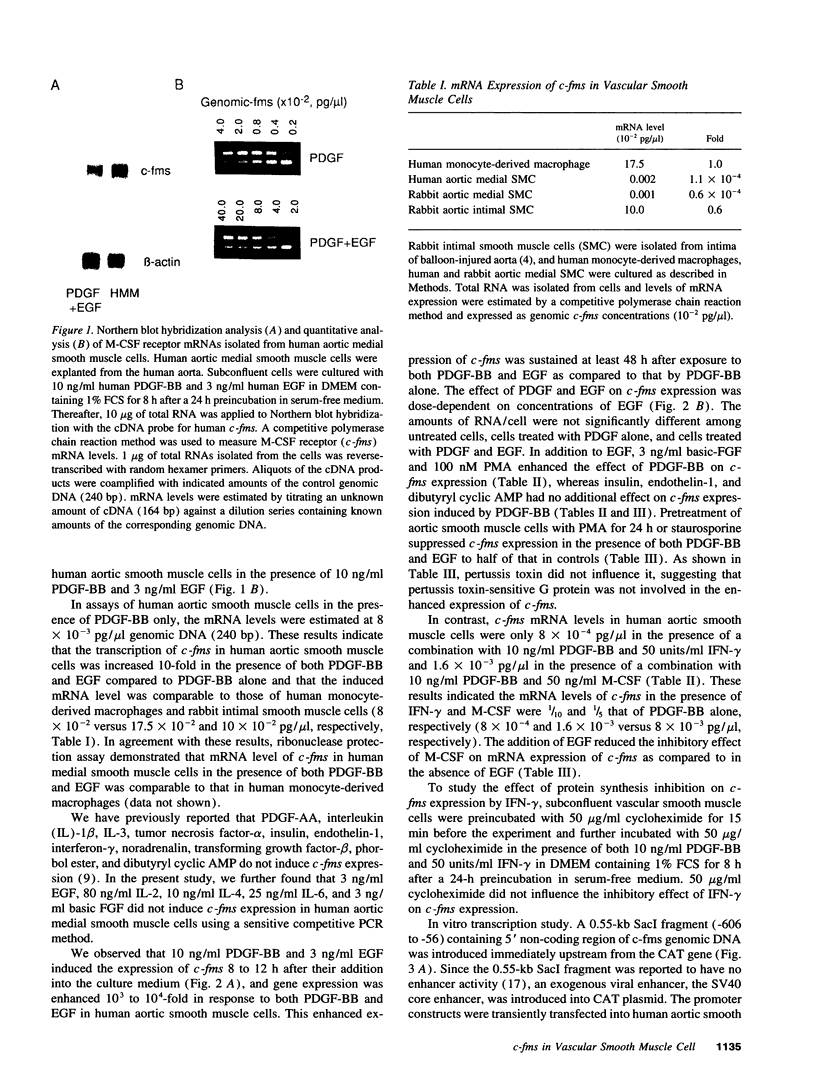
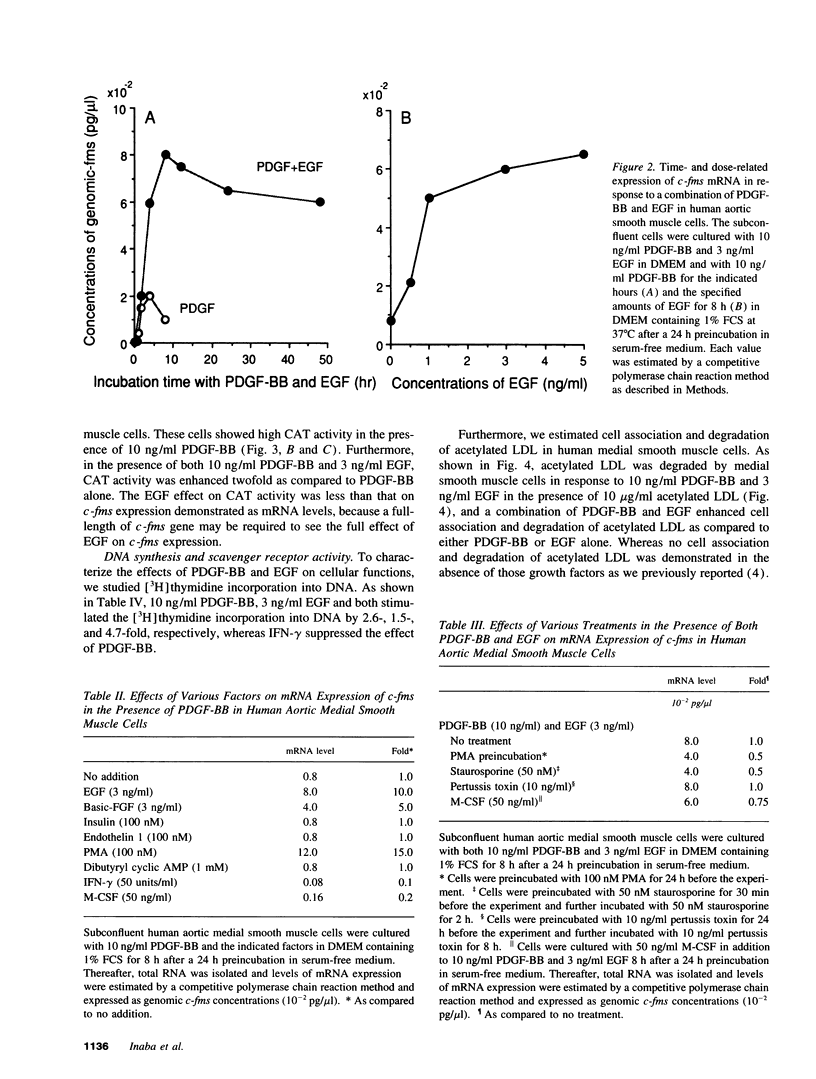
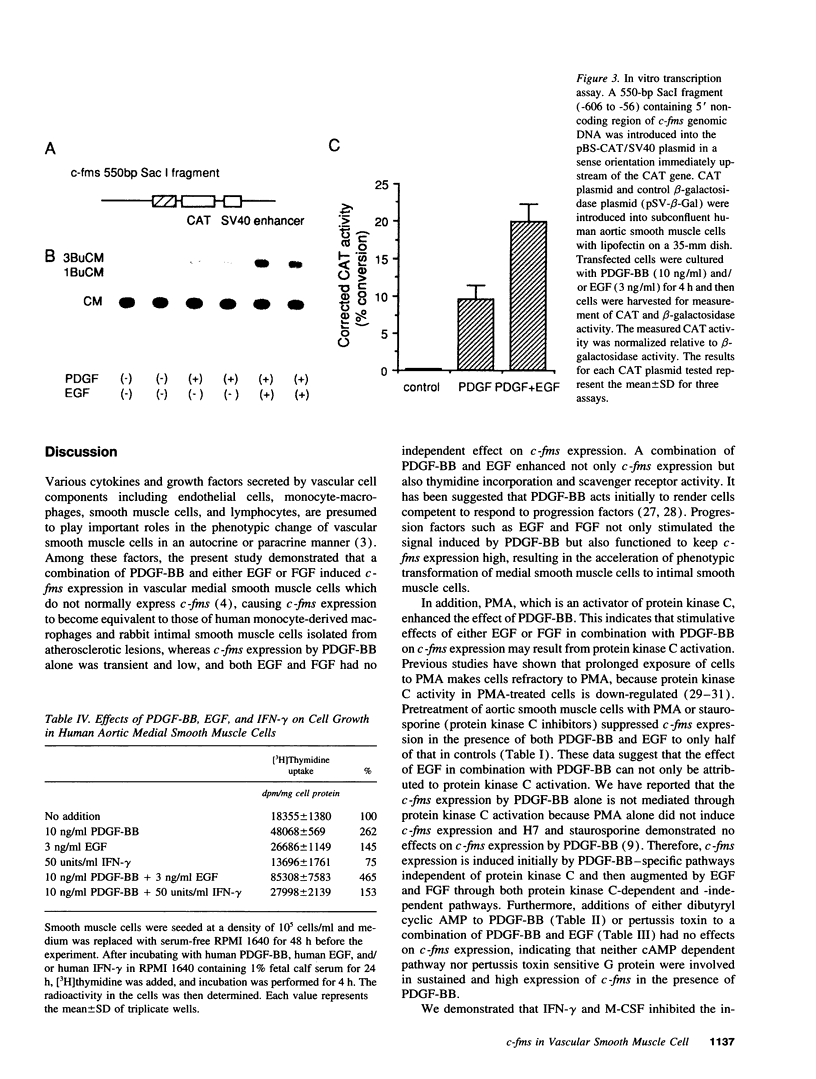
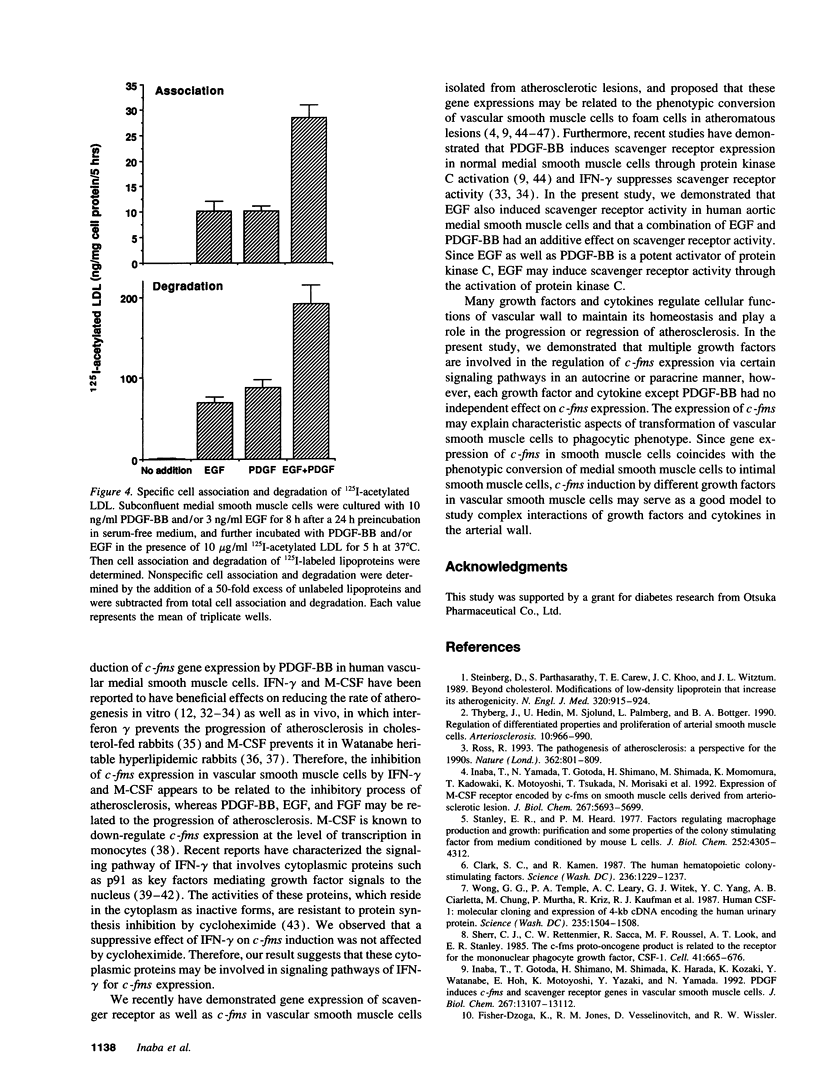
Vascular medial smooth muscle cells migrate, proliferate and transform to foam cells in the process of atherosclerosis. We have reported that the intimal smooth muscle cells express proto-oncogene c-fms, a characteristic gene of monocyte-macrophages, which is not normally expressed in medial smooth muscle cells. In the present study, we demonstrated that combinations of platelet-derived growth factor (PDGF)-BB and either epidermal growth factor (EGF) or fibroblast growth factor (FGF) induced high expression of c-fms in normal human medial smooth muscle cells to the level of intimal smooth muscle cells or monocyte-derived macrophages, whereas c-fms expression by PDGF-BB alone was 1/10 and both EGF and FGF had no independent effect on c-fms expression. By contrast, interferon (IFN)-gamma and macrophage colony-stimulating factor (M-CSF) suppressed the induction of c-fms expression. These results indicate that multiple growth factors and cytokines may play a role in the phenotypic transformation of medial smooth muscle cells to intimal smooth muscle cells in atherosclerotic lesions by altering c-fms expression.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bickel P. E., Freeman M. W. Rabbit aortic smooth muscle cells express inducible macrophage scavenger receptor messenger RNA that is absent from endothelial cells. J Clin Invest. 1992 Oct;90(4):1450–1457. doi: 10.1172/JCI116012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilheimer D. W., Eisenberg S., Levy R. I. The metabolism of very low density lipoprotein proteins. I. Preliminary in vitro and in vivo observations. Biochim Biophys Acta. 1972 Feb 21;260(2):212–221. doi: 10.1016/0005-2760(72)90034-3. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Clark S. C., Kamen R. The human hematopoietic colony-stimulating factors. Science. 1987 Jun 5;236(4806):1229–1237. doi: 10.1126/science.3296190. [DOI] [PubMed] [Google Scholar]
- Collins M. K., Rozengurt E. Binding of phorbol esters to high-affinity sites on murine fibroblastic cells elicits a mitogenic response. J Cell Physiol. 1982 Jul;112(1):42–50. doi: 10.1002/jcp.1041120108. [DOI] [PubMed] [Google Scholar]
- Dejager S., Mietus-Synder M., Pitas R. E. Oxidized low density lipoproteins bind to the scavenger receptor expressed by rabbit smooth muscle cells and macrophages. Arterioscler Thromb. 1993 Mar;13(3):371–378. doi: 10.1161/01.atv.13.3.371. [DOI] [PubMed] [Google Scholar]
- Fong L. G., Fong T. A., Cooper A. D. Inhibition of mouse macrophage degradation of acetyl-low density lipoprotein by interferon-gamma. J Biol Chem. 1990 Jul 15;265(20):11751–11760. [PubMed] [Google Scholar]
- Geng Y. J., Hansson G. K. Interferon-gamma inhibits scavenger receptor expression and foam cell formation in human monocyte-derived macrophages. J Clin Invest. 1992 Apr;89(4):1322–1330. doi: 10.1172/JCI115718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilliland G., Perrin S., Blanchard K., Bunn H. F. Analysis of cytokine mRNA and DNA: detection and quantitation by competitive polymerase chain reaction. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2725–2729. doi: 10.1073/pnas.87.7.2725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein J. L., Basu S. K., Brown M. S. Receptor-mediated endocytosis of low-density lipoprotein in cultured cells. Methods Enzymol. 1983;98:241–260. doi: 10.1016/0076-6879(83)98152-1. [DOI] [PubMed] [Google Scholar]
- Goldstein J. L., Ho Y. K., Basu S. K., Brown M. S. Binding site on macrophages that mediates uptake and degradation of acetylated low density lipoprotein, producing massive cholesterol deposition. Proc Natl Acad Sci U S A. 1979 Jan;76(1):333–337. doi: 10.1073/pnas.76.1.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotoda T., Yamada N., Kawamura M., Kozaki K., Mori N., Ishibashi S., Shimano H., Takaku F., Yazaki Y., Furuichi Y. Heterogeneous mutations in the human lipoprotein lipase gene in patients with familial lipoprotein lipase deficiency. J Clin Invest. 1991 Dec;88(6):1856–1864. doi: 10.1172/JCI115507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotoda T., Yamada N., Murase T., Inaba T., Ishibashi S., Shimano H., Koga S., Yazaki Y., Furuichi Y., Takaku F. Occurrence of multiple aberrantly spliced mRNAs upon a donor splice site mutation that causes familial lipoprotein lipase deficiency. J Biol Chem. 1991 Dec 25;266(36):24757–24762. [PubMed] [Google Scholar]
- HAVEL R. J., EDER H. A., BRAGDON J. H. The distribution and chemical composition of ultracentrifugally separated lipoproteins in human serum. J Clin Invest. 1955 Sep;34(9):1345–1353. doi: 10.1172/JCI103182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbomel P., Bourachot B., Yaniv M. Two distinct enhancers with different cell specificities coexist in the regulatory region of polyoma. Cell. 1984 Dec;39(3 Pt 2):653–662. doi: 10.1016/0092-8674(84)90472-0. [DOI] [PubMed] [Google Scholar]
- Inaba T., Gotoda T., Shimano H., Shimada M., Harada K., Kozaki K., Watanabe Y., Hoh E., Motoyoshi K., Yazaki Y. Platelet-derived growth factor induces c-fms and scavenger receptor genes in vascular smooth muscle cells. J Biol Chem. 1992 Jun 25;267(18):13107–13112. [PubMed] [Google Scholar]
- Inaba T., Shimano H., Gotoda T., Harada K., Shimada M., Kawamura M., Yazaki Y., Yamada N. Macrophage colony-stimulating factor regulates both activities of neutral and acidic cholesteryl ester hydrolases in human monocyte-derived macrophages. J Clin Invest. 1993 Aug;92(2):750–757. doi: 10.1172/JCI116646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inaba T., Yamada N., Gotoda T., Shimano H., Shimada M., Momomura K., Kadowaki T., Motoyoshi K., Tsukada T., Morisaki N. Expression of M-CSF receptor encoded by c-fms on smooth muscle cells derived from arteriosclerotic lesion. J Biol Chem. 1992 Mar 15;267(8):5693–5699. [PubMed] [Google Scholar]
- Inoue I., Inaba T., Motoyoshi K., Harada K., Shimano H., Kawamura M., Gotoda T., Oka T., Shiomi M., Watanabe Y. Macrophage colony stimulating factor prevents the progression of atherosclerosis in Watanabe heritable hyperlipidemic rabbits. Atherosclerosis. 1992 Apr;93(3):245–254. doi: 10.1016/0021-9150(92)90261-e. [DOI] [PubMed] [Google Scholar]
- Ishibashi S., Inaba T., Shimano H., Harada K., Inoue I., Mokuno H., Mori N., Gotoda T., Takaku F., Yamada N. Monocyte colony-stimulating factor enhances uptake and degradation of acetylated low density lipoproteins and cholesterol esterification in human monocyte-derived macrophages. J Biol Chem. 1990 Aug 25;265(24):14109–14117. [PubMed] [Google Scholar]
- Ishibashi S., Yamada N., Shimano H., Mori N., Mokuno H., Gotohda T., Kawakami M., Murase T., Takaku F. Apolipoprotein E and lipoprotein lipase secreted from human monocyte-derived macrophages modulate very low density lipoprotein uptake. J Biol Chem. 1990 Feb 25;265(6):3040–3047. [PubMed] [Google Scholar]
- Larner A. C., David M., Feldman G. M., Igarashi K., Hackett R. H., Webb D. S., Sweitzer S. M., Petricoin E. F., 3rd, Finbloom D. S. Tyrosine phosphorylation of DNA binding proteins by multiple cytokines. Science. 1993 Sep 24;261(5129):1730–1733. doi: 10.1126/science.8378773. [DOI] [PubMed] [Google Scholar]
- Lee L. S., Weinstein I. B. Mechanism of tumor promoter inhibition of cellular binding of epidermal growth factor. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5168–5172. doi: 10.1073/pnas.76.10.5168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppenheimer M. J., Oram J. F., Bierman E. L. Up-regulation of high density lipoprotein receptor activity by gamma-interferon associated with inhibition of cell proliferation. J Biol Chem. 1988 Dec 25;263(36):19318–19323. [PubMed] [Google Scholar]
- Pitas R. E. Expression of the acetyl low density lipoprotein receptor by rabbit fibroblasts and smooth muscle cells. Up-regulation by phorbol esters. J Biol Chem. 1990 Jul 25;265(21):12722–12727. [PubMed] [Google Scholar]
- Pitas R. E., Friera A., McGuire J., Dejager S. Further characterization of the acetyl LDL (scavenger) receptor expressed by rabbit smooth muscle cells and fibroblasts. Arterioscler Thromb. 1992 Nov;12(11):1235–1244. doi: 10.1161/01.atv.12.11.1235. [DOI] [PubMed] [Google Scholar]
- Pledger W. J., Stiles C. D., Antoniades H. N., Scher C. D. Induction of DNA synthesis in BALB/c 3T3 cells by serum components: reevaluation of the commitment process. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4481–4485. doi: 10.1073/pnas.74.10.4481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts W. M., Shapiro L. H., Ashmun R. A., Look A. T. Transcription of the human colony-stimulating factor-1 receptor gene is regulated by separate tissue-specific promoters. Blood. 1992 Feb 1;79(3):586–593. [PubMed] [Google Scholar]
- Ross R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature. 1993 Apr 29;362(6423):801–809. doi: 10.1038/362801a0. [DOI] [PubMed] [Google Scholar]
- Rozengurt E., Rodriguez-Pena M., Smith K. A. Phorbol esters, phospholipase C, and growth factors rapidly stimulate the phosphorylation of a Mr 80,000 protein in intact quiescent 3T3 cells. Proc Natl Acad Sci U S A. 1983 Dec;80(23):7244–7248. doi: 10.1073/pnas.80.23.7244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruff-Jamison S., Chen K., Cohen S. Induction by EGF and interferon-gamma of tyrosine phosphorylated DNA binding proteins in mouse liver nuclei. Science. 1993 Sep 24;261(5129):1733–1736. doi: 10.1126/science.8378774. [DOI] [PubMed] [Google Scholar]
- Sadowski H. B., Shuai K., Darnell J. E., Jr, Gilman M. Z. A common nuclear signal transduction pathway activated by growth factor and cytokine receptors. Science. 1993 Sep 24;261(5129):1739–1744. doi: 10.1126/science.8397445. [DOI] [PubMed] [Google Scholar]
- Sariban E., Imamura K., Sherman M., Rothwell V., Pantazis P., Kufe D. Downregulation of c-fms gene expression in human monocytes treated with phorbol esters and colony-stimulating factor 1. Blood. 1989 Jul;74(1):123–129. [PubMed] [Google Scholar]
- Schaub R. G., Bree M. P., Hayes L. L., Rudd M. A., Rabbani L., Loscalzo J., Clinton S. K. Recombinant human macrophage colony-stimulating factor reduces plasma cholesterol and carrageenan granuloma foam cell formation in Watanabe heritable hyperlipidemic rabbits. Arterioscler Thromb. 1994 Jan;14(1):70–76. doi: 10.1161/01.atv.14.1.70. [DOI] [PubMed] [Google Scholar]
- Sherr C. J., Rettenmier C. W., Sacca R., Roussel M. F., Look A. T., Stanley E. R. The c-fms proto-oncogene product is related to the receptor for the mononuclear phagocyte growth factor, CSF-1. Cell. 1985 Jul;41(3):665–676. doi: 10.1016/s0092-8674(85)80047-7. [DOI] [PubMed] [Google Scholar]
- Shuai K., Stark G. R., Kerr I. M., Darnell J. E., Jr A single phosphotyrosine residue of Stat91 required for gene activation by interferon-gamma. Science. 1993 Sep 24;261(5129):1744–1746. doi: 10.1126/science.7690989. [DOI] [PubMed] [Google Scholar]
- Silvennoinen O., Schindler C., Schlessinger J., Levy D. E. Ras-independent growth factor signaling by transcription factor tyrosine phosphorylation. Science. 1993 Sep 24;261(5129):1736–1739. doi: 10.1126/science.8378775. [DOI] [PubMed] [Google Scholar]
- Stanley E. R., Heard P. M. Factors regulating macrophage production and growth. Purification and some properties of the colony stimulating factor from medium conditioned by mouse L cells. J Biol Chem. 1977 Jun 25;252(12):4305–4312. [PubMed] [Google Scholar]
- Steinberg D., Parthasarathy S., Carew T. E., Khoo J. C., Witztum J. L. Beyond cholesterol. Modifications of low-density lipoprotein that increase its atherogenicity. N Engl J Med. 1989 Apr 6;320(14):915–924. doi: 10.1056/NEJM198904063201407. [DOI] [PubMed] [Google Scholar]
- Thyberg J., Hedin U., Sjölund M., Palmberg L., Bottger B. A. Regulation of differentiated properties and proliferation of arterial smooth muscle cells. Arteriosclerosis. 1990 Nov-Dec;10(6):966–990. doi: 10.1161/01.atv.10.6.966. [DOI] [PubMed] [Google Scholar]
- Wharton W., Leof E., Pledger W. J., O'Keefe E. J. Modulation of the epidermal growth factor receptor by platelet-derived growth factor and choleragen: effects on mitogenesis. Proc Natl Acad Sci U S A. 1982 Sep;79(18):5567–5571. doi: 10.1073/pnas.79.18.5567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson A. C., Schaub R. G., Goldstein R. C., Kuo P. T. Suppression of aortic atherosclerosis in cholesterol-fed rabbits by purified rabbit interferon. Arteriosclerosis. 1990 Mar-Apr;10(2):208–214. doi: 10.1161/01.atv.10.2.208. [DOI] [PubMed] [Google Scholar]
- Wong G. G., Temple P. A., Leary A. C., Witek-Giannotti J. S., Yang Y. C., Ciarletta A. B., Chung M., Murtha P., Kriz R., Kaufman R. J. Human CSF-1: molecular cloning and expression of 4-kb cDNA encoding the human urinary protein. Science. 1987 Mar 20;235(4795):1504–1508. doi: 10.1126/science.3493529. [DOI] [PubMed] [Google Scholar]
- Yamada N., Shames D. M., Stoudemire J. B., Havel R. J. Metabolism of lipoproteins containing apolipoprotein B-100 in blood plasma of rabbits: heterogeneity related to the presence of apolipoprotein E. Proc Natl Acad Sci U S A. 1986 May;83(10):3479–3483. doi: 10.1073/pnas.83.10.3479. [DOI] [PMC free article] [PubMed] [Google Scholar]




