Abstract
Study objective
We determine the cost-effectiveness of out-of-hospital continuous positive airway pressure (CPAP) compared with standard care for adults presenting to emergency medical services with acute respiratory failure.
Methods
We developed an economic model using a United Kingdom health care system perspective to compare the costs and health outcomes of out-of-hospital CPAP to standard care (inhospital noninvasive ventilation) when applied to a hypothetical cohort of patients with acute respiratory failure. The model assigned each patient a probability of intubation or death, depending on the patient’s characteristics and whether he or she had out-of-hospital CPAP or standard care. The patients who survived accrued lifetime quality-adjusted life-years (QALYs) and health care costs according to their age and sex. Costs were accrued through intervention and hospital treatment costs, which depended on patient outcomes. All results were converted into US dollars, using the Organisation for Economic Co-operation and Development purchasing power parities rates.
Results
Out-of-hospital CPAP was more effective than standard care but was also more expensive, with an incremental cost-effectiveness ratio of £20,514 per QALY ($29,720/QALY) and a 49.5% probability of being cost-effective at the £20,000 per QALY ($29,000/QALY) threshold. The probability of out-of-hospital CPAP’s being cost-effective at the £20,000 per QALY ($29,000/QALY) threshold depended on the incidence of eligible patients and varied from 35.4% when a low estimate of incidence was used to 93.8% with a high estimate. Variation in the incidence of eligible patients also had a marked influence on the expected value of sample information for a future randomized trial.
Conclusion
The cost-effectiveness of out-of-hospital CPAP is uncertain. The incidence of patients eligible for out-of-hospital CPAP appears to be the key determinant of cost-effectiveness.
Introduction
Background
Acute respiratory failure is a common but life-threatening medical emergency, especially in elderly patients with respiratory and cardiac diseases.1, 2, 3 Inhospital noninvasive ventilation is widely used to treat acute respiratory failure that is refractory to initial medical therapy.4, 5, 6, 7, 8, 9, 10, 11, 12 However, the delay in providing noninvasive ventilation until arrival at the hospital may be one factor explaining why the risk of death in patients with respiratory problems increases markedly with distance traveled to the hospital.13 It has been argued that noninvasive ventilation is more likely to be effective if used early in the course of respiratory failure, before fatigue develops.14 Recent reviews have indicated that out-of-hospital noninvasive ventilation is feasible and beneficial in selected patients with acute respiratory failure.15, 16, 17, 18 We have undertaken a meta-analysis19 suggesting that out-of-hospital continuous positive airway pressure (CPAP) has the best evidence of effectiveness. CPAP is also the most practical way of providing out-of-hospital noninvasive ventilation.
Editor’s Capsule Summary.
What is already known on this topic
Emergency medical services (EMS) often use continuous positive airway pressure (CPAP) to treat out-of-hospital acute respiratory failure.
What question this study addressed
Is out-of-hospital CPAP cost-effective?
What this study adds to our knowledge
In this cost-effectiveness analysis using a United Kingdom perspective, the incremental cost-effectiveness ratio (£20,514 [$29,720] per quality-adjusted life-year gained) was insufficient by British standards to support widespread CPAP implementation. Interpretation of cost-effectiveness may vary by country, including the US.
How this is relevant to clinical practice
Although not directly impacting clinical practice, these findings highlight factors that should be considered in the selection and implementation of EMS therapies.
Importance
In the United States, the National Association of Emergency Medical Services Physicians stated that noninvasive ventilation is an important treatment modality for the out-of-hospital management of acute dyspnea.20 The recent UK Ambulance Services Clinical Practice Guidelines 201321 recommended (for the first time) the use of CPAP in the out-of-hospital environment on the basis of expert consensus. However, use of out-of-hospital CPAP in the United Kingdom remains limited in practice, probably reflecting the significant costs of establishing this treatment. The decision to establish out-of-hospital CPAP depends on weighing the benefits of improved outcomes against the additional costs incurred by establishing the service and is fundamentally an issue of cost-effectiveness.
Goals of This Investigation
We aimed to estimate the incremental cost per quality-adjusted life-year (QALY) of out-of-hospital CPAP compared with standard care and determine whether out-of-hospital CPAP should be recommended for funding according to accepted thresholds for cost-effectiveness.22
Materials and Methods
Theoretical Model of the Problem
The cost-effectiveness of a more effective and expensive treatment, such as out-of-hospital CPAP, can be estimated by comparing the outcomes and costs associated with the treatment to an appropriate alternative, such as inhospital noninvasive ventilation. Modeling is used to estimate how better effectiveness leads to improved patient outcomes relative to the cost of the care, expressed in terms of QALYs gained by using the intervention. A QALY is equivalent to a year of life spent in full health and incorporates both quality of life and survival. If outcomes are measured as QALYs, then the incremental cost-effectiveness ratio, or cost per QALY gained, can be calculated and compared with alternative uses of health care funding. The incremental cost-effectiveness ratio can be crudely understood as the amount needed for the intervention to “buy” each additional QALY compared with standard care. In the United Kingdom, the National Institute for Health and Care Excellence typically recommends in favor of funding interventions with an incremental cost-effectiveness ratio below a threshold, widely accepted to be between £20,000 and £30,000 per QALY (ie, interventions that can be used to buy QALYs for less than £20,000 [$29,000] or £30,000 [$43,500] each) and recommends against funding interventions with an incremental cost-effectiveness ratio above these thresholds.22 Other countries use different thresholds; one of $50,000 is conventionally used in the United States. We used the National Institute for Health and Care Excellence threshold because our analysis took a UK perspective and was based on UK practice and unit costs. Appendix E1 (available online at http://www.annemergmed.com) describes the general principles of cost-effectiveness analysis.
Study Design
We developed an economic model with the statistical software program R23 (version 3.0.3) to explore the costs and health outcomes associated with the use of out-of-hospital CPAP to treat acute respiratory failure compared with standard care, and to calculate the incremental cost per QALY gained. We based the analysis on a UK health care system perspective using a lifetime horizon. We converted the results into US dollars, using the Organisation for Economic Co-operation and Development purchasing power parities rates (£1=$1.45).
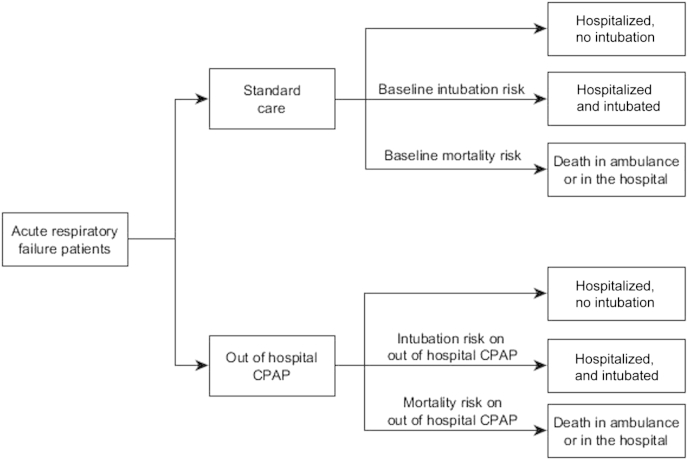
The structure of the model is shown in Figure 1 and the parameters used in the model are reported in Table 1. Patients in acute respiratory failure may receive either out-of-hospital CPAP or standard care, so these alternative treatments were applied to a hypothetical cohort of patients with acute respiratory failure who were eligible for noninvasive ventilation, ie, all patients receive out-of-hospital CPAP in the intervention group and standard care in the comparator group. Each patient in the model could have one of the following outcomes: hospitalized without intubation, hospitalized with intubation, or death in the ambulance or hospital. The probability of death and probability of intubation depended on whether the patient received out-of-hospital CPAP or standard care. The patients who survived accrued lifetime QALYs and health care costs according to their life expectancy. Costs were also accrued through costs of intervention (ie, out-of-hospital CPAP) and hospital treatment costs, which depended on whether the patient needed intubation. Details of each of these processes are outlined below.
Figure 1.
Model structure. CPAP, Continuous positive airway pressure.
Table 1.
Summary of model parameters.∗
| Parameter | Mean | Distribution | Source |
|---|---|---|---|
| Baseline risks | |||
| General population mean 30-day mortality probability | 0.118 | Beta (79, 589) | Nicholl et al13 |
| Risk of intubation | 0.029 | Beta (4.45, 150) | 3CPO,24 clinical opinion |
| OR for out-of-hospital CPAP | |||
| Mortality | 0.43 | Samples | Meta-analysis19 |
| Intubation | 0.32 | Samples | Meta-analysis19 |
| Life expectancy of patients | |||
| Lifetime years | 2.67 | Normal (2.67, 0.16) | 3CPO,24 clinical opinion |
| Health-related quality of life | |||
| Utility | 0.6 | Beta (640, 425) | 3CPO,24 clinical opinion |
| Costs, £ ($) | |||
| Out-of-hospital CPAP | 1,212 (1,740) | 1,500–1,000×β (2, 5) | Clinical input |
| Hospitalization | 2,250 (3,260) | Gamma (75, 30) | NHS reference costs30 |
| Intubation | 3,500 (5,075) | Gamma (70, 50) | NHS reference costs 2011–201230 |
| Annual | 5,360 (7,685) | Gamma (53, 100) | NHS reference costs 2011–201230 |
3CPO, Three Interventions in Cardiogenic Pulmonary Oedema24; NHS, National Health Service.
Beta (a,b) Distribution is a statistical distribution defined between 0 and 1; a and b parameters in the Beta distribution can be thought of as counts of the event of interest versus its complement, eg, Beta (79,589) for mortality represents 79 deaths in a population of 668 (ie, 79+589). Normal distribution is represented with mean and SD, with 95% of the values in the distribution lying between 2 SDs on either side of the mean, eg, normal (2.67, 0.16) implies that 95% of the samples lie between 2.35 and 2.99. Gamma (a,b) Distribution, where a is the shape parameter and b is the scale parameter, is typically used for skewed distributions and has a mean expected value of a×b, eg, the average value of the samples of distribution Gamma (75,30) is 2,250 (75×30).
We estimated the baseline risks of intubation and death (ie, for patients receiving standard care) with data from published studies.19 We modeled the mortality risk of emergency admissions with respiratory illness with a large cohort data set of 668 patients presenting with “respiratory disease” across 4 English ambulance services during a 4-year period, from 1997 to 2001.13 There were 79 deaths in 668 patients, which resulted in a mean mortality rate of 11.8%. This baseline mortality rate is similar to that in the more recent Three Interventions in Cardiogenic Pulmonary Oedema (3CPO) study.24
We modeled the risk of intubation for respiratory illness with the data from 3CPO study,24 a multicenter, open, prospective, randomized, controlled trial of 1,069 patients presenting with severe acute cardiogenic pulmonary edema at 26 emergency departments (EDs) in the UK. The study reported a mean intubation rate of 2.9%, which is similar to the intubation rates of 2.7% reported in British Thoracic Society national respiratory audit program annual report 2011/12.25
We estimated the effectiveness of out-of-hospital CPAP in reducing mortality and intubation as odds ratios (ORs) from a meta-analysis19 of 6 randomized trials, which are summarized in Table 1. The effectiveness estimates from our meta-analysis are similar to those of Williams et al18 but greater than those of Mal et al17 because the latter authors estimated effectiveness for all forms of out-of-hospital noninvasive ventilation together, whereas we estimated effectiveness of out-of-hospital CPAP and bi-level inspiratory positive airway pressure separately and used only the estimate for CPAP.
The patients who survived (ie, who avoided the short-term mortality risk) accrued QALYs, and these lifetime QALYs were estimated according to patients’ life expectancy and their utilities. The life expectancy of patients with acute respiratory failure and admitted to the hospital were captured from the 3CPO trial24 and parameterized as a normal distribution with a mean of 2.67 and SD of 0.16, after discussions with a clinical expert group (S.G., M.W., J.P.-A., and G.D.P.). The 3CPO study24 reported that the mean utility value (quality of life) was 0.6. The estimated QALYs for patients with acute respiratory failure were estimated by multiplying the life-years by the lifetime quality of life shown in Table 1. There was no evidence that patients who survived after receiving out-of-hospital CPAP experienced better health-related quality of life or lived longer compared with patients given standard care. We assumed that the lifetime QALYs were same for all survivors, irrespective of whether they were in standard care or the out-of-hospital CPAP arm.
The costs included in the model are for of out-of-hospital CPAP, intubation, hospitalization, and lifetime care for patients. Hubble et al26 reported a mean additional 5 days in length of stay associated with intubation compared with that for patients without intubation. We assumed that the additional 5 hospital days spent by the intubated patients would be in the ICU, according to the suggestions by the clinical expert group, at a cost of £700 ($1,015) per day. We estimated lifetime costs of survivors by using the annual costs and the discounted life expectancy of patients captured from the 3CPO trial.24 The study reported that mean costs in months 4 to 6 were £1,341 ($1,944.50), which resulted in mean annual costs of £5,360 ($7,685). The costs of standard care were not included in the model because the analysis is based on incremental costs, ie, we assumed that all initial treatment costs were the same in both arms, regardless of whether the patient received out-of-hospital CPAP.
We estimated the costs of out-of-hospital CPAP at an ambulance service level and converted these into a cost per patient according to a 5-year depreciation period. These costs included those for initial equipment, implementation, and ongoing maintenance. Although the costs of setting up the service are largely the same, there are substantially different estimates of incidence reported in different sources.25, 26, 27, 28, 29 The cost of out-of-hospital CPAP per patient in an ambulance service is estimated to be £189.93+£202,446/N ($275.50+$293,546/N), where N is the number of patients per ambulance service in a year. This information was synthesized into a mean cost of £1,212 ($1,740) per patient, according to our clinical expert input. Because there are different estimates of incidence, ranging from approximately 175 to 2,000 patients per ambulance service in a year, scenario analysis was also conducted for 3 different estimates for unit cost for performing out-of-hospital CPAP per patient (for different estimates of the eligible population), ie, a high-cost scenario with a unit cost of £1,400 ($2,030), a low-cost scenario with a unit cost of £745 ($1,080), and a lower-cost scenario with a unit cost of £300 ($435). Full details are provided in Appendix E2 (available online at http://www.annemergmed.com).
Primary Data Analysis
Probabilistic analysis incorporated uncertainty in the parameter estimates to provide a measure of precision and confidence in the estimates of the mean costs and QALYs. Additionally, we calculated the probability that each strategy would be the most cost-effective at different thresholds for willingness to pay for health gain. We constructed cost-effectiveness acceptability curves by plotting the probability of each strategy’s being cost-effective against willingness to pay. Furthermore, expected value of perfect information was estimated to identify whether the expected cost of future research would be valuable. Expected value of partial perfect information and expected value of sample information techniques were also used to identify the critical areas of uncertainty in which future research would produce the most benefit. Value-of-information analyses (expected value of perfect information, expected value of partial perfect information, and expected value of sample information) provide an estimate of the monetary value of further research to reduce uncertainty and, in particular, an estimate of how much we should be prepared to pay for a trial to reduce uncertainty.
Results
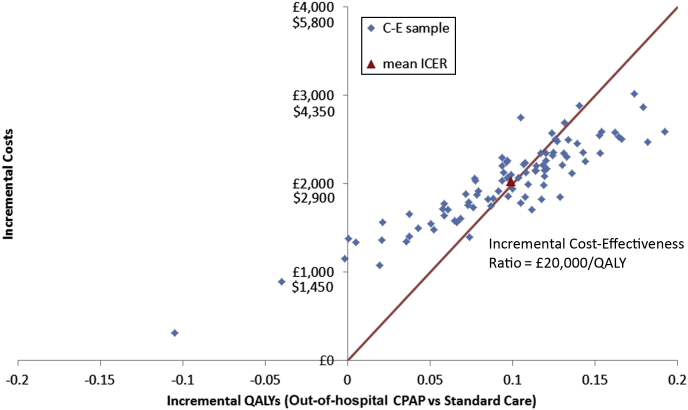
The total costs of out-of-hospital CPAP are higher than those of usual care (£16,895 versus £14,863, or $24,497 versus $21,551), but 0.099 QALYs are gained per patient treated (1.513 versus 1.414). The mean incremental cost-effectiveness ratio of out-of-hospital CPAP compared with standard care in the base case analysis is £20,514 per QALY ($29,720/QALY). It therefore costs the health service £20,514 ($29,720) to buy each additional QALY with out-of-hospital CPAP. Figure 2 shows the uncertainty associated with this estimate by plotting samples of mean incremental costs and QALYs. There is substantial uncertainty, with samples falling equally on either side of the red line, indicating the £20,000 per QALY ($29,000/QALY) threshold and a 49.5% probability of out-of-hospital CPAP’s being cost-effective at this threshold.
Figure 2.
Cost-effectiveness plane for the base-case economic analysis. ICER, Incremental cost-effectiveness ratio; QALY, quality-adjusted life-years.
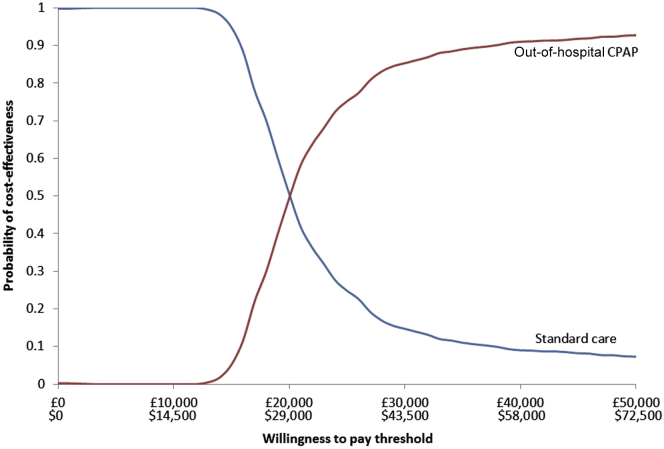
This is also observed in the cost-effectiveness analysis curve in Figure 3, which shows the proportion of model runs for which each strategy is cost-effective over a range of potential thresholds for willingness to pay. The more we are willing to pay for health gain (ie, the more we are willing to spend to buy a QALY), the more likely it is that out-of-hospital CPAP will be cost-effective, but there is substantial uncertainty between the thresholds of £20,000 per QALY ($29,000/QALY) and £30,000 per QALY ($43,500/QALY).
Figure 3.
Cost-effectiveness acceptability curve for the base-case economic analysis.
Scenario analysis was also conducted for 3 different estimates for unit cost for providing out-of-hospital CPAP per patient, ie, a high-cost scenario with a unit cost of £1,400 ($2,030), a low-cost scenario with a unit cost of £745 ($1,080), and a lower-cost scenario with a unit cost of £300 ($435). These estimates reflect variation in our estimates of the incidence of appropriate patients, with high estimates of incidence resulting in lower estimated costs. Table 2 compares each scenario with the base case and shows that out-of-hospital CPAP is more likely to be cost-effective (93.8% probability) if the incidence of appropriate patients is high and the resulting cost per patient low. Results from threshold analysis suggested that CPAP is unlikely to be cost-effective at £30,000 per QALY ($43,500/QALY) in an ambulance service is greater than if it costs more than £2,170 ($3,150) for out-of-hospital CPAP per patient.
Table 2.
Results for different cost scenarios.
| Scenario Type, £ ($) | Standard Care |
Out-of-Hospital CPAP |
Differences Between Out-of-Hospital CPAP and Standard Care |
ICER (per QALY), £ ($) | Probability of being Cost-effective | |||
|---|---|---|---|---|---|---|---|---|
| Total Costs, £ ($) | Total QALYs | Total Costs, £ ($) | Total QALYs | Costs, £ ($) | QALYs | |||
| Base case | 14,863 (21,551) | 1.414 | 16,895 (24,498) | 1.513 | 2,032 (2,946) | 0.099 | 20,514 (29,720) | 0.495 |
| High cost, 1,400 (2,030)∗ | 14,863 (21,551) | 1.414 | 17,078 (24,763) | 1.513 | 2,216 (3,213) | 0.099 | 22,368 (32,434) | 0.354 |
| Low cost, 745 (1,080)∗ | 14,863 (21,551) | 1.414 | 16,421 (23,810) | 1.513 | 1,558 (2,259) | 0.099 | 15,728 (22,805) | 0.798 |
| Lower cost, 300 (435)∗ | 14,863 (21,551) | 1.414 | 15,977 (23,166) | 1.513 | 1,114 (1,615) | 0.099 | 11,248 (16,309) | 0.938 |
Scenario analysis was conducted for different estimates for unit cost for performing out-of-hospital CPAP per patient (for different estimates of the eligible population). See Appendix E2 (available online at http://www.annemergmed.com) for more details.
The incidence of appropriate patients was also an important determinant of the expected value of information. The population expected value of perfect information at the threshold of £20,000 per QALY ($29,000/QALY) is £1.9 million ($2.75 million) at a low estimate of incidence of 3.5 per 100,000 population per year and £22.5 million ($32.5 million) at a higher incidence of 40.8 per 100,000 population per year. This value is defined as the maximum investment a decisionmaker would be willing to pay to eliminate all parameter uncertainty from the decision problem and reflects the amount we should be willing to pay for research to reduce uncertainty. Expected value of partial perfect information analysis suggested baseline mortality, out-of-hospital CPAP mortality OR, and costs of out-of-hospital CPAP as the key parameters driving uncertainty. The population expected value of partial perfect information for the 3 parameters together at the threshold is estimated as £1.83 million ($2.65 million) at the low incidence and £21.3 million ($30.8 million) at the higher one, both of which are very close to the population expected value of perfect information values, suggesting that most of the uncertainty in the decision problem is from these 3 parameters. The population expected value of sample information value for baseline mortality and out-of-hospital CPAP mortality OR parameters, assuming a randomized controlled trial with 100 patients in each arm, is estimated as £1.08 million ($1.56 million) at low incidence and £12.67 million ($18.37 million) at the higher one. It is cost-effective to conduct the trial to address the uncertainty if the population expected value of sample information of a proposed trial at a given sample size is greater than the costs of the trial.
Limitations
This model is generally based on robust data sources, with the estimates of effectiveness of out-of-hospital CPAP being derived from a meta-analysis of randomized trials and most cost estimates being derived from UK National Health Service reference costs. However, there are some limitations to the data. The trials in the meta-analysis were generally small and had potentially selected study populations, which may not have compared out-of-hospital CPAP with best alternative care. The model parameters were estimated from different sources, which may include different patient populations; for example, the baseline mortality risk was estimated for patients with respiratory disease, whereas the intubation risk was based on data for patients with a diagnosis of severe acute cardiogenic pulmonary edema.
The perspective of the analysis was the English health service, UK cost estimates were used, key model parameters were estimated from UK sources, and the thresholds for cost-effectiveness were those used in the United Kingdom by the National Institute for Health and Care Excellence. Costs may differ in other countries; for example, the unit cost of the CPAP system may be lower in the United States. The cost-effectiveness of out-of-hospital CPAP may be more certain in health services with different parameters of willingness to pay for health gain. In particular, cost-effectiveness appears to be more certain when compared against a US threshold of $50,000 per QALY. However, our analysis used UK estimates of costs and resource use. If costs and resource use are higher in the United States, the cost-effectiveness will be less certain.
The cost per patient of providing out-of-hospital CPAP was calculated by dividing the total cost of setting up and running the service by the total number of patients treated, which means that the cost per patient was determined by the incidence of patients who were likely to benefit from out-of-hospital CPAP. We identified a number of sources for our estimate of this parameter, but these estimates varied markedly. Sensitivity analysis showed that cost per patient is an important determinant of cost-effectiveness, so an accurate estimate of the incidence of patients likely to benefit from out-of-hospital CPAP is required to accurately estimate cost-effectiveness.
We have assumed in the analysis that the ambulance service has a 1-tiered response. However, some services may have different tiers of response that may allow more efficient use of equipment and trained staff. We assumed that out-of-hospital CPAP had a constant effect on mortality and intubation rate, according to estimates from meta-analysis. Effectiveness may depend on distance traveled to the hospital, being more effective in settings with long distances to the hospital. Unfortunately, distance to the hospital was not consistently collected in primary studies, so this factor could not be explored in the individual patient data meta-analysis.19
Discussion
The economic analysis showed that out-of-hospital CPAP was more effective than standard care, with 0.099 QALYs gained per patient treated, but was more expensive, with an additional cost of £2,032 ($2,934) per patient treated. The incremental cost-effectiveness ratio for out-of-hospital CPAP was £20,514 per QALY ($29,720/QALY) compared with standard care, with 49.5% probability of being cost-effective at the £20,000 per QALY threshold. These findings suggest that even if the apparent effectiveness of out-of-hospital CPAP reported by recent meta-analysis17, 18 were confirmed, the cost-effectiveness of this treatment is uncertain when compared with a UK cost-effectiveness threshold. It is therefore unlikely that out-of-hospital CPAP would be recommended for widespread implementation in the United Kingdom on the basis of this analysis.
In developing the economic model, we identified marked variation between estimates from different sources of the incidence of patients likely to benefit from out-of-hospital CPAP. Sensitivity analysis showed that this parameter was an important determinant of cost-effectiveness, with the probability of out-of-hospital CPAP’s being cost-effective at the £20,000 per QALY ($29,000/QALY) threshold varying from 35.4% to 93.8%. Most of the costs of providing out-of-hospital CPAP are incurred in setting up the service. If only a small number of patients will benefit from out-of-hospital CPAP, then the cost per patient will be high and cost-effectiveness is unlikely.
We identified only 1 previous economic analysis of out-of-hospital CPAP.25 This was undertaken in the United States and estimated that out-of-hospital CPAP would save 0.75 additional lives per 1,000 patients, at a cost of $490 per life saved. This analysis had a number of limitations. Treatment effect estimates were based on trial of inhospital rather than out-of-hospital CPAP, so the analysis effectively compared CPAP with no noninvasive ventilation, rather than comparing out-of-hospital CPAP with inhospital noninvasive ventilation. Outcomes were valued as lives saved rather than QALYs, and the model used only a 1-year time horizon. One-way sensitivity analysis was performed, but the authors did not perform a probabilistic sensitivity analysis. The estimate that out-of-hospital CPAP would be used 4 times per 1,000 patients seems high compared with our estimates of patient eligibility. As a consequence, although this analysis suggested that out-of-hospital CPAP is cost-effective, it is unlikely to convince purchasers of health care.
Expected value of information analysis was undertaken to explore uncertainty and determine the value of further research. It showed that the value of undertaking a trial depends on the estimated incidence of eligible patients. The maximum cost at which it would be cost-effective to conduct a trial with 100 patients in each arm is only £1.08 million ($1.56 million) if there were a low estimated incidence (of 3.5 per 100,000 population per year) of eligible patients, but would be £12.67 million ($18.37 million) if there were a high estimated incidence (of 40.8 per 100,000 population per year). A more precise estimate of the incidence of eligible patients is therefore required to determine the cost-effectiveness of a future trial of out-of-hospital CPAP. Of course, the feasibility of a future trial would also depend on the incidence of eligible patients.
Our model can be used to determine whether out-of-hospital CPAP is likely to be cost-effective in a particular system, given the estimate of the incidence of eligible patients. Our analysis indicates that out-of-hospital CPAP has uncertain cost-effectiveness. Establishing out-of-hospital CPAP as a standard treatment of acute respiratory failure will require substantial resources, and there is a relatively high probability that outcomes improvements from out-of-hospital CPAP may not be justified by the financial investment. A large pragmatic randomized trial could improve our estimates of effectiveness and cost-effectiveness, but this would also require substantial funding, with uncertain value. Our analysis suggests that the incidence of patients eligible for out-of-hospital CPAP is an important determinant of the cost-effectiveness of out-of-hospital CPAP itself and of a future trial of it. Health systems considering implementing out-of-hospital CPAP should first estimate the incidence of potentially eligible patients. If the incidence is low, then implementation of out-of-hospital CPAP is not likely to be cost-effective. If the incidence is high, then implementation or further research with a large pragmatic randomized trial may be appropriate. If incidence varies between health care systems, then out-of-hospital CPAP may be cost-effective in some systems but not others.
Acknowledgments
The authors acknowledge John Stevens, PhD, and Shiije Ren, PhD, for providing meta-analysis data and Kathryn MacKellar for clerical assistance.
Footnotes
Please see page 557 for the Editor’s Capsule Summary of this article.
Supervising editor: Henry E. Wang, MD, MS
Author contributions: SG conceived the original idea for the study. PT and SG designed the study. PT developed the model. SG, MW, JP-A, and GDP provided clinical expertise in model development. All authors interpreted the results. PT wrote the first draft of the article. All authors contributed to redrafting and approved the final version of the article. PT takes responsibility for the paper as a whole.
Funding and support: By Annals policy, all authors are required to disclose any and all commercial, financial, and other relationships in any way related to the subject of this article as per ICMJE conflict of interest guidelines (see www.icmje.org). The authors have stated that no such relationships exist and provided the following details: This study was funded by the United Kingdom National Institute for Health Research Health Technology Assessment (NIHR HTA) Programme (project 11/36/09).
The views expressed in this article are those of the authors and not necessarily those of the NIHR HTA Programme. Any errors are the responsibility of the authors. The funders had no role in the study design; collection, analysis, and interpretation of data; writing of the article; or decision to submit the article for publication.
A feedback survey is available with each research article published on the Web at www.annemergmed.com.
A podcast for this article is available at www.annemergmed.com.
Appendix E1
Methods to estimate cost-effectiveness
The cost-effectiveness of the different interventions was estimated with both the ICER and the net benefit approaches. Uncertainty was incorporated in the modeling by performing probabilistic sensitivity analysis. Descriptions of these terms and approaches are provided in the sections below.
Definitions of Cost-effectiveness Terms
The ICER measures the relative value of 2 strategies and is calculated as the mean incremental cost divided by the mean incremental benefits. A strategy is dominated when another strategy accrues more QALYs for less cost. Extended dominance occurs when a combination of 2 alternative strategies can produce the same QALYs as a chosen strategy but at a lower cost. Strategies that are neither dominated nor extendedly dominated constitute the cost-effectiveness frontier, and the ICER is reported for these strategies compared with the next least effective strategy. The willingness-to-pay threshold is the amount of money that the decisionmaker is willing to pay to gain 1 additional QALY. The usual threshold for decisionmaking at the United Kingdom National Institute for Care Excellence is considered to be approximately £20,000 to £30,000 per QALY. The net monetary benefit is defined as the QALYs multiplied by a value for the QALYs (eg, £20,000) minus the costs of obtaining them, that is, net monetary benefit=QALYs×λ–cost, where λ is the willingness-to-pay threshold. The net monetary benefit approach is simpler to calculate and gives equivalent findings (but requires an explicit assumption about the value of λ).
Uncertainty Analysis
The results presented in the following section include the effects of accounting for uncertainty in the model parameters (the costs, utilities, risks, and ORs for mortality and intubation), characterized as probability distributions. Probabilistic sensitivity analysis is undertaken whereby the model is rerun (1,000 times), each time with a different value for the risks, ORs, costs, and utilities, sampled from the probability distributions. The cost-effectiveness plane shows the incremental costs (y axis) and incremental QALYs (x axis) compared with usual care. In this chart, if a model run for a strategy had exactly the same costs and QALYs as usual care, then the “sample” for that model run would appear at the origin. Samples plotted to the right of the y axis have more QALYs than usual care and samples plotted above the x axis have more costs. Samples plotted to the right of a straight line with slope λ passing through the origin are cost-effective, whereas those plotted to the left are not. The cost-effectiveness acceptability curve shows the proportion of model runs for which each strategy is cost-effective over a range of potential willingness-to-pay thresholds (ie, λ).
Value of Information Analysis
Another measure of uncertainty is the overall expected value of perfect information (EVPI). This calculation is carried out according to the theory that the decisionmaker will choose the most cost-effective option but could acquire additional evidence to reduce the uncertainties in the decision; for example, know exactly what the ORs for mortality and hospitalizations are for each treatment. In the EVPI calculation, it can be estimated how often making the decision based on current evidence could be wrong, and also how many QALYs (and costs) would be lost by choosing the strategy that is expected to be most cost-effective, given current evidence, when in fact one of the other strategies is truly the most cost-effective. This can be multiplied by the number of patients per year and the expected lifetime of the decision to estimate the population EVPI. The interpretation of population EVPI is that if one could fund research to eliminate the uncertainty in effectiveness for all of the parameters for each strategy (eg, by a large or infinitely large clinical trial), then the value of eliminating the uncertainty through such research would be expected to be the population EVPI. This can be thought of as the maximum that the health care system should be willing to pay for additional evidence to inform the decision in the future and thus is an upper bound on the value of conducting further research, ie, if the population EVPI exceeds the expected costs of additional research, then it is potentially cost-effective to conduct further research.
Expected value of partial perfect information (EVPPI) is similar to EVPI, but instead of evaluating the uncertainty associated with all parameters it focuses on the uncertainty associated with a subset of one or more parameters, allowing the decisionmakers to conclude in which variables further research would be most beneficial. The computational time required for EVPPI is markedly more than for EVPI because the process essentially requires 2 iterations of probabilistic analyses, as standard probabilistic sensitivity analyses are undertaken for each sampled parameter value for the variable(s) under analysis. If the population EVPPI for a subset of parameters exceeds the expected costs of additional research, then it is potentially cost-effective to conduct further research to estimate those parameters.
Expected value of sample information (EVSI) addresses the limitation of EVPI, which assumes parameters can be ascertained without uncertainty (ie, effectively assuming an infinite trial size), and seeks to provide an optimal number of patients to study within a future trial. In addition, EVSI also allows the evaluation of marginal returns associated with increased sample size formally taking uncertainty into account (eg, say that an additional 100 patients when only 500 have been recruited would be likely to provide more value than when 20,000 have been recruited). Within EVSI, the costs of the trial are compared with the benefits achieved to find the maximum expected net benefit, which would correspond with the recommended trial size. If the population EVSI of a proposed trial is greater than the costs of the trial, then it is cost-effective to conduct the trial to address the uncertainty.
Appendix E2
Costs of out-of-hospital CPAP
There are a number of costs involved in providing out-of-hospital CPAP, such as initial equipment costs, implementation costs, and ongoing maintenance costs. These costs were converted into a cost per patient according to a 5-year depreciation period (ie, assuming that new out-of-hospital noninvasive ventilation equipment will be required in 5 years) and sharing the overall costs out among the number of patients who would benefit from the service during this period.
Total Costs of Out-of-Hospital CPAP to the Ambulance Service
The costs were often missing from the studies in literature, and thus bottom-up costing methods were used to estimate the costs of out-of-hospital CPAP. The breakdown of the costs for out-of-hospital CPAP is shown in Table E1 and is split into 3 main components:
-
•
initial costs of the out-of-hospital CPAP devices
-
•
setup/implementation costs, ie, staff training costs and service reconfiguration costs
-
•
maintenance costs of the service, ie, consumables and depreciation
The costs of the out-of-hospital CPAP devices were elicited from the expert advisory group. The costs of implementation on provider organizations were estimated with bottom-up costing methods, assuming a typical ambulance service. The maintenance costs were estimated with activity-based costing for the resources spent on consumables, according to evidence from the literature.
The out-of-hospital CPAP device can take different levels of complexity, and the cost of the device is based on this complexity. For example, the costs are different for the noninvasive positive pressure ventilation devices and CPAP/bi-level inspiratory positive airway pressure devices. Furthermore, the costs also depend on whether the devices use a cylinder or whether they are electrically or mechanically powered. The costs of the device were elicited from the expert advisory group, assuming a close-fitting face mask CPAP device with Boussignac CPAP system manufactured by Vygon as the representative of a typical CPAP system. The Boussignac Hospital CPAP kit costs £513.49 ($744.5) and contains the equipment required to deliver out-of-hospital CPAP. We assumed that each ambulance would have the equipment and 10% would need replacing during the 5-year period.
The costs of implementation on provider organizations was estimated with bottom-up costing methods, assuming a typical ambulance service, according to the mean size of National Health Service ambulance services in the United Kingdom. It was assumed that a typical ambulance service would have an average capacity of 1,500 paramedics, which was deemed sensible by the expert advisory group. They also suggested that an average of 2 days needed to be dedicated for paramedic training. The costs associated with training were estimated by multiplying this paramedic time with their daily rate according to the Personal and Social Services Research Unit (PSSRU 2012).6 The daily cost per working day was estimated as £150 ($217.5), assuming an average salary of £40,000 ($58,000), including overheads if they are in band 6/7, on suggestion by the clinical advisory group. Service reconfiguration costs were estimated as a 1-off cost of £100,000 ($145,000), and this included the cost of developing new guidelines and pathways. Installation costs were assumed to be zero because the CPAP set under consideration is a disposable system.
The maintenance costs were estimated by using costing for the resources spent on consumables according to information from the manufacturers that the facial mask, oxygen tubing, and valve (costing £189.93 [$275]) would need to be replaced after each use, whereas the rest of the equipment was reusable. The expert advisory group suggested that an average of an additional day halfway through the 5-year period would be required by the paramedics for updating their training.
Table E1.
Breakdown of out-of-hospital CPAP costs.
| Number of Devices | Source | Unit Cost, £ ($) | Source | Total Cost, £ ($) | ||||
|---|---|---|---|---|---|---|---|---|
| Device costs | ||||||||
| Out-of-hospital CPAP device | Number of ambulances that need the CPAP device (420) | Expert advisory input | 513.49 (744.50) | Vygon: hospital CPAP kit | 513.49×420 | |||
| Assuming 10% new CPAP devices during 5-y usage (42) | Expert advisory input | 513.49 (744.50) | Vygon: hospital CPAP kit | 513.49×42 | ||||
| Total cost of the device | 237,232 | |||||||
| Setup/implementation costs | ||||||||
| Resource Usage | ||||||||
| Initial training | 1,500 paramedics for 2 days each | Expert advisory input | 150 (217.50) per day | Expert advisory input | 450,000 (652,500) | |||
| Service reconfiguration | 1-off cost for reconfiguration | Expert advisory input | 100,000 (145,000) | |||||
| Total setup/implementation costs | 550,000 (797,500) | |||||||
| Maintenance costs | ||||||||
| Resource Usage | ||||||||
| Consumables | Number of patients during 5 y=5×N | Expert advisory input | 189.93 (275) per use | Vygon: facial mask, oxygen tubing, and valve | 189.93×5×N (275×5×N) | |||
| Ongoing training | 1,500 paramedics for 1 day each | Expert advisory input | 150 (217.50) per day | Expert advisory input | 225,000 (326,500) | |||
| Total maintenance costs | 225,000+949.65×N (326,500+1,375×N) |
|||||||
| Total costs of out-of-hospital CPAP | 1,012,232+949.65×N | |||||||
| Total number of patients (N patients per year times depreciation period of 5 y (ie, assuming new out-of-hospital CPAP equipment will be required in 5 y) | 5×N | |||||||
| Cost of out-of-hospital CPAP per patient | 189.93+202,446/N (275.50+293,546/N) |
|||||||
Number of Patients Receiving Out-of-Hospital CPAP in a Typical Ambulance Service
The incidence of patients who will benefit from out-of-hospital CPAP is one of the key parameters in the model because the unit cost of out-of-hospital CPAP is estimated by dividing the total costs of out-of-hospital CPAP to the ambulance service by the number of patients treated. There are different estimates of incidence reported in different sources, as reported below, and these are synthesized to achieve a distribution for the costs of out-of-hospital CPAP.
Spijker et al1 reported that 16 patients received out-of-hospital CPAP across 11 months in an ambulance service covering a population of 500,000, which amounts into 3.5 potentially eligible cases per 100,000/year. This study identified only patients with acute cardiogenic pulmonary edema, and many eligible patients did not receive treatment (which admittedly may reflect real life); hence, this could be an underestimate of true incidence. Similarly, Aguilar et al2 reported that 175 patients received out-of-hospital noninvasive ventilation across 22 months in an ambulance service covering a population of 1.3 million, which amounts to 7.3 potentially eligible cases per 100,000/year.
Luhr et al3 estimated 77.6 cases of acute respiratory failure per 100,000/year population. Of these, 13.7% were due to chronic obstructive pulmonary disease and 9.2% were due to cardiogenic pulmonary edema (ie, cases with potential to benefit from out-of-hospital noninvasive ventilation). Thus, the incidence can be estimated as 17.8 potentially eligible cases per 100,000/year. However, they are relatively old data and include patients who developed acute respiratory failure in the hospital, so they may be an overestimate.
The British Thoracic Society National Respiratory Audit Programme 2011/124 reported that 130 hospitals submitted data on 2,490 patients with noninvasive ventilation between February 1 and March 31, 2012 (ie, 2 months). This amounts to 19.15 (2,490/130) NIV patients per hospital per month, which in yearly terms is 115 per hospital. There are 168 acute hospitals in England serving a population of 53.01 million, which yields an incidence of 36.4 per 100,000 population. However, the details of the audit were not clear and may be subject to bias. Furthermore, it included patients who developed acute respiratory failure in the hospital so may be an overestimate.
In the Sheffield Teaching Hospital ED, there were 255 sets of noninvasive ventilation equipment used during 1 year. This hospital serves a population of 551,800, which amounts to 46.2 potentially eligible cases per 100,000/year. However, the equipment may not actually have been used for patient care, or multiple pieces of equipment may have been used for the same patient, so this is likely to be an overestimate.
Hubble et al5 estimated that there were 4 per 1,000 patients transported by ambulance who were eligible for noninvasive ventilation. In 2011 to 2012, there were 4.53 million emergency ambulance transfers to a Level I or II ED in England (population 53.01 million). If 4 per 1,000 of these were eligible, this suggests an incidence of 34.2 per 100,000 population.
Scenarios for Costs of Out-of-Hospital CPAP to the Ambulance Service
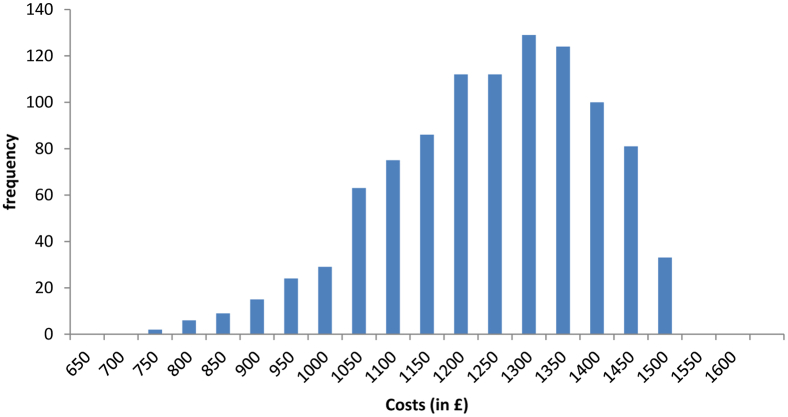
As stated above, there are substantially different estimates of incidence reported in different sources, and they are summarized in Table E2. A typical ambulance service caters to a population of approximately 5 million, which suggests a range from approximately 175 to 2,000 patients per ambulance service in a year, depending on the estimate of the incidence. Thus, scenario analysis was conducted by estimating the unit cost for providing out-of-hospital CPAP for these different estimates of the eligible population. The unit costs for the different scenarios are presented in Table E3. This information was synthesized into an expression for the out-of-hospital CPAP costs as £1,500 to £1,000×β (2, 5), shown in Figure E1. This was chosen because our clinical experts believed that most of the samples of costs will fall between £1,400 ($2,030) and £800 ($1,160), with only a few instances in which the costs are lower than £800 ($1,160).
Table E2.
Scenarios for unit costs of out-of-hospital CPAP.
| Source | Incidence of Eligible Patients per 100,000 | Annual Eligible Patients in an Ambulance Service | Unit Cost of Out-of-Hospital CPAP, £ ($)∗ |
|---|---|---|---|
| Spijker et al1 | 3.5 | 175 | 1,346.76 (1,952.80) |
| Aguilar et al2 | 7.3 | 365 | 744.58 (1,096.64) |
| Luhr et al3 | 17.8 | 890 | 417.40 (605.20) |
| Hubble et al5 | 34.2 | 1,700 | 309.02 (448.08) |
| BTS audit4 | 36.1 | 1,800 | 302.40 (438.48) |
| STH ED data | 40.8 | 2,000 | 291.15 (422.15) |
BTS, British Thoracic Society; STH, Sheffield Teaching Hospital.
Using the formula unit cost=£189.93+£202,446/N ($275.5+$293,546/N), where N is the number of patients per year.
Figure E1.
Histogram of the distribution of CPAP costs.
High-Cost Scenario
Scenario analysis was conducted with a different estimate for unit cost for performing out-of-hospital CPAP per patient (assuming 175 patients in the ambulance service as the eligible population for CPAP), a high-cost scenario with a unit cost of £1,400 ($2,030). The distribution used is normal (£1,400, £100), ie, normal ($2,030, $145).
Low-Cost Scenario
Scenario analysis was also conducted with a different estimate for unit cost for performing out-of-hospital CPAP per patient (assuming 365 patients in the ambulance service as the eligible population for CPAP), a low-cost scenario with a unit cost of £745 ($1,080). The distribution used is normal (£745, £100), ie, normal ($1,080, $145).
Lower-Cost Scenario
Scenario analysis was also conducted with a different estimate for unit cost for performing out-of-hospital CPAP per patient (assuming 1,700 to 2,000 patients in the ambulance service as the eligible population for CPAP), a low-cost scenario with a unit cost of £300 ($435). The distribution used is normal (£300, £50), ie, normal ($435, $72.50).
Table E3.
Summary of costs.
| Scenario | Mean Value, £ ($) | Distribution, £ ($) |
|---|---|---|
| Baseline | 1,212 (1,740) | 1,500–1,000×β (2, 5) |
| High cost | 1,400 (2,030) | Normal (1,400, 100) [normal (2,030, 145)] |
| Low cost | 745 (1,080) | Normal (745, 100) [normal (1,080, 145)] |
| Lower cost | 300 (435) | Normal (300, 50) [normal (435, 72.50)] |
Economic evaluation∗
| Section/Item | Item No. | Recommendation | Reported on Page No. |
|---|---|---|---|
| Title and abstract | |||
| Title | 1 | Identify the study as an economic evaluation or use more specific terms such as “cost-effectiveness analysis,” and describe the interventions compared. | 1 |
| Abstract | 2 | Provide a structured summary of objectives, perspective, setting, methods (including study design and inputs), results (including base case and uncertainty analyses), and conclusions. | 1 |
| Introduction | |||
| Background and objectives |
3 | Provide an explicit statement of the broader context for the study. Present the study question and its relevance for health policy or practice decisions. |
2 |
| Methods | |||
| Target population and subgroups | 4 | Describe characteristics of the base-case population and subgroups analyzed, including why they were chosen. | 2 |
| Setting and location | 5 | State relevant aspects of the system(s) in which the decision(s) need(s) to be made. | 3 |
| Study perspective | 6 | Describe the perspective of the study and relate this to the costs being evaluated. | 3 |
| Comparators | 7 | Describe the interventions or strategies being compared and state why they were chosen. | 3 |
| Time horizon | 8 | State the time horizon(s) over which costs and consequences are being evaluated and say why appropriate. | 3 |
| Discount rate | 9 | Report the choice of discount rate(s) used for costs and outcomes and say why appropriate. | 4 |
| Choice of health outcomes | 10 | Describe what outcomes were used as the measure(s) of benefit in the evaluation and their relevance for the type of analysis performed. | 3 |
| Measurement of effectiveness | 11a | Single-study-based estimates: Describe fully the design features of the single effectiveness study and why the single study was a sufficient source of clinical effectiveness data. | 4 |
| Measurement and valuation of preference-based outcome | 12 | If applicable, describe the population and methods used to elicit preferences for outcomes. | NA |
| Estimating resources and costs | 13a | Single-study-based economic evaluation: Describe approaches used to estimate resource use associated with the alternative interventions. Describe primary or secondary research methods for valuing each resource item in terms of its unit cost. Describe any adjustments made to approximate to opportunity costs. | 4, 5 Appendix |
| Currency, price date, and conversion | 14 | Report the dates of the estimated resource quantities and unit costs. Describe methods for adjusting estimated unit costs to the year of reported costs if necessary. Describe methods for converting costs into a common currency base and the exchange rate. | 3, 4, 5 |
| Choice of model | 15 | Describe and give reasons for the specific type of decision analytical model used. Providing a figure to show model structure is strongly recommended. | 3 |
| Assumptions | 16 | Describe all structural or other assumptions underpinning the decision-analytical model. | 3, 4, 5 |
| Analytical methods | 17 | Describe all analytical methods supporting the evaluation. This could include methods for dealing with skewed, missing, or censored data; extrapolation methods; methods for pooling data; approaches to validate or make adjustments (such as half cycle corrections) to a model; and methods for handling population heterogeneity and uncertainty. | 3, 4, 5 Appendix |
| Results | |||
| Study parameters | 18 | Report the values, ranges, references, and, if used, probability distributions for all parameters. Report reasons or sources for distributions used to represent uncertainty where appropriate. Providing a table to show the input values is strongly recommended. | Table 1 |
| Incremental costs and outcomes | 19 | For each intervention, report mean values for the main categories of estimated costs and outcomes of interest, as well as mean differences between the comparator groups. If applicable, report ICERs. | Table 2 |
| Characterizing uncertainty | 20a | Single-study-based economic evaluation: Describe the effects of sampling uncertainty for the estimated incremental cost and incremental effectiveness parameters, together with the impact of methodological assumptions (such as discount rate and study perspective). | Figure 2, Figure 3 |
| Characterizing heterogeneity | 21 | If applicable, report differences in costs, outcomes, or cost-effectiveness that can be explained by variations between subgroups of patients with different baseline characteristics or other observed variability in effects that are not reducible by more information. | Table 2 |
| Discussion | |||
| Study findings, limitations, generalizability, and current knowledge | 22 | Summarize key study findings and describe how they support the conclusions reached. Discuss limitations and the generalizability of the findings and how the findings fit with current knowledge. | s 6,7 |
| Other | |||
| Source of funding | 23 | Describe how the study was funded and the role of the funder in the identification, design, conduct, and reporting of the analysis. Describe other nonmonetary sources of support. | tbc |
| Conflicts of interest | 24 | Describe any potential for conflict of interest of study contributors in accordance with journal policy. In the absence of a journal policy, we recommend authors comply with International Committee of Medical Journal Editors recommendations. | tbc |
Husereau D, Drummond M, Petrou S, et al. Consolidated Health Economic Evaluation Reporting Standards (CHEERS)—explanation and elaboration: a report of the ISPOR Health Economic Evaluations Publication Guidelines Good Reporting Practices Task Force. Value Health. 2013;16:231-250.
References
- 1.Delmere S., Ray P. Acute respiratory failure in the elderly: diagnosis and prognosis. Age Ageing. 2008;37:251–257. doi: 10.1093/ageing/afn060. [DOI] [PubMed] [Google Scholar]
- 2.Aminzadeh F., Dalziel W.B. Older adults in the emergency department: a systematic review of patterns of use, adverse outcomes, and effectiveness of interventions. Ann Emerg Med. 2002;39:238–247. doi: 10.1067/mem.2002.121523. [DOI] [PubMed] [Google Scholar]
- 3.Ray P., Birolleau S., Lefort Y., et al. Acute respiratory failure in the elderly: etiology, emergency diagnosis and prognosis. Crit Care. 2006;10:R82. doi: 10.1186/cc4926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baudouin S., Blumenthal S., Cooper B., et al. Non-invasive ventilation in acute respiratory failure—British Thoracic Society Standards of Care Committee. Thorax. 2002;57:192–211. doi: 10.1136/thorax.57.3.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berlac P., Hyldmo P.K., Kongstad P., et al. Pre-hospital airway management: guidelines from a task force from the Scandinavian Society for Anaesthesiology and Intensive Care Medicine. Acta Anaesthesiol Scand. 2008;52:897–907. doi: 10.1111/j.1399-6576.2008.01673.x. [DOI] [PubMed] [Google Scholar]
- 6.Fisher J.D., Brown S.N., Cooke M.W. Joint Royal College Ambulance Liaison Committee; London, England: 2006. UK Ambulance Service Clinical Practice Guidelines (2006) [Google Scholar]
- 7.Keenan S.P., Sinuff T., Burns K.E.A., et al. Clinical practice guidelines for the use of noninvasive positive-pressure ventilation and noninvasive continuous positive airway pressure in the acute care setting. CMAJ. 2011;183:E195–E214. doi: 10.1503/cmaj.100071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kunze R. Standards and guidelines for prehospital ventilation management. J Anasth Intensivbehandlung. 2007;14:2007. [in German] [Google Scholar]
- 9.Llorens P., Miro O., Martin-Sanchez F.J., et al. Guidelines for emergency management of acute heart failure: consensus of the Acute Heart Failure Working Group of the Spanish Society of Emergency Medicine (ICA-SEMES) in 2011. Emergencias. 2011;23:119–139. [Google Scholar]
- 10.Roberts C.M., Brown J.L., Reinhardt A.K., et al. Non-invasive ventilation in chronic obstructive pulmonary disease: management of acute type 2 respiratory failure. Clin Med. 2008;8:517–521. doi: 10.7861/clinmedicine.8-5-517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schonhofer B., Kuhlen R., Neumann P., et al. Non-invasive ventilation as treatment for acute respiratory insufficiency. Essentials from the new S3 guidelines. Anaesthesist. 2008;57:1091–1102. doi: 10.1007/s00101-008-1449-0. [DOI] [PubMed] [Google Scholar]
- 12.Sinuff T., Keenan S.P. Clinical practice guideline for the use of noninvasive positive pressure ventilation in COPD patients with acute respiratory failure. J Crit Care. 2004;19:82–91. doi: 10.1016/j.jcrc.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 13.Nicholl J., West J., Goodacre S., et al. The relationship between distance to hospital and patient mortality in emergencies: an observational study. Emerg Med J. 2007;24:665–668. doi: 10.1136/emj.2007.047654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Masip J., Mebazaa A., Filippatos G. Noninvasive ventilation in acute cardiogenic pulmonary edema. N Engl J Med. 2008;359:2068–2069. [correspondence] [PubMed] [Google Scholar]
- 15.Simpson P.M., Bendall J.C. Prehospital non-invasive ventilation for acute cardiogenic pulmonary oedema: an evidence-based review. Emerg Med J. 2011;28:609–612. doi: 10.1136/emj.2010.092296. [review] [DOI] [PubMed] [Google Scholar]
- 16.Williams B., Boyle M., Robertson N., et al. When pressure is positive: a literature review of the prehospital use of continuous positive airway pressure. Prehosp Disaster Med. 2013;28:52–60. doi: 10.1017/S1049023X12001562. [review] [DOI] [PubMed] [Google Scholar]
- 17.Mal S., McLeod S., Iansavichene A., et al. The impact of prehospital non-invasive positive pressure support ventilation in adult patients with severe respiratory distress: a systematic review and meta-analysis. Ann Emerg Med. 2014;63:600–607. doi: 10.1016/j.annemergmed.2013.11.013. [DOI] [PubMed] [Google Scholar]
- 18.Williams T.A., Finn J., Perkins G.D., et al. Prehospital continuous positive airway pressure for acute respiratory failure: a systematic review and meta-analysis. Prehosp Emerg Care. 2013;17:261–273. doi: 10.3109/10903127.2012.749967. [DOI] [PubMed] [Google Scholar]
- 19.Goodacre S., Stevens J.W., Pandor A., et al. Pre-hospital non-invasive ventilation for acute respiratory failure: a systematic review and network meta-analysis. Acad Emerg Med. 2014;21:949–959. doi: 10.1111/acem.12466. [DOI] [PubMed] [Google Scholar]
- 20.Daily J.C., Wang H.E. Noninvasive positive pressure ventilation: resource document for the National Association of EMS Physicians position statement. Prehosp Emerg Care. 2011;15:432–438. doi: 10.3109/10903127.2011.569851. [DOI] [PubMed] [Google Scholar]
- 21.Association of Ambulance Chief Executives . 4th ed. Association of Ambulance Chief Executives; Basingstoke, England: 2013. UK Ambulance Services Clinical Practice Guidelines 2013. [Google Scholar]
- 22.National Institute for Health and Clinical Excellence. Guide to the methods of technology appraisal. 2013. Available at: http://www.nice.org.uk/article/pmg9/chapter/foreword. Accessed October 1, 2013. [PubMed]
- 23.R Development Core Team. R: A Language and Environment for Statistical Computing. 2008. Avilable at: http://www.r-project.org/. Accessed March 1, 2013.
- 24.Gray A.J., Goodacre S., Newby D.E., et al. A multicentre randomised controlled trial of the use of continuous positive airway pressure and non-invasive positive pressure ventilation in the early treatment of patients presenting to the emergency department with severe acute cardiogenic pulmonary oedema: the 3CPO trial. Health Technol Assess. 2009;13:1–106. doi: 10.3310/hta13330. [DOI] [PubMed] [Google Scholar]
- 25.British Thoracic Society. National Respiratory Audit Programme annual report. 2012. Available at: https://www.brit-thoracic.org.uk/document-library/audit-and-quality-improvement/audit-reports/bts-national-respiratory-audit-programme-annual-report-2011-2012/. Accessed May 1, 2013.
- 26.Hubble M.W., Richards M.E., Wilfong D.A. Estimates of cost-effectiveness of prehospital continuous positive airway pressure in the management of acute pulmonary edema. Prehospital Emerg Care. 2008;12:277–285. doi: 10.1080/10903120801949275. [DOI] [PubMed] [Google Scholar]
- 27.Aguilar S.A., Lee J., Castillo E., et al. Assessment of the addition of prehospital continuous positive airway pressure (CPAP) to an urban emergency medical services (EMS) system in persons with severe respiratory distress. J Emerg Med. 2013;45:210–219. doi: 10.1016/j.jemermed.2013.01.044. [DOI] [PubMed] [Google Scholar]
- 28.Luhr O.R., Antonsen K., Karlsson M., et al. Incidence and mortality after acute respiratory failure and acute respiratory distress syndrome in Sweden, Denmark, and Iceland. The ARF Study Group. Am J Respir Crit Care Med. 1999;159:1849–1861. doi: 10.1164/ajrccm.159.6.9808136. [DOI] [PubMed] [Google Scholar]
- 29.Spijker E.E., de Bont M., Bax M., et al. Practical use, effects and complications of prehospital treatment of acute cardiogenic pulmonary edema using the Boussignac CPAP system. Int J Emerg Med. 2013;6:8. doi: 10.1186/1865-1380-6-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Department of Health. NHS Reference Costs 2011-2012. London, England: 2013.
References
- 1.Spijker E.E., de Bont M., Bax M., et al. Practical use, effects and complications of prehospital treatment of acute cardiogenic pulmonary edema using the Boussignac CPAP system. Int J Emerg Med. 2013;6:8. doi: 10.1186/1865-1380-6-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aguilar S.A., Lee J., Castillo E., et al. Assessment of the addition of prehospital continuous positive airway pressure (CPAP) to an urban emergency medical services (EMS) system in persons with severe respiratory distress. J Emerg Med. 2013;45:210–219. doi: 10.1016/j.jemermed.2013.01.044. [DOI] [PubMed] [Google Scholar]
- 3.Luhr O.R., Antonsen K., Karlsson M., et al. Incidence and mortality after acute respiratory failure and acute respiratory distress syndrome in Sweden, Denmark, and Iceland. The ARF Study Group. Am J Respir Crit Care Med. 1999;159:1849–1861. doi: 10.1164/ajrccm.159.6.9808136. [DOI] [PubMed] [Google Scholar]
- 4.British Thoracic Society. National Respiratory Audit Programme annual report. 2012. Available at: https://www.brit-thoracic.org.uk/document-library/audit-and-quality-improvement/audit-reports/bts-national-respiratory-audit-programme-annual-report-2011-2012/. Accessed May 1, 2013.
- 5.Hubble M.W., Richards M.E., Wilfong D.A. Estimates of cost-effectiveness of prehospital continuous positive airway pressure in the management of acute pulmonary edema. Prehosp Emerg Care. 2008;12:277–285. doi: 10.1080/10903120801949275. [DOI] [PubMed] [Google Scholar]
- 6.Personal Social Services. Unit costs of health and social care 2012. Available at: http://www.pssru.ac.uk/archive/pdf/uc/uc2012/full-with-covers.pdf. Accessed May 1, 2013.