Abstract
Objective
To assess the methodological quality of published network meta-analysis.
Design
Systematic review.
Methods
We searched the medical literature for network meta-analyses of pharmaceuticals. We assessed general study characteristics, study transparency and reproducibility, methodological approach, and reporting of findings. We compared studies published in journals with lower impact factors with those published in journals with higher impact factors, studies published prior to January 1st, 2013 with those published after that date, and studies supported financially by industry with those supported by non-profit institutions or that received no support.
Results
The systematic literature search identified 854 citations. Three hundred and eighteen studies met our inclusion criteria. The number of network meta-analyses has grown rapidly, with 48% of studies published since January 2013. The majority of network meta-analyses were supported by a non-profit institution or received no support (68%). We found considerable inconsistencies among reviewed studies. Eighty percent reported search terms, 61% a network diagram, 65% sufficient data to replicate the analysis, and 90% the characteristics of included trials. Seventy percent performed a risk of bias assessment of included trials, 40% an assessment of model fit, and 56% a sensitivity analysis. Among studies with a closed loop, 69% examined the consistency of direct and indirect evidence. Sixty-four percent of studies presented the full matrix of head-to-head treatment comparisons. For Bayesian studies, 41% reported the probability that each treatment was best, 31% reported treatment ranking, and 16% included the model code or referenced publicly-available code. Network meta-analyses published in higher impact factors journals and those that did not receive industry support performed better across the assessment criteria. We found few differences between older and newer studies.
Conclusions
There is substantial variation in the network meta-analysis literature. Consensus among guidelines is needed improve the methodological quality, transparency, and consistency of study conduct and reporting.
Introduction
Network meta-analysis (NMA) is increasingly accepted as a valuable methodology by clinical researchers and health care decision makers.[1–3] Network meta-analysis has been described as the “new generation” of evidence synthesis and the number of published studies is growing rapidly.[4] The approach has been embraced by national health technology assessment agencies in a number of countries, including Australia, Canada, and the UK.[5–7] The Cochrane Collaboration has also introduced a new type of review called ‘Overviews of Reviews’, which summarize the relative effectiveness of multiple competing interventions for a single indication.[8,9] A report by the Agency for Healthcare Research and Quality in the US identified 25 publicly-available guidance documents on how to conduct and report network meta-analyses.[10] National and regional health technology assessment agencies prepared the majority of these documents, although the guidance issued by the International Society for Pharmacoeconomics and Outcomes Research (ISPOR) was considered to be most comprehensive.[10]
The objective of this study was to assess the methodological quality of published NMAs. Previous studies have documented the growth and study characteristics of the NMA literature through July 2012.[3,4,11–14] These reviews investigated the validity assumptions and methods of using NMAs. Our study builds on this body of literature in several ways. First, we update the evidence by including articles published up to July 2014. Second, we investigate the methods, the transparency and reproducibility, and the presentation of findings in these studies. Finally, we compare the characteristics of studies published in journals with lower impact factors with those published in journals with higher impact factors, studies published prior to January 1st, 2013 with those published after that date, and studies supported financially by industry with those supported by non-profit or that received no support.
Methods
We systematically searched for all published network meta-analyses in the Ovid-MEDLINE database using the following search terms: network meta-analysis; indirect treatment comparison; mixed treatment comparison; and, multiple treatments meta-analysis. The date of our last search was July 30th, 2014. We limited our search to studies including randomized controlled trials with human participants; we only included articles published in English-language journals. We excluded NMAs submitted to national health technology assessment agencies unless they were subsequently published in the medical literature.
Two trained reviewers reviewed each abstract independently using predefined inclusion criteria. We only included NMAs with at least one pharmaceutical in the set of treatments examined and data from at least three clinical trials; we also only included NMAs that compared the efficacy of the treatments. We excluded methodological studies, cost-effectiveness studies, editorials, and letters to the editor. Two trained reviewers independently extracted data from each included study; the reviewers resolved disagreements through consensus. We reviewed all published material related to each NMA, including the online supplementary material and appendices. When the source of study funding was undeclared or unclear, we contacted the corresponding author for the information.
Using a standardized data collection form, we collected information for each NMA on general study characteristics, study methods, transparency, and presentation of findings. We reported the following study characteristics: journal name; study authors; publication year; study support (industry or other); journal impact factor (as reported on Journal Citation Reports)[15]; country (determined by the corresponding author’s affiliation); number of included active treatments; number of clinical trials included; total number of patients included; and indication and patient population studied. We also noted all of the comparator treatments, including non-pharmaceutical treatments and different modes of administration of the same pharmaceutical.
We applied objective assessment criteria from the “checklist of good research practices” in the ISPOR guidance for interpreting and conducting indirect-treatment-comparison and network-meta-analysis studies.[16,17] Our assessment criteria included the following:
- Study method assessment criteria
- Was a Bayesian or a frequentist framework used?
- Did the analysis include adjustments for model covariates?
- Was a fixed or random effects model used? Or, were the findings of both fixed and random effects models presented?
- Was an assessment of model fit reported?
- Was a sensitivity analysis performed? (e.g., varied the included clinical studies to evaluate the robustness of the findings)
- For studies with at least one closed loop, was the consistency of direct evidence and indirect evidence evaluated? (i.e., presented and compared the findings from the traditional meta-analysis and the network meta-analysis)
- Study transparency and reproducibility assessment criteria
- Were the search terms reported?
- Was a network diagram of included treatments presented?
- Was data from the included clinical studies necessary to reproduce the network meta-analysis presented?
- Was a table of key clinical study characteristics presented?
- Was the model code presented or source cited? (reported for studies performed using a Bayesian framework only)
- Presentation of study findings
- Were pairwise comparisons of all included treatments presented?
- Was the probability of each treatment being best reported? (reported for studies performed using a Bayesian framework only)
- Was a ranking of treatments in terms of effectiveness reported? (reported for studies performed using a Bayesian framework only)
To compare studies published in high- and low-impact journals, we evenly split the sample based on the reported journal impact factors (cut-off point: impact factor above and below 3.534). To compare older and newer studies, we again divided the sample in two (cut-off point: published before and after January 1st, 2013). We used our categorization of study support to compare industry-supported studies with studies with and non-industry-supported studies. We used a Chi-square test and a student’s t-test to evaluate categorical and continuous variables, respectively. We considered p-values below the 5% level as statistically significant. Analyses were undertaken using Stata 13.
Results
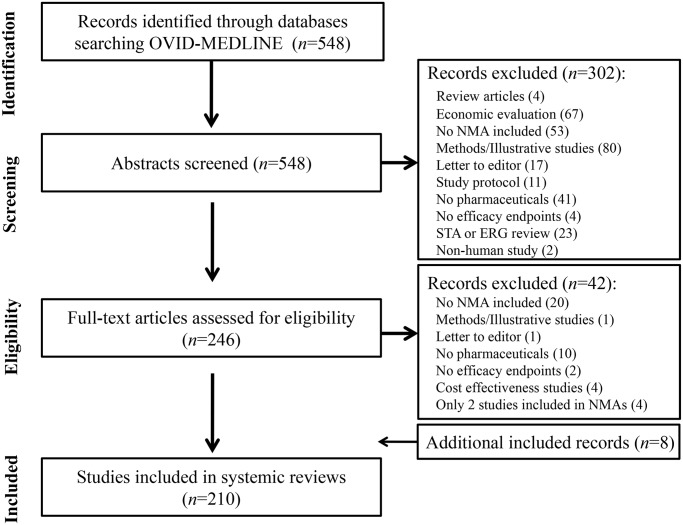
Our systematic literature search identified 854 citations. We reviewed the full texts of 386 abstracts met the inclusion criteria. Based on the full text reviews, we excluded another 68 studies. We included 318 studies in our final sample (Fig 1).
Fig 1. Identification of network meta-analyses included in review.
Table 1 presents the frequency of published NMAs by year, indication, and country; it also presents the types of included pharmaceutical included. The number of NMAs has increased rapidly each year; about 68% and 48% of studies were published after January 2012 and January 2013, respectively. Studies have evaluated treatments for a range of indications, most commonly those affecting the circulatory (n = 64, 20%) and musculoskeletal systems (n = 45, 14%). Analyses were most often performed by researchers based in the USA (n = 81, 26%), UK (n = 79, 25%), and Canada (n = 28, 9%). The majority of studies adopted a Bayesian framework (n = 214, 67%) and either received non-profit or no support (n = 215, 68%).
Table 1. Frequency of network meta-analyses (n = 210) by year, indication, and country.
| Year study published † | n |
|---|---|
| 1997 | 1 (0.3%) |
| 2003 | 3 (0.9%) |
| 2004 | 1 (0.3%) |
| 2006 | 3 (0.9%) |
| 2007 | 3 (0.9%) |
| 2008 | 9 (2.8%) |
| 2009 | 16 (5.0%) |
| 2010 | 21 (6.9%) |
| 2011 | 44 (13.8%) |
| 2012 | 66 (20.4%) |
| 2013 | 43 (24.5%) |
| 2014 (through July 31st) | 73 (23.0%) |
| International Statistical Classification of Diseases (ICD) disease categories | n |
| Blood Disease | 3 (0.9%) |
| Circulatory System | 64 (20.1%) |
| Digestive System | 13 (4.1%) |
| Endocrine, Nutritional, Metabolic, and Immunity | 28 (8.8%) |
| Genitourinary System | 7 (2.2%) |
| Infectious and Parasite Disease | 14 (4.4%) |
| Mental and Behavioral Disorder | 13 (4.1%) |
| Musculoskeletal System and Connective Tissue | 45 (14.2%) |
| Neoplasm | 39 (12.3%) |
| Nervous System and Sensory Organs | 33 (10.4%) |
| Respiratory System | 20 (6.3%) |
| Skin and Subcutaneous Tissues | 9 (2.8%) |
| Other | 30 (9.4%) |
| Country | n |
| USA | 81 (25.5%) |
| UK | 79 (24.8%) |
| Canada | 28 (8.8%) |
| Italy | 21 (6.6%) |
| China | 16 (5.0%) |
| France | 14 (4.4%) |
| The Netherlands | 10 (3.1%) |
| Germany | 8 (2.5%) |
| Brazil | 6 (1.9%) |
| Switzerland | 6 (1.9%) |
| Taiwan | 6 (1.9%) |
| Greece | 5 (1.6%) |
| Spain | 4 (1.3%) |
| Other | 34 (10.7%) |
| Type of pharmaceutical intervention included | n |
| Multiple pharmaceuticals compared | 304 (95.6%) |
| Study included a non pharmaceutical treatment (e.g., surgery, exercise, counselling, etc) | 30 (9.4%) |
| Different strengths of the same pharmaceutical compared (e.g., simvastatin 20mg vs. 40mg) | 82 (25.8%) |
| Treatments in the same drug class grouped together as a comparator (e.g., beta-blockers, or statins) | 75 (23.6%) |
| Multiple modes of administration of a drug compared (e.g., oral, sublingual, intramuscular, etc) | 10 (3.1%) |
† We limited our literature search to studies published in the medical literature. We did not include NMAs submitted to national health technology assessment agencies unless also published in the Ovid-MEDLINE database.
* ‘Other countries’ includes Greece, Ireland, Singapore, Australia, Cameroon, Denmark, Finland, Hong Kong, Korea, Norway, Poland, and Portugal.
The vast majority of network meta-analyses compared multiple pharmaceuticals (n = 304, 96%), while a minority included a non-pharmaceutical comparator treatment (n = 30, 9%). Some researchers grouped treatments in the same therapeutic class, e.g., beta-blockers or statins, as a single comparator treatment (n = 75, 24%). Different dosages, e.g., simvastatin 20 mg vs. 40 mg, were compared in 82 (26%) studies, and ten studies (3%) compared different modes of administration of the same pharmaceutical, e.g., oral vs. intramuscular injection.
We found many differences among the included studies (Table 2). In terms of study methods, 30% of studies did not perform a risk of bias assessment of included studies, 60% did not report an assessment of model fit, and 44% did not include a sensitivity analysis. Among studies with a closed loop, i.e., three or more included treatments had been compared in head-to-head trials, 40% did not report the consistency of direct and indirect evidence. In terms of study transparency and reproducibility, 20% did not report the systematic review search terms, 39% did not present a network diagram, 35% did not present sufficient data from contributing studies to replicate the analysis, and 10% did not provide details of the key characteristics of included trials. Thirty-six percent of included studies did not present the full matrix of head-to-head treatment comparisons. For studies performed using a Bayesian framework, 41% reported the probability that each included treatment was best, 31% reported a ranking of included treatments, and 16% included or directed readers to the model code.
Table 2. Assessment of network meta-analysis study characteristics.
| Journal quality (n = 301)* | Date of study publication (n = 318) | Source of study support (n = 315)** | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Assessment criteria | All studies (n = 318) | Low impact factor (<3.534) (n = 147) | High impact factor (≥3.534) (n = 154) | p-value | Older studies (published prior to 2013) (n = 167) | Recent studies (2013, 2014) (n = 151) | p-value | Industry support (n = 98) | Non-Industry support/ no support (n = 217) | p-value |
| General study characteristics | ||||||||||
| Number of treatments compared | 6.3 (±6.4) | 6.8 (±8.5) | 6.0 (±3.9) | 0.3136 | 6.0 (±4.2) | 6.7 (±8.2) | 0.3816 | 5.9 (±3.6) | 6.5 (±7.3) | 0.446 |
| Total number of studies | 32.9 (±45.5) | 28.3 (±38.6) | 36.5 (±46.9) | 0.0992 | 30.5 (±50.2) | 35.5 (±50.2) | 0.3341 | 22.7 (±29.4) | 37.4 (±50.5) | 0.0079 |
| Total number of patients | 26875 (±65936) | 21938 (±46061) | 33292 (±82859) | 0.1549 | 23711 (±49899) | 30460 (±80375) | 0.3732 | 10945 (±13183) | 33864 (±77635) | 0.005 |
| HTA region (UK, AUS and Canada) † | 110 (35%) | 50 (34%) | 56 (36%) | 0.6709 | 68 (47%) | 42 (28%) | 0.0156 | 48 (49%) | 62 (28%) | 0.0003 |
| Journal impact factor | 5.5 (±6.2) | NA | NA | NA | 5.8 (±6.5) | 5.2 (±5.9) | 0.3791 | 3.1 (±1.7) | 6.5 (±7.1) | <0.0001 |
| Study method | ||||||||||
| Bayesian framework | 214 (67%) | 91 (62%) | 109 (71%) | 0.1038 | 106 (63%) | 108 (72%) | 0.1273 | 75 (77%) | 139 (63%) | 0.0191 |
| Risk of bias assessment of included studies | 223 (70%) | 100 (68%) | 111 (72%) | 0.4446 | 103 (62%) | 120 (79%) | 0.0005 | 53 (54%) | 170 (77%) | <0.0001 |
| Adjustment for covariates | 92 (29%) | 35 (24%) | 51 (33%) | 0.0744 | 54 (32%) | 38 (25%) | 0.1601 | 37 (38%) | 55 (25%) | 0.0205 |
| Random effects model*** | 221 (70%) | 98 (67%) | 114 (75%) | 0.1609 | 116 (69%) | 106 (71%) | 0.7453 | 67 (68%) | 155 (71%) | 0.6243 |
| Assessment of model fit | 127 (40%) | 53 (36%) | 70 (45%) | 0.0979 | 69 (41%) | 58 (38%) | 0.5985 | 46 (47%) | 81 (37%) | 0.0894 |
| Sensitivity analysis | 179 (56%) | 73 (50%) | 96 (62%) | 0.0267 | 88 (53%) | 91 (60%) | 0.1752 | 57 (58%) | 122 (58%) | 0.6542 |
| Consistency of direct and indirect evidence reported**** (closed loop studies only, n = 167) | 116 (69%) | 39 (57%) | 73 (79%) | 0.0017 | 57 (66%) | 59 (73%) | 0.3606 | 16 (39%) | 100 (79%) | <0.0001 |
| Study transparency and reproducibility | ||||||||||
| Search terms reported | 254 (80%) | 112 (76%) | 129 (84%) | 0.1007 | 129 (77%) | 125 (83%) | 0.2201 | 61 (62%) | 193 (88%) | <0.0001 |
| Network diagram | 194 (61%) | 85 (58%) | 101 (66%) | 0.1671 | 103 (62%) | 91 (60%) | 0.7974 | 62 (63%) | 132 (60%) | 0.5829 |
| Extracted data from contributing clinical studies | 206 (65%) | 87 (60%) | 106 (69%) | 0.0955 | 116 (69%) | 91 (61%) | 0.1011 | 58 (60%) | 149 (68%) | 0.1726 |
| Table of key clinical study characteristics | 286 (90%) | 128 (87%) | 141 (92%) | 0.2084 | 145 (87%) | 141 (93%) | 0.0527 | 89 (91%) | 197 (90%) | 0.729 |
| Model code (Bayesian framework only, n = 214) | 35 (16%) | 9 (6%) | 24 (16%) | 0.0085 | 24 (14%) | 11 (7%) | 0.0439 | 8 (8%) | 27 (12%) | 0.2811 |
| Presentation of study findings | ||||||||||
| Full matrix of head-to-head comparisons | 203 (64%) | 84 (57%) | 108 (70%) | 0.0191 | 110 (66%) | 93 (62%) | 0.4294 | 44 (45%) | 159 (73%) | <0.0001 |
| Reported probability of being best (Bayesian framework only, n = 214) | 87 (41%) | 32 (22%) | 51 (33%) | 0.0277 | 41 (25%) | 46 (30%) | 0.2389 | 25 (26%) | 62 (28%) | 0.623 |
| Ranking of included treatments (Bayesian framework only, n = 214) | 67 (31%) | 26 (18%) | 40 (26%) | 0.0829 | 29 (17%) | 39 (26%) | 0.0664 | 11 (11%) | 56 (26%) | 0.0031 |
† Regions in which submissions to HTA agencies generally require a NMA;
* 17 studies published in journals with no associated impact factor;
** 3 studies for which source of study support was unclear;
*** 77 studies reported both fixed and random effects models, 38 studies did not report models used;
**** Consistency only reported for studies with a closed loop
Journal quality
We found that NMAs published in journals with higher impact factors more often performed a sensitivity analysis (62% versus 50%, p = 0.0267) and reported the full matrix of head-to-head comparisons (70% versus 57%, p = 0.0191). For studies with a closed loop, those published in journals with higher impact factors more often compared the consistency of direct and indirect evidence (79% versus 57%, p = 0.0017). For studies performed using a Bayesian framework, studies published in higher impact factor journals more often reported the probability of each treatment being best (33% versus 22%, p = 0.0277), and the model code (16% versus 6%, p = 0.0085).
Publication date
We found that studies published prior to January 1st, 2013 were more often authored by researchers based in regions where HTA agencies include NMAs in their assessments (47% versus 28%, p = 0.0156). Studies published prior to January 1st, 2013 less often performed a risk of bias assessment of included trials (62% versus 79%, p = 0.005), but, for Bayesian studies, more often reported the model code or cited publicly-available code (14% versus 7%, p = 0.0439).
Source of financial support
We found a number of differences between industry-supported studies and non-industry supported studies. Industry-supported studies included fewer trials (22.7 versus 37.4, p = 0.0079) and a smaller total number of patients (10945 versus 33864, p = 0.0050). A greater proportion of industry-funded studies were authored by researchers based in regions in which HTA agencies often include NMA in their assessments (49% versus 28%, p = 0.0079). Industry-supported studies were on average published in journals with lower impact factors (3.1 versus 6.5, p<0.0001).
Industry-supported studies more often used a Bayesian framework (77% versus 63%, p = 0.0191), and adjusted for study covariates (38% versus 25%, p = 0.0251); however, they less often performed a risk of bias assessment of included studies (54% versus 77%, p<0.0001), and, for Bayesian studies, less often compared the consistency of direct and indirect evidence (39% versus 79%, p<0.0001).
Industry-supported studies less often reported the search terms used in the systematic literature review (62% versus 88%, p<0.0001), and reported the full matrix of head-to-head comparisons (45% versus 73%, p<0.0001). For studies performed using a Bayesian framework, industry-supported studies less often reported a ranking of treatments (11% versus 26%, p = 0.0031).
Discussion
Network meta-analyses are increasingly recognized as offering valuable information to the medical community. Growth in the NMA literature has been rapid, with roughly half of studies in our dataset published since January 1st, 2013. Our dataset includes studies by researchers based in 30 countries, confirming NMAs as a global research enterprise. We found notable variation across published network meta-analyses with respect to methods, transparency and reproducibility, and presentation of findings.
As expected, we found that studies published in higher-impact journals performed better across our assessment criteria, suggesting that higher-quality journals publish higher quality NMAs. Our findings may also point to a marginal improvement in the conduct and reporting of NMAs over time, with more recently published studies more often performing a risk of bias assessment of included trials. Interestingly, more recent Bayesian studies less often reported the model code, perhaps reflecting the fact that example model code has become easily accessible and freely available.[20] It is notable that a smaller proportion of studies are now authored by researchers based in regions where HTA agencies include network meta-analysis in their assessments. This likely reflects the widespread acceptance of NMA and the growing international use of the technique by researchers.
We also found differences among NMAs according to source of financial support. For instance, we found that industry-supported studies tended to be published in journals with a lower impact factor. Similarly, industry-supported studies were more often authored by researchers based in HTA regions, which is likely due to the publication of NMAs that were previously included in a submission to a regulatory agency. An interesting finding is that industry-sponsored studies less often used a Bayesian framework, the reason for which is unclear.
Consistent with the studies published in journals with higher impact factors, we found that industry-supported studies less often reported the full matrix of head-to-head comparisons. For NMAs with a closed loop, they less often compared the consistency of direct and indirect evidence. Like older analyses, industry-supported studies were less likely to perform a risk of bias assessment of included trials.
Other identified differences between industry-supported and non-industry-supported studies do not appear to be explained by journal quality or the data of publication. For instance, industry-supported studies included fewer contributing trials and total patients, and less often reported the systematic review search terms. Industry sponsored Bayesian network meta-analyses less often reported a ranking of included drugs based on efficacy, although they more often adjusted for covariates. However, as we emphasize below, these findings should be interpreted with caution due to limitations of our assessment criteria.
Study limitations
While our assessment criteria were based on published guidelines for the conduct and reporting of network meta-analysis, they may have been insufficient to fully assess study quality. For example, while we determined whether a study reported a sensitivity analysis or assessed the model fit, we did not judge whether the approaches were appropriate or sufficient. We also did not evaluate whether the set of included treatments was appropriate or comprehensive. This is a salient consideration as the inappropriate inclusion or exclusion of treatments can significantly affect study findings.
Furthermore, a study may have not met an assessment criterion, or appear to have performed poorly on a given criterion, for good reason. For instance, while industry-supported studies included fewer contributing studies and total patients, this may reflect the precision of the study research question, rather than the how comprehensively the question was addressed. Similarly, while guidelines recommend assessing included trials for risk of bias, the influence of individual design components of individual randomized trials on the findings is variable. [21–23] While it is recommended that the consistency between direct and indirect evidence should be assessed, researchers may be more inclined to do so when there is apparent heterogeneity among the included trials.[24–28]
For indications for which the relative efficacy of existing treatments has been established by multiple NMAs, a researcher may choose to compare a newly-introduced treatment to existing interventions, rather than to report the full matrix. Moreover, it is reported that treatment rankings may at times be an unreliable metric for comparative efficacy and safety, and authors may choose not to present these findings.[29]
Our comparison of NMAs on the basis of study support has a number of additional limitations. First, while we took great care when categorizing studies as ‘industry-supported’ and contacted authors when source of support was ambiguous, we predominantly relied on the reported disclosures. Second, we did not compare the conclusions of industry-and non-industry-supported NMAs. To determine if industry-supported studies tend to produce more favourable conclusions about the study sponsors’ drugs, it would be necessary to identify and compare industry sponsored and non-industry sponsored trials that evaluate the same clinical research questions.[30,31] Unfortunately, while various NMAs have been conducted for some indications, these studies often considered different research questions and included different treatments. This prevented us from evaluating the NMA literature in this way.
Moving forward
This research highlights the need for greater consistency in the conduct and reporting of NMAs. In addition to the ISPOR recommendations used here, there exist a multitude of guidelines and recommendations with notable differences. This suggests that a consensus, on which NMA methods are most appropriate has not been reached.[10] [32] While the development of guidelines advances the field, inconsistencies between guidelines may impede standardization. For example, while all guidelines are unanimous in stating the consistency between direct and indirect evidence should be assessed, there is disagreement with respect to the importance of testing model fit, the use of fixed- or random-effects models, and the exclusion or inclusion of covariates in the model.[10]
As NMAs are an extension of traditional meta-analyses, it seems logical that they should be held to similar methodological and reporting standards. For systematic reviews and traditional meta-analysis, the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) protocol—which consists of a 27-item checklist and a four-phase flow diagram—has emerged as the standard reporting approach.[33] The forthcoming extension of the PRISMA guidelines for network meta-analysis may consolidate existing recommendations, and serve as the authoritative guideline.
Still, our study underscores that checklists should be considered only as guides to study quality. A NMA that satisfies all criteria on a given checklist may not necessarily be a high-quality study, and a study that does not meet various criteria may not necessarily be a low-quality study. Indeed, the recent ISPOR-AMCP-NPC Good Practice Task Force Report is a questionnaire, not a checklist, reflecting the challenge of creating a ‘one size fits all’ checklist for network meta-analyses.[34] It is important that users of network meta-analyses critique the research, and make their own judgment regarding study quality and accuracy. Journal editors should ensure that published network meta-analyses are transparent and reproducible, and that these studies present findings in an unambiguous and comprehensive manner.
Data Availability
All relevant data are within the paper.
Funding Statement
The authors have no support or funding to report.
References
- 1. Ioannidis JP. Indirect comparisons: the mesh and mess of clinical trials. Lancet. 2006. October 28;368(9546):1470–2. [DOI] [PubMed] [Google Scholar]
- 2. Piccini JP, Kong DF. Mixed treatment comparisons for atrial fibrillation: evidence network or bewildering entanglement? Europace. 2011. March;13(3):295–6. 10.1093/europace/eur029 [DOI] [PubMed] [Google Scholar]
- 3. Edwards SJ, Clarke MJ, Wordsworth S, Borrill J. Indirect comparisons of treatments based on systematic reviews of randomised controlled trials. Int J Clin Pract. 2009. June;63(6):841–54. 10.1111/j.1742-1241.2009.02072.x [DOI] [PubMed] [Google Scholar]
- 4. Salanti G. Indirect and mixed-treatment comparison, network, or multiple-treatments meta-analysis: many names, many benefits, many concerns for the next generation evidence synthesis tool. Research synthesis methods 2012; 3:80–97. [DOI] [PubMed] [Google Scholar]
- 5.National Institute for Health and Care Excellence [Internet]. Guide to the methods of technology appraisal [updated 2013 April 4; cited 2014 Dec 11]. Available from: http://www.nice.org.uk/article/pmg9/resources/non-guidance-guide-to-the-methods-of-technology-appraisal-2013-pdf [PubMed]
- 6.Wells GA, Sultan SA, Chen L, Khan M, Coyle D. [Internet]. Indirect Evidence: Indirect Treatment Comparisons in Meta-Analysis. [updated 2009 March; cited 2014 Dec 11] Available from: http://www.cadth.ca/en/products/health-technology-assessment/publication/884
- 7.Pharmaceutical Benefits Advisory Committee [Internet]. Guidelines for preparing submissions to the Pharmaceutical Benefits Advisory Committee (Version 4.4) [updated 2013 June; cited 2014 Dec 11] Available: http://www.pbac.pbs.gov.au/content/information/printable-files/pbacg-book.pdf
- 8. Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011; cited 2014 Dec 11]. Available: www.cochrane-handbook.org. [Google Scholar]
- 9.The Cochrane Collaboration. [Internet] Comparing Multiple Interventions Methods Group. [updated 2013 Dec 18; cited 2014 Dec 11] Available: http://cmimg.cochrane.org/
- 10. Coleman CI, Phung OJ, Cappelleri JC, Baker WL, Kluger J, White CM, et al. Use of Mixed Treatment Comparisons in Systematic Reviews Methods Research Report. (Prepared by the University of Connecticut/Hartford Evidence-based Practice Center; under Contract No.290-2007-10067-I.) AHRQ Publication No. 12-EHC119-EF. [updated 2012 Aug; cited 2014 Dec 11] Available: www.effectivehealthcare.ahrq.gov/reports/final.cfm. [Google Scholar]
- 11. Bafeta A, Trinquart L, Seror R, Ravaud P. Analysis of the systematic reviews process in reports of network meta-analyses: methodological systematic review. BMJ. 2013. July 1;347:f3675 10.1136/bmj.f3675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sobieraj DM, Cappelleri JC, Baker WL, Phung OJ, White CM, Coleman CI. Methods used to conduct and report Bayesian mixed treatment comparisons published in the medical literature: a systematic review. BMJ Open. 2013. July 21;3(7). pii: e003111 10.1136/bmjopen-2013-003111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Song F, Loke YK, Walsh T, Glenny AM, Eastwood AJ, Altman DG. Methodological problems in the use of indirect comparisons for evaluating healthcare interventions: survey of published systematic reviews. BMJ. 2009. April 3;338:b1147 10.1136/bmj.b1147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Donegan S, Williamson P, Gamble C, Tudur-Smith C. Indirect comparisons: a review of reporting and methodological quality. PLoS One. 2010. November 10;5(11):e11054 10.1371/journal.pone.0011054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thomson Reuters. 2013 Journal Citation Reports. Available: http://thomsonreuters.com/journal-citation-reports/
- 16. Hoaglin DC, Hawkins N, Jansen JP, Scott DA, Itzler R, Cappelleri JC, et al. Conducting indirect-treatment-comparison and network-meta-analysis studies: report of the ISPOR Task Force on Indirect Treatment Comparisons Good Research Practices: part 2. Value Health. 2011. June;14(4):429–37. 10.1016/j.jval.2011.01.011 [DOI] [PubMed] [Google Scholar]
- 17. Jansen JP, Fleurence R, Devine B, Itzler R, Barrett A, Hawkins N, et al. Interpreting indirect treatment comparisons and network meta-analysis for health-care decision making: report of the ISPOR Task Force on Indirect Treatment Comparisons Good Research Practices: part 1. Value Health. 2011. June;14(4):417–28. 10.1016/j.jval.2011.04.002 [DOI] [PubMed] [Google Scholar]
- 18. Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011. October 18;343:d5928 10.1136/bmj.d5928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996. February;17(1):1–12. [DOI] [PubMed] [Google Scholar]
- 20.National Institute for Health and Care Excellence [Internet]. Evidence Synthesis Technical Support Documents Series [updated 2014 April; cited 2014 Dec 11]. Available: http://www.nicedsu.org.uk/evidence-synthesis-tsd-series%282391675%29.htm.
- 21. Savović J, Jones HE, Altman DG, Harris RJ, Jüni P, Pildal J, et al. Influence of reported study design characteristics on intervention effect estimates from randomized, controlled trials. Ann Intern Med. 2012. September 18;157(6):429–38. [DOI] [PubMed] [Google Scholar]
- 22. Dias S, Welton NJ, Marinho VCC, Salanti G, Higgins JP, Ades AE. Estimation and adjustment of bias in randomized evidence by using mixed treatment comparison meta-analysis. J R Stat Soc. 2010;173:613–29. [Google Scholar]
- 23. Wood L, Egger M, Gluud LL, Schulz KF, Jüni P, Altman DG, et al. Empirical evidence of bias in treatment effect estimates in controlled trials with different interventions and outcomes: meta-epidemiological study. BMJ. 2008. March 15;336(7644):601–5. 10.1136/bmj.39465.451748.AD [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Salanti G, Higgins JP, Ades AE, Ioannidis JP. Evaluation of networks of randomized trials. Stat Methods Med Res 2008;17: 279–301. [DOI] [PubMed] [Google Scholar]
- 25. Lu G, Ades AE. Combination of direct and indirect evidence in mixed treatment comparisons. Stat Med. 2004. October 30;23(20):3105–24. [DOI] [PubMed] [Google Scholar]
- 26. Cooper NJ, Sutton AJ, Morris D, Ades AE, Welton NJ. Addressing between-study heterogeneity and inconsistency in mixed treatment comparisons: Application to stroke prevention treatments in individuals with non-rheumatic atrial fibrillation. Stat Med. 2009. June 30;28(14):1861–81. 10.1002/sim.3594 [DOI] [PubMed] [Google Scholar]
- 27. Ades AE. A chain of evidence with mixed comparisons: models for multi-parameter synthesis and consistency of evidence. Stat Med. 2003. October 15;22(19):2995–3016. [DOI] [PubMed] [Google Scholar]
- 28. Dias S, Welton NJ, Caldwell DM, Ades AE. Checking consistency in mixed treatment comparison meta-analysis. Stat Med. 2010. March 30;29(7–8):932–44. [DOI] [PubMed] [Google Scholar]
- 29. Cipriani A, Higgins JP, Geddes JR, Salanti G. Conceptual and technical challenges in network meta-analysis. Ann Intern Med. 2013. July 16;159(2):130–7 10.7326/0003-4819-159-2-201307160-00008 [DOI] [PubMed] [Google Scholar]
- 30. Jorgensen AW, Hilden J, Gotzsche PC. Cochrane reviews compared with industry supported meta-analyses and other meta-analyses of the same drugs: systematic review. BMJ. 2006. October 14;333(7572):782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Jorgensen AW, Maric KL, Tendal B, Faurschou A, Gotzsche PC. Industry-supported meta-analyses compared with meta-analyses with non-profit or no support: differences in methodological quality and conclusions. BMC Med Res Methodol. 2008. September 9;8:60 10.1186/1471-2288-8-60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hutton B, Salanti G, Chaimani A, Caldwell DM, Schmid C, Thorlund K, et al. The quality of reporting methods and results in network meta-analyses: an overview of reviews and suggestions for improvement. PLoS One. 2014. March 26;9(3):e92508 10.1371/journal.pone.0092508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009. July 21;339:b2700 10.1136/bmj.b2700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Jansen JP, Trikalinos T, Cappelleri JC, Daw J, Andes S, Eldessouki R, et al. Indirect treatment comparison/network meta-analysis study questionnaire to assess relevance and credibility to inform health care decision making: an ISPOR-AMCP-NPC Good Practice Task Force report. Value Health. 2014. March;17(2):157–73. 10.1016/j.jval.2014.01.004 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.