Abstract
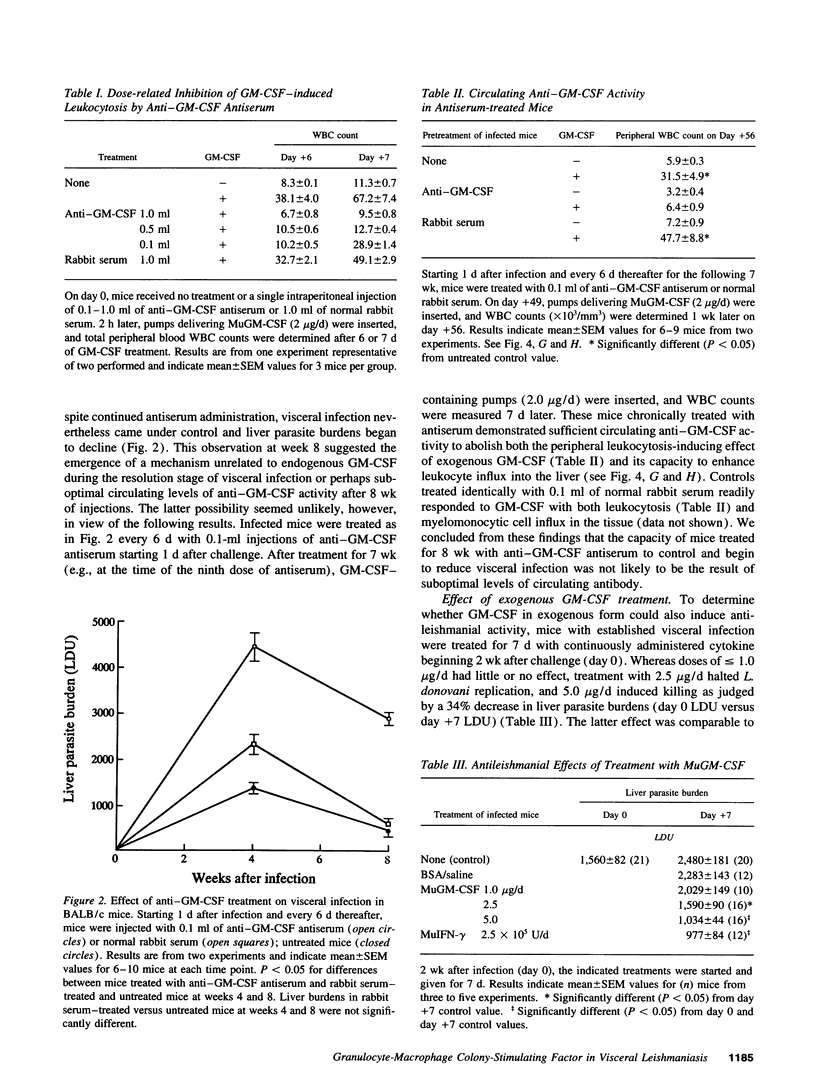
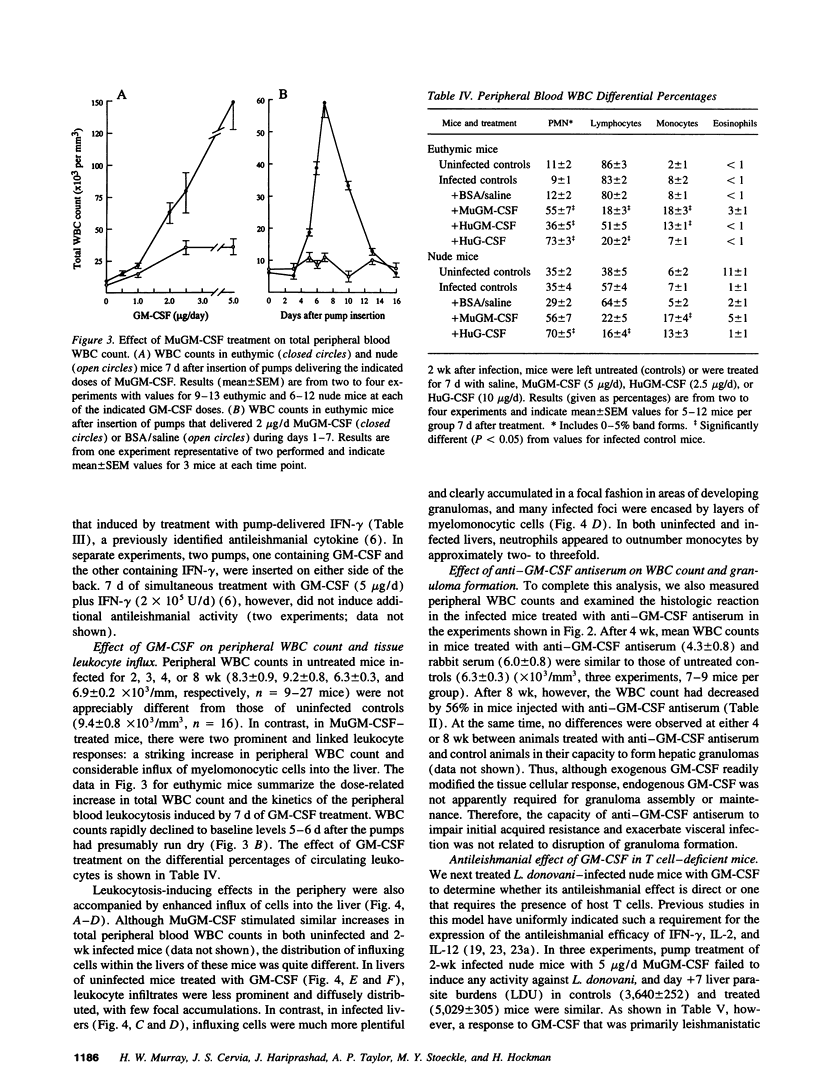
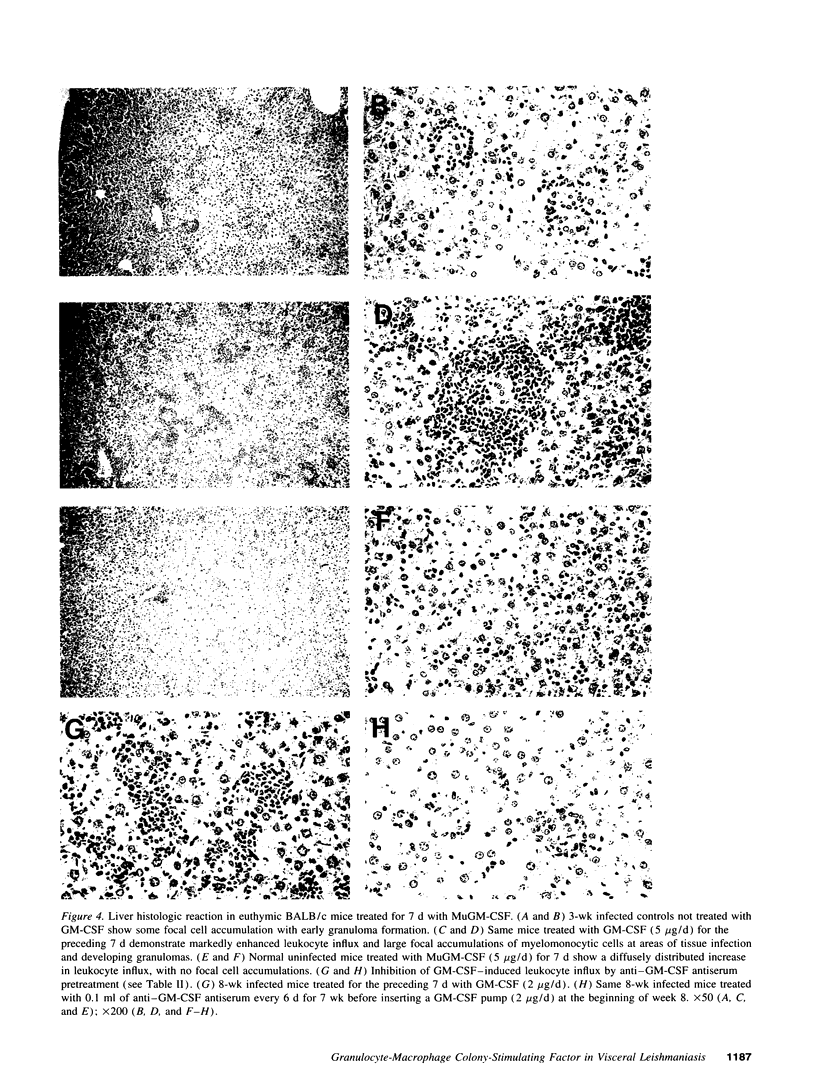
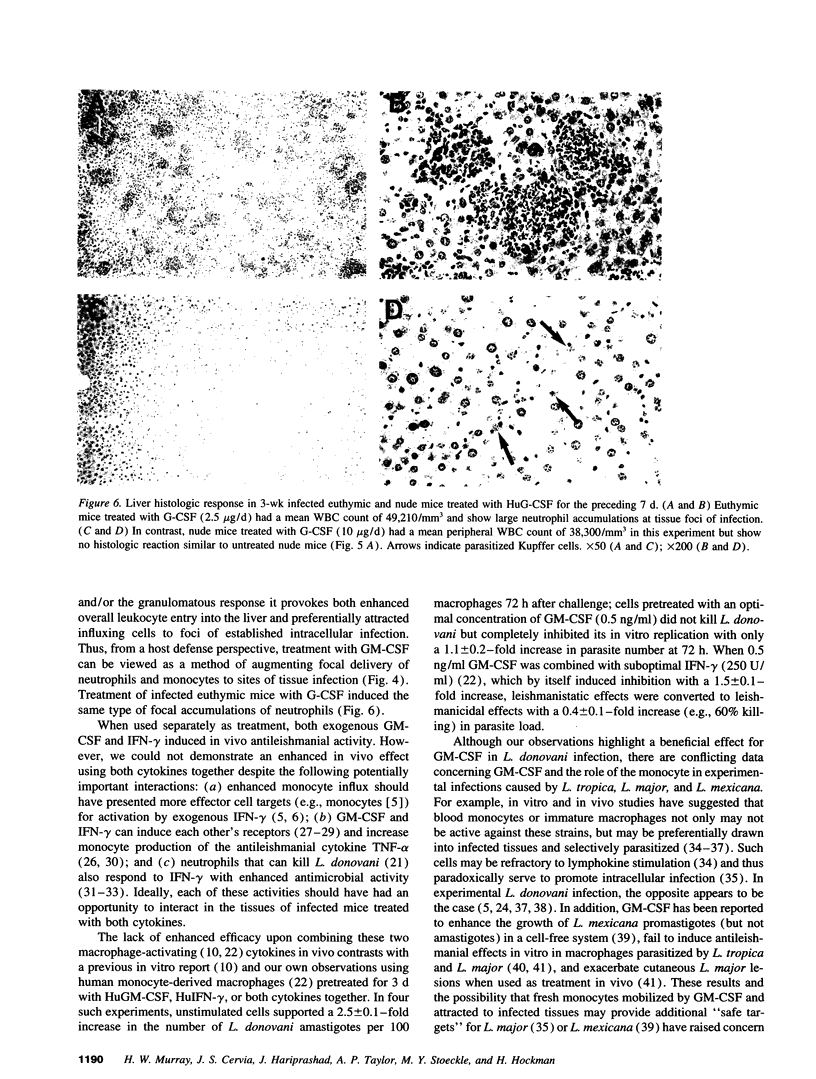
GM-CSF induces three effects potentially beneficial in visceral leishmaniasis: blood monocyte mobilization, macrophage activation, and amelioration of granulocytopenia. To determine the experimental role and effect of GM-CSF in this intracellular infection, livers from Leishmania donovani-infected BALB/c mice were tested for GM-CSF mRNA expression and mice were treated with anti-GM-CSF antiserum or GM-CSF. L. donovani infection upregulated hepatic GM-CSF mRNA expression by 10-fold, and anti-GM-CSF treatment exacerbated visceral infection and tripled liver parasite burdens 4 wk after challenge. In euthymic mice with established infection, treatment with 1-5 micrograms/d murine GM-CSF induced three dose-related effects: peripheral blood leukocytosis, preferential accumulation of myelomonocytic cells at visceral foci of infection, and leishmanicidal activity comparable to that achieved by IFN-gamma. These effects were either largely or entirely T cell dependent. Treatment with human GM-CSF also induced anti-leishmanial activity but with little effect on peripheral leukocyte number or tissue myelomonocytic cell influx; human G-CSF stimulated marked peripheral granulocytosis and neutrophil tissue accumulation but induced little antileishmanial effect. These results identify a role for endogenous GM-CSF in the initial host defense response to L. donovani, reemphasize the influxing monocyte as an effector cell, and indicate that GM-CSF can be used as an antileishmanial treatment.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Badaró R., Nascimento C., Carvalho J. S., Badaró F., Russo D., Ho J. L., Reed S. G., Johnson W. D., Jr, Jones T. C. Recombinant human granulocyte-macrophage colony-stimulating factor reverses neutropenia and reduces secondary infections in visceral leishmaniasis. J Infect Dis. 1994 Aug;170(2):413–418. doi: 10.1093/infdis/170.2.413. [DOI] [PubMed] [Google Scholar]
- Barcinski M. A., Schechtman D., Quintao L. G., Costa D. de A., Soares L. R., Moreira M. E., Charlab R. Granulocyte-macrophage colony-stimulating factor increases the infectivity of Leishmania amazonensis by protecting promastigotes from heat-induced death. Infect Immun. 1992 Sep;60(9):3523–3527. doi: 10.1128/iai.60.9.3523-3527.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bermudez L. E., Martinelli J., Petrofsky M., Kolonoski P., Young L. S. Recombinant granulocyte-macrophage colony-stimulating factor enhances the effects of antibiotics against Mycobacterium avium complex infection in the beige mouse model. J Infect Dis. 1994 Mar;169(3):575–580. doi: 10.1093/infdis/169.3.575. [DOI] [PubMed] [Google Scholar]
- Cervia J. S., Rosen H., Murray H. W. Effector role of blood monocytes in experimental visceral leishmaniasis. Infect Immun. 1993 Apr;61(4):1330–1333. doi: 10.1128/iai.61.4.1330-1333.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang K. P. Leishmanicidal mechanisms of human polymorphonuclear phagocytes. Am J Trop Med Hyg. 1981 Mar;30(2):322–333. doi: 10.4269/ajtmh.1981.30.322. [DOI] [PubMed] [Google Scholar]
- Chen G. H., Curtis J. L., Mody C. H., Christensen P. J., Armstrong L. R., Toews G. B. Effect of granulocyte-macrophage colony-stimulating factor on rat alveolar macrophage anticryptococcal activity in vitro. J Immunol. 1994 Jan 15;152(2):724–734. [PubMed] [Google Scholar]
- Coffman R. L., Varkila K., Scott P., Chatelain R. Role of cytokines in the differentiation of CD4+ T-cell subsets in vivo. Immunol Rev. 1991 Oct;123:189–207. doi: 10.1111/j.1600-065x.1991.tb00611.x. [DOI] [PubMed] [Google Scholar]
- Corcoran L. M., Metcalf D., Edwards S. J., Handman E. GM-CSF produced by recombinant vaccinia virus or in GM-CSF transgenic mice has no effect in vivo on murine cutaneous leishmaniasis. J Parasitol. 1988 Oct;74(5):763–767. [PubMed] [Google Scholar]
- Davies E. V., Singleton A. M., Blackwell J. M. Differences in Lsh gene control over systemic Leishmania major and Leishmania donovani or Leishmania mexicana mexicana infections are caused by differential targeting to infiltrating and resident liver macrophage populations. Infect Immun. 1988 May;56(5):1128–1134. doi: 10.1128/iai.56.5.1128-1134.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty T. M., Coffman R. L. Leishmania antigens presented by GM-CSF-derived macrophages protect susceptible mice against challenge with Leishmania major. J Immunol. 1993 Jun 15;150(12):5476–5483. [PubMed] [Google Scholar]
- Finbloom D. S., Larner A. C., Nakagawa Y., Hoover D. L. Culture of human monocytes with granulocyte-macrophage colony-stimulating factor results in enhancement of IFN-gamma receptors but suppression of IFN-gamma-induced expression of the gene IP-10. J Immunol. 1993 Mar 15;150(6):2383–2390. [PubMed] [Google Scholar]
- Fischer T., Wiegmann K., Böttinger H., Morens K., Burmester G., Pfizenmaier K. Regulation of IFN-gamma-receptor expression in human monocytes by granulocyte-macrophage colony-stimulating factor. J Immunol. 1990 Nov 1;145(9):2914–2919. [PubMed] [Google Scholar]
- Greil J., Bodendorfer B., Röllinghoff M., Solbach W. Application of recombinant granulocyte-macrophage colony-stimulating factor has a detrimental effect in experimental murine leishmaniasis. Eur J Immunol. 1988 Oct;18(10):1527–1533. doi: 10.1002/eji.1830181009. [DOI] [PubMed] [Google Scholar]
- Hallek M., Lepisto E. M., Slattery K. E., Griffin J. D., Ernst T. J. Interferon-gamma increases the expression of the gene encoding the beta subunit of the granulocyte-macrophage colony-stimulating factor receptor. Blood. 1992 Oct 1;80(7):1736–1742. [PubMed] [Google Scholar]
- Handman E., Burgess A. W. Stimulation by granulocyte-macrophage colony-stimulating factor of Leishmania tropica killing by macrophages. J Immunol. 1979 Mar;122(3):1134–1137. [PubMed] [Google Scholar]
- Hart P. H., Whitty G. A., Piccoli D. S., Hamilton J. A. Synergistic activation of human monocytes by granulocyte-macrophage colony-stimulating factor and IFN-gamma. Increased TNF-alpha but not IL-1 activity. J Immunol. 1988 Sep 1;141(5):1516–1521. [PubMed] [Google Scholar]
- Ho J. L., Reed S. G., Wick E. A., Giordano M. Granulocyte-macrophage and macrophage colony-stimulating factors activate intramacrophage killing of Leishmania mexicana amazonensis. J Infect Dis. 1990 Jul;162(1):224–230. doi: 10.1093/infdis/162.1.224. [DOI] [PubMed] [Google Scholar]
- Hoover D. L., Nacy C. A. Macrophage activation to kill Leishmania tropica: defective intracellular killing of amastigotes by macrophages elicited with sterile inflammatory agents. J Immunol. 1984 Mar;132(3):1487–1493. [PubMed] [Google Scholar]
- Klebanoff S. J., Olszowski S., Van Voorhis W. C., Ledbetter J. A., Waltersdorph A. M., Schlechte K. G. Effects of gamma-interferon on human neutrophils: protection from deterioration on storage. Blood. 1992 Jul 1;80(1):225–234. [PubMed] [Google Scholar]
- Lieschke G. J., Burgess A. W. Granulocyte colony-stimulating factor and granulocyte-macrophage colony-stimulating factor (1). N Engl J Med. 1992 Jul 2;327(1):28–35. doi: 10.1056/NEJM199207023270106. [DOI] [PubMed] [Google Scholar]
- Magee D. M., Wing E. J. Secretion of colony-stimulating factors by T cell clones. Role in adoptive protection against Listeria monocytogenes. J Immunol. 1989 Oct 1;143(7):2336–2341. [PubMed] [Google Scholar]
- McElrath M. J., Murray H. W., Cohn Z. A. The dynamics of granuloma formation in experimental visceral leishmaniasis. J Exp Med. 1988 Jun 1;167(6):1927–1937. doi: 10.1084/jem.167.6.1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miralles G. D., Stoeckle M. Y., McDermott D. F., Finkelman F. D., Murray H. W. Th1 and Th2 cell-associated cytokines in experimental visceral leishmaniasis. Infect Immun. 1994 Mar;62(3):1058–1063. doi: 10.1128/iai.62.3.1058-1063.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirkovich A. M., Galelli A., Allison A. C., Modabber F. Z. Increased myelopoiesis during Leishmania major infection in mice: generation of 'safe targets', a possible way to evade the effector immune mechanism. Clin Exp Immunol. 1986 Apr;64(1):1–7. [PMC free article] [PubMed] [Google Scholar]
- Mochizuki D. Y., Eisenman J. R., Conlon P. J., Park L. S., Urdal D. L. Development and characterization of antiserum to murine granulocyte-macrophage colony-stimulating factor. J Immunol. 1986 May 15;136(10):3706–3709. [PubMed] [Google Scholar]
- Morrissey P. J., Charrier K. GM-CSF administration augments the survival of ity-resistant A/J mice, but not ity-susceptible C57BL/6 mice, to a lethal challenge with Salmonella typhimurium. J Immunol. 1990 Jan 15;144(2):557–561. [PubMed] [Google Scholar]
- Murray H. W. Blood monocytes: differing effector role in experimental visceral versus cutaneous leishmaniasis. Parasitol Today. 1994 Jun;10(6):220–223. doi: 10.1016/0169-4758(94)90117-1. [DOI] [PubMed] [Google Scholar]
- Murray H. W., Cartelli D. M. Killing of intracellular Leishmania donovani by human mononuclear phagocytes. Evidence for oxygen-dependent and -independent leishmanicidal activity. J Clin Invest. 1983 Jul;72(1):32–44. doi: 10.1172/JCI110972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray H. W. Effect of continuous administration of interferon-gamma in experimental visceral leishmaniasis. J Infect Dis. 1990 May;161(5):992–994. doi: 10.1093/infdis/161.5.992. [DOI] [PubMed] [Google Scholar]
- Murray H. W., Hariprashad J. Interleukin 12 is effective treatment for an established systemic intracellular infection: experimental visceral leishmaniasis. J Exp Med. 1995 Jan 1;181(1):387–391. doi: 10.1084/jem.181.1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray H. W., Miralles G. D., Stoeckle M. Y., McDermott D. F. Role and effect of IL-2 in experimental visceral leishmaniasis. J Immunol. 1993 Jul 15;151(2):929–938. [PubMed] [Google Scholar]
- Murray H. W., Rubin B. Y., Rothermel C. D. Killing of intracellular Leishmania donovani by lymphokine-stimulated human mononuclear phagocytes. Evidence that interferon-gamma is the activating lymphokine. J Clin Invest. 1983 Oct;72(4):1506–1510. doi: 10.1172/JCI111107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray H. W., Stern J. J., Welte K., Rubin B. Y., Carriero S. M., Nathan C. F. Experimental visceral leishmaniasis: production of interleukin 2 and interferon-gamma, tissue immune reaction, and response to treatment with interleukin 2 and interferon-gamma. J Immunol. 1987 Apr 1;138(7):2290–2297. [PubMed] [Google Scholar]
- Newman S. L., Gootee L. Colony-stimulating factors activate human macrophages to inhibit intracellular growth of Histoplasma capsulatum yeasts. Infect Immun. 1992 Nov;60(11):4593–4597. doi: 10.1128/iai.60.11.4593-4597.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralph P., Nacy C. A., Meltzer M. S., Williams N., Nakoinz I., Leonard E. J. Colony-stimulating factors and regulation of macrophage tumoricidal and microbicidal activities. Cell Immunol. 1983 Feb 15;76(1):10–21. doi: 10.1016/0008-8749(83)90343-x. [DOI] [PubMed] [Google Scholar]
- Reed S. G., Nathan C. F., Pihl D. L., Rodricks P., Shanebeck K., Conlon P. J., Grabstein K. H. Recombinant granulocyte/macrophage colony-stimulating factor activates macrophages to inhibit Trypanosoma cruzi and release hydrogen peroxide. Comparison with interferon gamma. J Exp Med. 1987 Dec 1;166(6):1734–1746. doi: 10.1084/jem.166.6.1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roilides E., Uhlig K., Venzon D., Pizzo P. A., Walsh T. J. Enhancement of oxidative response and damage caused by human neutrophils to Aspergillus fumigatus hyphae by granulocyte colony-stimulating factor and gamma interferon. Infect Immun. 1993 Apr;61(4):1185–1193. doi: 10.1128/iai.61.4.1185-1193.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruef C., Coleman D. L. Granulocyte-macrophage colony-stimulating factor: pleiotropic cytokine with potential clinical usefulness. Rev Infect Dis. 1990 Jan-Feb;12(1):41–62. doi: 10.1093/clinids/12.1.41. [DOI] [PubMed] [Google Scholar]
- Sechler J. M., Malech H. L., White C. J., Gallin J. I. Recombinant human interferon-gamma reconstitutes defective phagocyte function in patients with chronic granulomatous disease of childhood. Proc Natl Acad Sci U S A. 1988 Jul;85(13):4874–4878. doi: 10.1073/pnas.85.13.4874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith P. D., Lamerson C. L., Banks S. M., Saini S. S., Wahl L. M., Calderone R. A., Wahl S. M. Granulocyte-macrophage colony-stimulating factor augments human monocyte fungicidal activity for Candida albicans. J Infect Dis. 1990 May;161(5):999–1005. doi: 10.1093/infdis/161.5.999. [DOI] [PubMed] [Google Scholar]
- Squires K. E., Schreiber R. D., McElrath M. J., Rubin B. Y., Anderson S. L., Murray H. W. Experimental visceral leishmaniasis: role of endogenous IFN-gamma in host defense and tissue granulomatous response. J Immunol. 1989 Dec 15;143(12):4244–4249. [PubMed] [Google Scholar]
- Stern J. J., Oca M. J., Rubin B. Y., Anderson S. L., Murray H. W. Role of L3T4+ and LyT-2+ cells in experimental visceral leishmaniasis. J Immunol. 1988 Jun 1;140(11):3971–3977. [PubMed] [Google Scholar]
- Sunderkötter C., Kunz M., Steinbrink K., Meinardus-Hager G., Goebeler M., Bildau H., Sorg C. Resistance of mice to experimental leishmaniasis is associated with more rapid appearance of mature macrophages in vitro and in vivo. J Immunol. 1993 Nov 1;151(9):4891–4901. [PubMed] [Google Scholar]
- Tumang M. C., Keogh C., Moldawer L. L., Helfgott D. C., Teitelbaum R., Hariprashad J., Murray H. W. Role and effect of TNF-alpha in experimental visceral leishmaniasis. J Immunol. 1994 Jul 15;153(2):768–775. [PubMed] [Google Scholar]
- Weiser W. Y., Van Niel A., Clark S. C., David J. R., Remold H. G. Recombinant human granulocyte/macrophage colony-stimulating factor activates intracellular killing of Leishmania donovani by human monocyte-derived macrophages. J Exp Med. 1987 Nov 1;166(5):1436–1446. doi: 10.1084/jem.166.5.1436. [DOI] [PMC free article] [PubMed] [Google Scholar]






