Abstract
Cross-resistance between cisplatin (DDP) and metalloid salts in human cells was sought on the basis that mechanisms that mediate metalloid salt cross-resistance in prokaryotes are evolutionarily conserved. Two ovarian and two head and neck carcinoma cell lines selected for DDP resistance were found to be cross-resistant to antimony potassium tartrate, which contains trivalent antimony. The DDP-resistant variant 2008/A was also cross-resistant to arsenite but not to stibogluconate, which contains pentavalent antimony. A variant selected for resistance to antimony potassium tartrate was cross-resistant to DDP and arsenite. Resistance to antimony potassium tartrate and arsenite was of a similar magnitude (3-7-fold), whereas the level of resistance to DDP was greater (17-fold), irrespective of whether the cells were selected by exposure to DDP or to antimony potassium tartrate. In the resistant sublines, uptake of [3H]-dichloro(ethylenediamine) platinum(II) was reduced to 41-52% of control, and a similar deficit was observed in the accumulation of arsenite. We conclude that DDP, antimony potassium tartrate, and arsenite all share a common mechanism of resistance in human cells and that this is due in part to an accumulation defect.
Full text
PDF
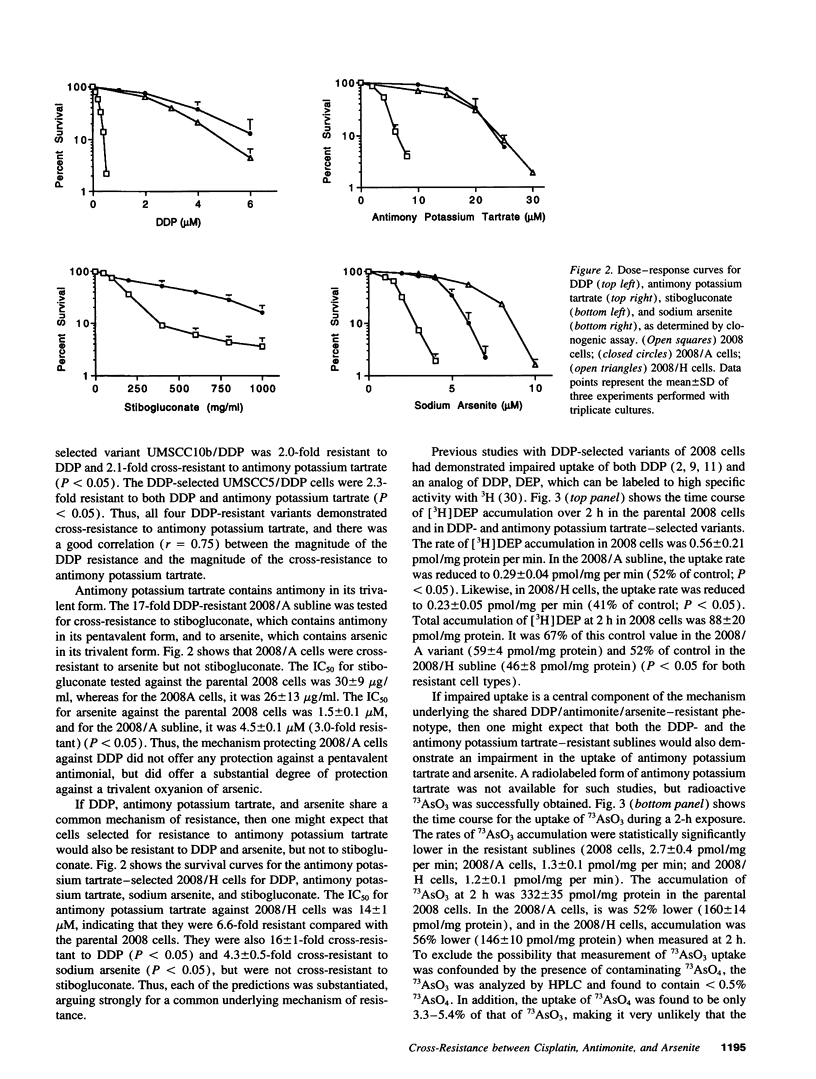
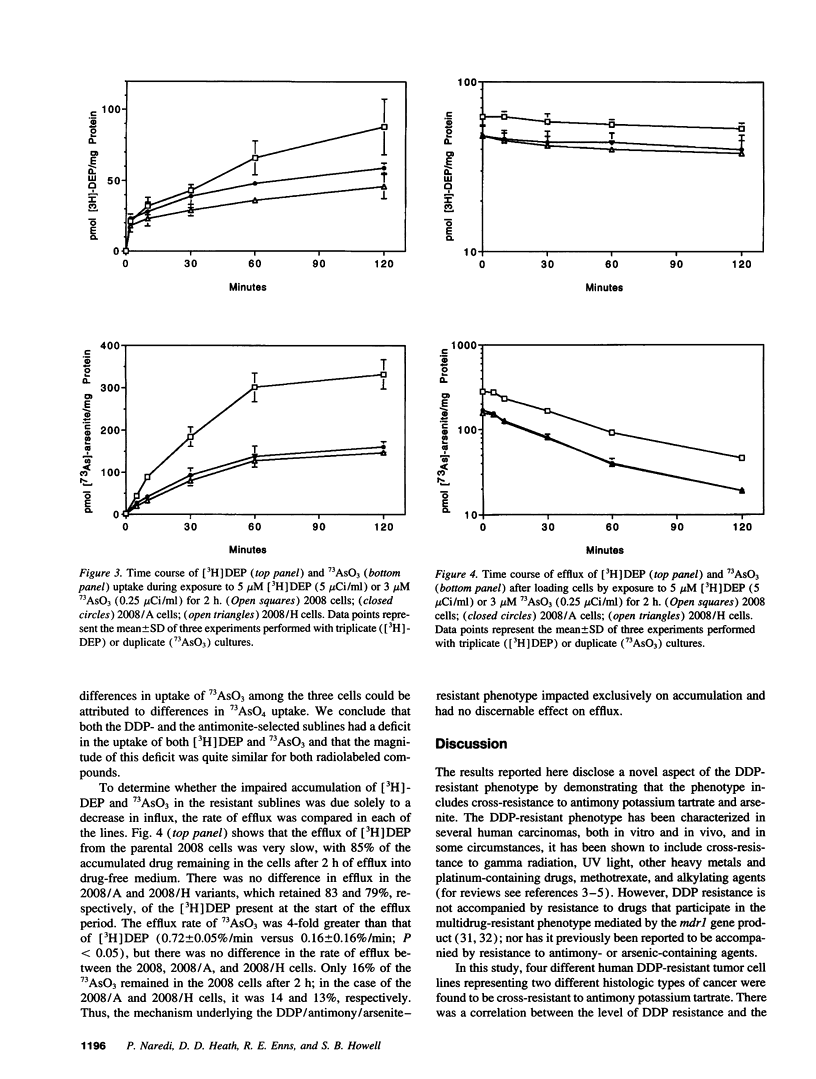


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews P. A., Howell S. B. Cellular pharmacology of cisplatin: perspectives on mechanisms of acquired resistance. Cancer Cells. 1990 Feb;2(2):35–43. [PubMed] [Google Scholar]
- Andrews P. A., Jones J. A., Varki N. M., Howell S. B. Rapid emergence of acquired cis-diamminedichloroplatinum(II) resistance in an in vivo model of human ovarian carcinoma. Cancer Commun. 1990;2(2):93–100. doi: 10.3727/095535490820874641. [DOI] [PubMed] [Google Scholar]
- Andrews P. A., Mann S. C., Huynh H. H., Albright K. D. Role of the Na+, K(+)-adenosine triphosphatase in the accumulation of cis-diamminedichloroplatinum(II) in human ovarian carcinoma cells. Cancer Res. 1991 Jul 15;51(14):3677–3681. [PubMed] [Google Scholar]
- Andrews P. A., Velury S., Mann S. C., Howell S. B. cis-Diamminedichloroplatinum(II) accumulation in sensitive and resistant human ovarian carcinoma cells. Cancer Res. 1988 Jan 1;48(1):68–73. [PubMed] [Google Scholar]
- Behrens B. C., Hamilton T. C., Masuda H., Grotzinger K. R., Whang-Peng J., Louie K. G., Knutsen T., McKoy W. M., Young R. C., Ozols R. F. Characterization of a cis-diamminedichloroplatinum(II)-resistant human ovarian cancer cell line and its use in evaluation of platinum analogues. Cancer Res. 1987 Jan 15;47(2):414–418. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Bröer S., Ji G., Bröer A., Silver S. Arsenic efflux governed by the arsenic resistance determinant of Staphylococcus aureus plasmid pI258. J Bacteriol. 1993 Jun;175(11):3480–3485. doi: 10.1128/jb.175.11.3480-3485.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callahan H. L., Beverley S. M. Heavy metal resistance: a new role for P-glycoproteins in Leishmania. J Biol Chem. 1991 Oct 5;266(28):18427–18430. [PubMed] [Google Scholar]
- Christen R. D., Jekunen A. P., Jones J. A., Thiebaut F., Shalinsky D. R., Howell S. B. In vitro modulation of cisplatin accumulation in human ovarian carcinoma cells by pharmacologic alteration of microtubules. J Clin Invest. 1993 Jul;92(1):431–440. doi: 10.1172/JCI116585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conklin D. S., McMaster J. A., Culbertson M. R., Kung C. COT1, a gene involved in cobalt accumulation in Saccharomyces cerevisiae. Mol Cell Biol. 1992 Sep;12(9):3678–3688. doi: 10.1128/mcb.12.9.3678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiSaia P. J., Sinkovics J. G., Rutledge F. N., Smith J. P. Cell-mediated immunity to human malignant cells. A brief review and further studies with two gynecologic tumors. Am J Obstet Gynecol. 1972 Dec 1;114(7):979–989. doi: 10.1016/0002-9378(72)90109-3. [DOI] [PubMed] [Google Scholar]
- Dornish J. M., Pettersen E. O., Oftebro R. Modifying effect of cinnamaldehyde and cinnamaldehyde derivatives on cell inactivation and cellular uptake of cis-diamminedichloroplatinum(II) in human NHIK 3025 cells. Cancer Res. 1989 Jul 15;49(14):3917–3921. [PubMed] [Google Scholar]
- Eastman A. Characterization of the adducts produced in DNA by cis-diamminedichloroplatinum(II) and cis-dichloro(ethylenediamine)platinum(II). Biochemistry. 1983 Aug 2;22(16):3927–3933. doi: 10.1021/bi00285a031. [DOI] [PubMed] [Google Scholar]
- Gately D. P., Howell S. B. Cellular accumulation of the anticancer agent cisplatin: a review. Br J Cancer. 1993 Jun;67(6):1171–1176. doi: 10.1038/bjc.1993.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grenman R., Burk D., Virolainen E., Buick R. N., Church J., Schwartz D. R., Carey T. E. Clonogenic cell assay for anchorage-dependent squamous carcinoma cell lines using limiting dilution. Int J Cancer. 1989 Jul 15;44(1):131–136. doi: 10.1002/ijc.2910440123. [DOI] [PubMed] [Google Scholar]
- Grogl M., Martin R. K., Oduola A. M., Milhous W. K., Kyle D. E. Characteristics of multidrug resistance in Plasmodium and Leishmania: detection of P-glycoprotein-like components. Am J Trop Med Hyg. 1991 Jul;45(1):98–111. doi: 10.4269/ajtmh.1991.45.98. [DOI] [PubMed] [Google Scholar]
- Hromas R. A., North J. A., Burns C. P. Decreased cisplatin uptake by resistant L1210 leukemia cells. Cancer Lett. 1987 Aug;36(2):197–201. doi: 10.1016/0304-3835(87)90091-7. [DOI] [PubMed] [Google Scholar]
- Kamizono A., Nishizawa M., Teranishi Y., Murata K., Kimura A. Identification of a gene conferring resistance to zinc and cadmium ions in the yeast Saccharomyces cerevisiae. Mol Gen Genet. 1989 Oct;219(1-2):161–167. doi: 10.1007/BF00261172. [DOI] [PubMed] [Google Scholar]
- Kaur P., Rosen B. P. Plasmid-encoded resistance to arsenic and antimony. Plasmid. 1992 Jan;27(1):29–40. doi: 10.1016/0147-619x(92)90004-t. [DOI] [PubMed] [Google Scholar]
- Krause C. J., Carey T. E., Ott R. W., Hurbis C., McClatchey K. D., Regezi J. A. Human squamous cell carcinoma. Establishment and characterization of new permanent cell lines. Arch Otolaryngol. 1981 Nov;107(11):703–710. doi: 10.1001/archotol.1981.00790470051012. [DOI] [PubMed] [Google Scholar]
- Licht T., Fiebig H. H., Bross K. J., Herrmann F., Berger D. P., Shoemaker R., Mertelsmann R. Induction of multiple-drug resistance during anti-neoplastic chemotherapy in vitro. Int J Cancer. 1991 Oct 21;49(4):630–637. doi: 10.1002/ijc.2910490427. [DOI] [PubMed] [Google Scholar]
- Loehrer P. J., Einhorn L. H. Drugs five years later. Cisplatin. Ann Intern Med. 1984 May;100(5):704–713. doi: 10.7326/0003-4819-100-5-704. [DOI] [PubMed] [Google Scholar]
- Mann S. C., Andrews P. A., Howell S. B. Comparison of lipid content, surface membrane fluidity, and temperature dependence of cis-diamminedichloroplatinum(II) accumulation in sensitive and resistant human ovarian carcinoma cells. Anticancer Res. 1988 Nov-Dec;8(6):1211–1215. [PubMed] [Google Scholar]
- Mann S. C., Andrews P. A., Howell S. B. Modulation of cis-diamminedichloroplatinum(II) accumulation and sensitivity by forskolin and 3-isobutyl-1-methylxanthine in sensitive and resistant human ovarian carcinoma cells. Int J Cancer. 1991 Jul 30;48(6):866–872. doi: 10.1002/ijc.2910480613. [DOI] [PubMed] [Google Scholar]
- Mann S. C., Andrews P. A., Howell S. B. Short-term cis-diamminedichloroplatinum(II) accumulation in sensitive and resistant human ovarian carcinoma cells. Cancer Chemother Pharmacol. 1990;25(4):236–240. doi: 10.1007/BF00684878. [DOI] [PubMed] [Google Scholar]
- Neal R. A., van Bueren J., McCoy N. G., Iwobi M. Reversal of drug resistance in Trypanosoma cruzi and Leishmania donovani by verapamil. Trans R Soc Trop Med Hyg. 1989 Mar-Apr;83(2):197–198. doi: 10.1016/0035-9203(89)90642-1. [DOI] [PubMed] [Google Scholar]
- Nies D. H. CzcR and CzcD, gene products affecting regulation of resistance to cobalt, zinc, and cadmium (czc system) in Alcaligenes eutrophus. J Bacteriol. 1992 Dec;174(24):8102–8110. doi: 10.1128/jb.174.24.8102-8110.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nies D. H. Resistance to cadmium, cobalt, zinc, and nickel in microbes. Plasmid. 1992 Jan;27(1):17–28. doi: 10.1016/0147-619x(92)90003-s. [DOI] [PubMed] [Google Scholar]
- Ortiz D. F., Kreppel L., Speiser D. M., Scheel G., McDonald G., Ow D. W. Heavy metal tolerance in the fission yeast requires an ATP-binding cassette-type vacuolar membrane transporter. EMBO J. 1992 Oct;11(10):3491–3499. doi: 10.1002/j.1460-2075.1992.tb05431.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisoni R. L. Characterization of a phosphate transport system in human fibroblast lysosomes. J Biol Chem. 1991 Jan 15;266(2):979–985. [PubMed] [Google Scholar]
- Rosen B. P., Borbolla M. G. A plasmid-encoded arsenite pump produces arsenite resistance in Escherichia coli. Biochem Biophys Res Commun. 1984 Nov 14;124(3):760–765. doi: 10.1016/0006-291x(84)91023-4. [DOI] [PubMed] [Google Scholar]
- Shionoya S., Lu Y., Scanlon K. J. Properties of amino acid transport systems in K562 cells sensitive and resistant to cis-diamminedichloroplatinum(II). Cancer Res. 1986 Jul;46(7):3445–3448. [PubMed] [Google Scholar]
- Timmer-Bosscha H., Mulder N. H., de Vries E. G. Modulation of cis-diamminedichloroplatinum(II) resistance: a review. Br J Cancer. 1992 Aug;66(2):227–238. doi: 10.1038/bjc.1992.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toffoli G., Viel A., Tumiotto L., Biscontin G., Rossi C., Boiocchi M. Pleiotropic-resistant phenotype is a multifactorial phenomenon in human colon carcinoma cell lines. Br J Cancer. 1991 Jan;63(1):51–56. doi: 10.1038/bjc.1991.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waud W. R. Differential uptake of cis-diamminedichloroplatinum (II) by sensitive and resistant murine L1210 leukemia cells. Cancer Res. 1987 Dec 15;47(24 Pt 1):6549–6555. [PubMed] [Google Scholar]
- Wu J., Rosen B. P. Metalloregulated expression of the ars operon. J Biol Chem. 1993 Jan 5;268(1):52–58. [PubMed] [Google Scholar]


