Abstract
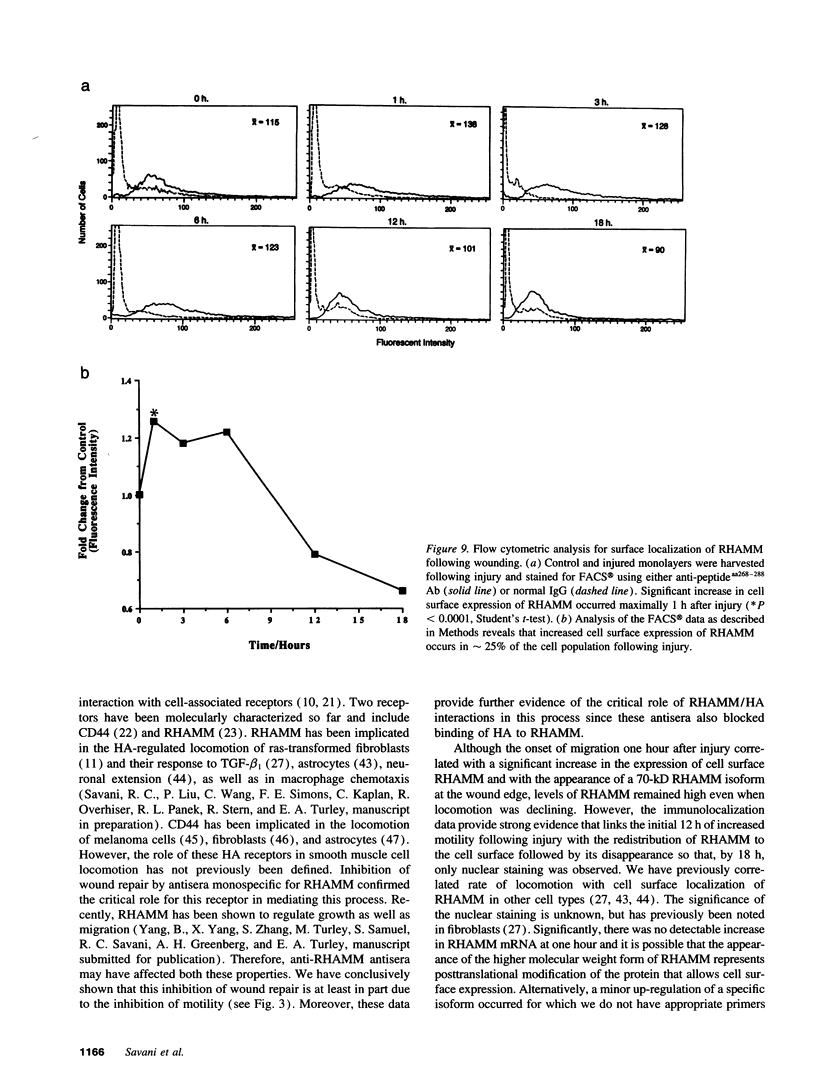
The migration of smooth muscle cells is a critical event in the pathogenesis of vascular diseases. We have investigated the role of hyaluronan (HA) and the hyaluronan receptor RHAMM in the migration of adult bovine aortic smooth muscle cells (BASMC). Cultured BASMC migrated from the leading edge of a single scratch wound with increased velocity between 1 and 24 h. Polyclonal anti-RHAMM antisera that block HA binding with this receptor abolished smooth muscle cell migration following injury. HA stimulated the random locomotion of BASMC and its association with the cell monolayer increased following wounding injury. Immunoblot analysis of wounded monolayers demonstrated a novel RHAMM protein isoform that appeared within one hour after injury. At the time of increased cell motility after wounding, FACS analysis demonstrated an increase in the membrane localization in approximately 25% of the cell population. Confocal microscopy of injured monolayers confirmed that membrane expression of this receptor was limited to cells at the wound edge. Collectively, these data demonstrate that RHAMM is necessary for the migration of smooth muscle cells and that expression and distribution of this receptor is tightly regulated following wounding of BASMC monolayers.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asher R., Bignami A. Hyaluronate binding and CD44 expression in human glioblastoma cells and astrocytes. Exp Cell Res. 1992 Nov;203(1):80–90. doi: 10.1016/0014-4827(92)90042-7. [DOI] [PubMed] [Google Scholar]
- Ausprunk D. H., Boudreau C. L., Nelson D. A. Proteoglycans in the microvascular. II. Histochemical localization in proliferating capillaries of the rabbit cornea. Am J Pathol. 1981 Jun;103(3):367–375. [PMC free article] [PubMed] [Google Scholar]
- Banerjee S. D., Toole B. P. Hyaluronan-binding protein in endothelial cell morphogenesis. J Cell Biol. 1992 Nov;119(3):643–652. doi: 10.1083/jcb.119.3.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee S. D., Toole B. P. Monoclonal antibody to chick embryo hyaluronan-binding protein: changes in distribution of binding protein during early brain development. Dev Biol. 1991 Jul;146(1):186–197. doi: 10.1016/0012-1606(91)90459-g. [DOI] [PubMed] [Google Scholar]
- Basson C. T., Knowles W. J., Bell L., Albelda S. M., Castronovo V., Liotta L. A., Madri J. A. Spatiotemporal segregation of endothelial cell integrin and nonintegrin extracellular matrix-binding proteins during adhesion events. J Cell Biol. 1990 Mar;110(3):789–801. doi: 10.1083/jcb.110.3.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell L., Madri J. A. Effect of platelet factors on migration of cultured bovine aortic endothelial and smooth muscle cells. Circ Res. 1989 Oct;65(4):1057–1065. doi: 10.1161/01.res.65.4.1057. [DOI] [PubMed] [Google Scholar]
- Bell L., Madri J. A. Influence of the angiotensin system on endothelial and smooth muscle cell migration. Am J Pathol. 1990 Jul;137(1):7–12. [PMC free article] [PubMed] [Google Scholar]
- Boudreau N., Rabinovitch M. Developmentally regulated changes in extracellular matrix in endothelial and smooth muscle cells in the ductus arteriosus may be related to intimal proliferation. Lab Invest. 1991 Feb;64(2):187–199. [PubMed] [Google Scholar]
- Boudreau N., Turley E., Rabinovitch M. Fibronectin, hyaluronan, and a hyaluronan binding protein contribute to increased ductus arteriosus smooth muscle cell migration. Dev Biol. 1991 Feb;143(2):235–247. doi: 10.1016/0012-1606(91)90074-d. [DOI] [PubMed] [Google Scholar]
- Bray B. A., Sampson P. M., Osman M., Giandomenico A., Turino G. M. Early changes in lung tissue hyaluronan (hyaluronic acid) and hyaluronidase in bleomycin-induced alveolitis in hamsters. Am Rev Respir Dis. 1991 Feb;143(2):284–288. doi: 10.1164/ajrccm/143.2.284. [DOI] [PubMed] [Google Scholar]
- Casscells W. Migration of smooth muscle and endothelial cells. Critical events in restenosis. Circulation. 1992 Sep;86(3):723–729. doi: 10.1161/01.cir.86.3.723. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Hardwick C., Hoare K., Owens R., Hohn H. P., Hook M., Moore D., Cripps V., Austen L., Nance D. M., Turley E. A. Molecular cloning of a novel hyaluronan receptor that mediates tumor cell motility. J Cell Biol. 1992 Jun;117(6):1343–1350. doi: 10.1083/jcb.117.6.1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes B. F., Liao H. X., Patton K. L. The transmembrane hyaluronate receptor (CD44): multiple functions, multiple forms. Cancer Cells. 1991 Sep;3(9):347–350. [PubMed] [Google Scholar]
- Hoare K., Savani R. C., Wang C., Yang B., Turley E. A. Identification of hyaluronan binding proteins using a biotinylated hyaluronan probe. Connect Tissue Res. 1993;30(2):117–126. doi: 10.3109/03008209309041327. [DOI] [PubMed] [Google Scholar]
- Hällgren R., Samuelsson T., Laurent T. C., Modig J. Accumulation of hyaluronan (hyaluronic acid) in the lung in adult respiratory distress syndrome. Am Rev Respir Dis. 1989 Mar;139(3):682–687. doi: 10.1164/ajrccm/139.3.682. [DOI] [PubMed] [Google Scholar]
- Klewes L., Turley E. A., Prehm P. The hyaluronate synthase from a eukaryotic cell line. Biochem J. 1993 Mar 15;290(Pt 3):791–795. doi: 10.1042/bj2900791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurent T. C., Fraser J. R. Hyaluronan. FASEB J. 1992 Apr;6(7):2397–2404. [PubMed] [Google Scholar]
- Madri J. A., Basson M. D. Extracellular matrix-cell interactions: dynamic modulators of cell, tissue and organism structure and function. Lab Invest. 1992 May;66(5):519–521. [PubMed] [Google Scholar]
- Madri J. A., Kocher O., Merwin J. R., Bell L., Tucker A., Basson C. T. Interactions of vascular cells with transforming growth factors-beta. Ann N Y Acad Sci. 1990;593:243–258. doi: 10.1111/j.1749-6632.1990.tb16116.x. [DOI] [PubMed] [Google Scholar]
- Madri J. A., Kocher O., Merwin J. R., Bell L., Yannariello-Brown J. The interactions of vascular cells with solid phase (matrix) and soluble factors. J Cardiovasc Pharmacol. 1989;14 (Suppl 6):S70–S75. [PubMed] [Google Scholar]
- Majack R. A., Clowes A. W. Inhibition of vascular smooth muscle cell migration by heparin-like glycosaminoglycans. J Cell Physiol. 1984 Mar;118(3):253–256. doi: 10.1002/jcp.1041180306. [DOI] [PubMed] [Google Scholar]
- Messadi D. V., Bertolami C. N. CD44 and hyaluronan expression in human cutaneous scar fibroblasts. Am J Pathol. 1993 Apr;142(4):1041–1049. [PMC free article] [PubMed] [Google Scholar]
- Moscatelli D., Rifkin D. B. Membrane and matrix localization of proteinases: a common theme in tumor cell invasion and angiogenesis. Biochim Biophys Acta. 1988 Aug 3;948(1):67–85. doi: 10.1016/0304-419x(88)90005-4. [DOI] [PubMed] [Google Scholar]
- Murphy J. D., Rabinovitch M., Goldstein J. D., Reid L. M. The structural basis of persistent pulmonary hypertension of the newborn infant. J Pediatr. 1981 Jun;98(6):962–967. doi: 10.1016/s0022-3476(81)80605-1. [DOI] [PubMed] [Google Scholar]
- Naito M., Hayashi T., Kuzuya M., Funaki C., Asai K., Kuzuya F. Effects of fibrinogen and fibrin on the migration of vascular smooth muscle cells in vitro. Atherosclerosis. 1990 Jul;83(1):9–14. doi: 10.1016/0021-9150(90)90124-2. [DOI] [PubMed] [Google Scholar]
- Nettelbladt O., Bergh J., Schenholm M., Tengblad A., Hällgren R. Accumulation of hyaluronic acid in the alveolar interstitial tissue in bleomycin-induced alveolitis. Am Rev Respir Dis. 1989 Mar;139(3):759–762. doi: 10.1164/ajrccm/139.3.759. [DOI] [PubMed] [Google Scholar]
- Nicholson-Weller A., Wang C. E. Structure and function of decay accelerating factor CD55. J Lab Clin Med. 1994 Apr;123(4):485–491. [PubMed] [Google Scholar]
- Ripellino J. A., Klinger M. M., Margolis R. U., Margolis R. K. The hyaluronic acid binding region as a specific probe for the localization of hyaluronic acid in tissue sections. Application to chick embryo and rat brain. J Histochem Cytochem. 1985 Oct;33(10):1060–1066. doi: 10.1177/33.10.4045184. [DOI] [PubMed] [Google Scholar]
- Ross R., Glomset J. A. The pathogenesis of atherosclerosis (second of two parts). N Engl J Med. 1976 Aug 19;295(8):420–425. doi: 10.1056/NEJM197608192950805. [DOI] [PubMed] [Google Scholar]
- Ross R., Raines E. W., Bowen-Pope D. F. The biology of platelet-derived growth factor. Cell. 1986 Jul 18;46(2):155–169. doi: 10.1016/0092-8674(86)90733-6. [DOI] [PubMed] [Google Scholar]
- Ross R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature. 1993 Apr 29;362(6423):801–809. doi: 10.1038/362801a0. [DOI] [PubMed] [Google Scholar]
- Samuel S. K., Hurta R. A., Spearman M. A., Wright J. A., Turley E. A., Greenberg A. H. TGF-beta 1 stimulation of cell locomotion utilizes the hyaluronan receptor RHAMM and hyaluronan. J Cell Biol. 1993 Nov;123(3):749–758. doi: 10.1083/jcb.123.3.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas L., Byers H. R., Vink J., Stamenkovic I. CD44H regulates tumor cell migration on hyaluronate-coated substrate. J Cell Biol. 1992 Aug;118(4):971–977. doi: 10.1083/jcb.118.4.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toole B. P. Developmental role of hyaluronate. Connect Tissue Res. 1982;10(1):93–100. doi: 10.3109/03008208209034409. [DOI] [PubMed] [Google Scholar]
- Toole B. P. Hyaluronan and its binding proteins, the hyaladherins. Curr Opin Cell Biol. 1990 Oct;2(5):839–844. doi: 10.1016/0955-0674(90)90081-o. [DOI] [PubMed] [Google Scholar]
- Turley E. A., Austen L., Vandeligt K., Clary C. Hyaluronan and a cell-associated hyaluronan binding protein regulate the locomotion of ras-transformed cells. J Cell Biol. 1991 Mar;112(5):1041–1047. doi: 10.1083/jcb.112.5.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turley E. A. Hyaluronan and cell locomotion. Cancer Metastasis Rev. 1992 Mar;11(1):21–30. doi: 10.1007/BF00047600. [DOI] [PubMed] [Google Scholar]
- Turley E. A., Moore D., Hayden L. J. Characterization of hyaluronate binding proteins isolated from 3T3 and murine sarcoma virus transformed 3T3 cells. Biochemistry. 1987 Jun 2;26(11):2997–3005. doi: 10.1021/bi00385a007. [DOI] [PubMed] [Google Scholar]
- Turley E. A. Purification of a hyaluronate-binding protein fraction that modifies cell social behavior. Biochem Biophys Res Commun. 1982 Oct 15;108(3):1016–1024. doi: 10.1016/0006-291x(82)92101-5. [DOI] [PubMed] [Google Scholar]
- Turley E. A. The role of a cell-associated hyaluronan-binding protein in fibroblast behaviour. Ciba Found Symp. 1989;143:121-33; discussion 133-7, 281-5. doi: 10.1002/9780470513774.ch8. [DOI] [PubMed] [Google Scholar]
- Turley E., Auersperg N. A hyaluronate binding protein transiently codistributes with p21k-ras in cultured cell lines. Exp Cell Res. 1989 Jun;182(2):340–348. doi: 10.1016/0014-4827(89)90239-5. [DOI] [PubMed] [Google Scholar]
- Waldenström A., Martinussen H. J., Gerdin B., Hällgren R. Accumulation of hyaluronan and tissue edema in experimental myocardial infarction. J Clin Invest. 1991 Nov;88(5):1622–1628. doi: 10.1172/JCI115475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigel P. H., Fuller G. M., LeBoeuf R. D. A model for the role of hyaluronic acid and fibrin in the early events during the inflammatory response and wound healing. J Theor Biol. 1986 Mar 21;119(2):219–234. doi: 10.1016/s0022-5193(86)80076-5. [DOI] [PubMed] [Google Scholar]
- West D. C., Hampson I. N., Arnold F., Kumar S. Angiogenesis induced by degradation products of hyaluronic acid. Science. 1985 Jun 14;228(4705):1324–1326. doi: 10.1126/science.2408340. [DOI] [PubMed] [Google Scholar]
- West D. C., Kumar S. The effect of hyaluronate and its oligosaccharides on endothelial cell proliferation and monolayer integrity. Exp Cell Res. 1989 Jul;183(1):179–196. doi: 10.1016/0014-4827(89)90428-x. [DOI] [PubMed] [Google Scholar]
- Yang B., Zhang L., Turley E. A. Identification of two hyaluronan-binding domains in the hyaluronan receptor RHAMM. J Biol Chem. 1993 Apr 25;268(12):8617–8623. [PubMed] [Google Scholar]