Abstract
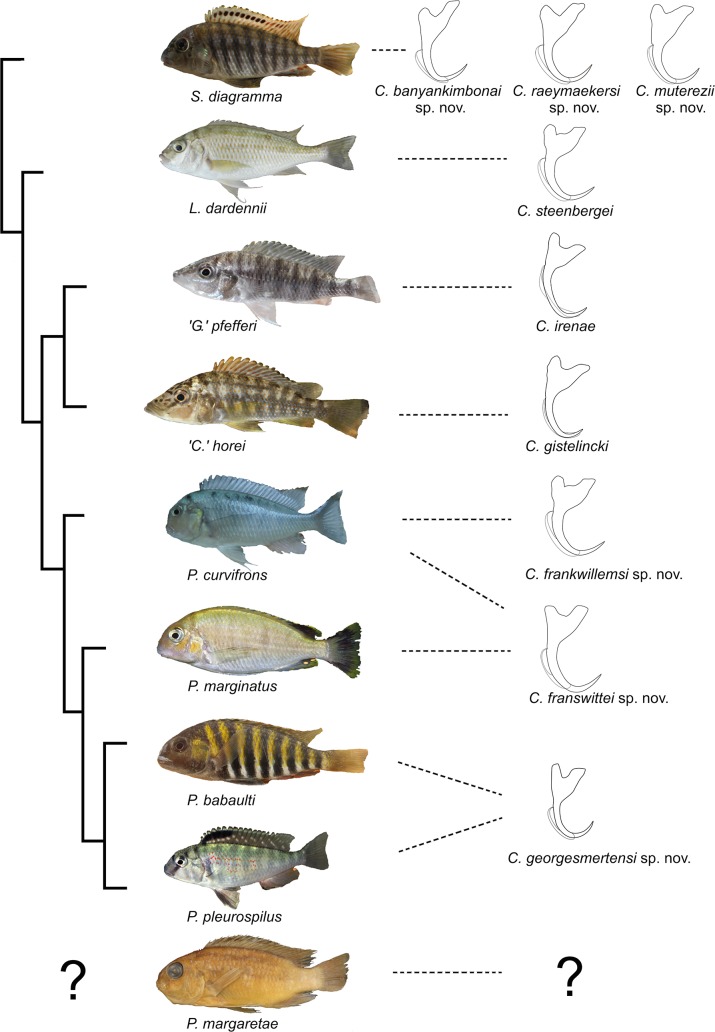
The unparalleled biodiversity of Lake Tanganyika (Africa) has fascinated biologists for over a century; its unique cichlid communities are a preferred model for evolutionary research. Although species delineation is, in most cases, relatively straightforward, higher-order classifications were shown not to agree with monophyletic groups. Here, traditional morphological methods meet their limitations. A typical example are the tropheine cichlids currently belonging to Simochromis and Pseudosimochromis. The affiliations of these widespread and abundant cichlids are poorly understood. Molecular work suggested that genus and species boundaries should be revised. Moreover, previous morphological results indicated that intraspecific variation should be considered to delineate species in Lake Tanganyika cichlids. We review the genera Simochromis and Pseudosimochromis using an integrative approach. Besides a morphometric study and a barcoding approach, monogenean Cichlidogyrus (Platyhelminthes: Ancyrocephalidae) gill parasites, often highly species-specific, are used as complementary markers. Six new species are described. Cichlidogyrus raeymaekersi sp. nov., C. muterezii sp. nov. and C. banyankimbonai sp. nov. infect S. diagramma. Cichlidogyrus georgesmertensi sp. nov. was found on S. babaulti and S. pleurospilus, C. franswittei sp. nov. on both S. marginatus and P. curvifrons and C. frankwillemsi sp. nov. only on P. curvifrons. As relatedness between Cichlidogyrus species usually reflects relatedness between hosts, we considered Simochromis monotypic because the three Cichlidogyrus species found on S. diagramma belonged to a different morphotype than those found on the other Simochromis. The transfer of S. babaulti, S. marginatus, S. pleurospilus and S. margaretae to Pseudosimochromis was justified by the similarity of their Cichlidogyrus fauna and the intermediate morphology of S. margaretae. Finally parasite data also supported the synonymy between S. pleurospilus and S. babaulti, a species that contains a large amount of geographical morphological variation.
Introduction
Systematics is the science of the world’s biodiversity and its interrelationships. Its language is provided by taxonomy, the theory and practise of identifying, describing and classifying organisms [1]. Traditionally, taxonomy relied on morphological characters, yet, given the limitations of morphology-based methods in e.g. distinguishing between cryptic species or in interpreting intra-specific variation, an integrative approach of taxonomy has been proposed [2]. A review of the literature, however, revealed a lack of consensus on what methodologies should be included in integrative taxonomy and on how different characters should be weighthed against each other [3]. Schlick-Steiner et al. [1] suggested the use of at least three independent datasets: morphology, nuclear DNA and supporting evidence from one additional discipline. Disciplines that have earned their merits in systematic studies include fields as diverse as mating trails [4–5], bioacoustics [6–7], electric signals [8], cuticular chemistry [9], cytogenetics [10] and ecological niche modeling [11–12].
As species interact with one another, their evolutionary trajectories often run in parallel. Hence, systematic knowledge of one group can provide additional information on a group with which it interacts. Nevertheless, the number of systematic studies that use knowledge of symbionts is limited (but see e.g. [13–14]). Few biological interactions are as intimate as parasitism [15]. Given the co-evolutionary arms race that may occur between a parasite and its host, parasites [16] and parasitoids [17] are often highly species-rich and species-specific. Hence, the distribution of parasites provides additional information about the systematics of their hosts [18–19]. An additional advantage of using parasites relates to their often shorter generation time leading to potentially higher genetic and/or morphological differentiation than observed in their hosts. Therefore, species-specific parasites can provide a magnifying glass that can be used to resolve their hosts’ taxonomy [20]. This is especially promising for hosts belonging to recently or rapidly formed radiations, the taxonomy of which is often difficult to unravel. For such taxa, an integrative approach is almost essential as different datasets often reflect alternative evolutionary scenarios [1].
The cichlid flocks of the East African Great Lakes form the most spectacular vertebrate radiations [21]. Diversification in Great Lake cichlids often occurred in rapid cladogenetic events [22–23], which hampers phylogenetic reconstruction [24–25]. Moreover, the high degree of morphological convergence in species-rich communities [26] also provides a challenge to morphology-based higher order classifications [27]. Lake Tanganyika is, with an estimated age of 9–12 million years [28], the oldest of the East African Great Lakes. Its 250 endemic cichlids form the morphologically, behaviourally and phylogenetically most diverse lacustrine cichlid fauna worldwide, although not the most species-rich [29]. Compared to the radiations of Lake Victoria and Malawi, the systematics of Lake Tanganyika cichlids is relatively well known, yet many problems remain, not in the least at the generic level [27].
In this study, we focus on two genera of Lake Tanganyika cichlids: Simochromis Boulenger, 1898 and Pseudosimochromis Nelissen, 1977, which belong to the endemic Lake Tanganyika tribe Tropheini. Tropheini are a moderately species-rich and ecologically diverse lineage of Lake Tanganyika cichlids containing 24 valid species in seven genera [30–31]. The tribe has a unique phylogenetic position as it is the sister taxon of the megadiverse assemblage that contains the haplochromine radiations of Lake Victoria and Malawi [32]. Many tropheine species are popular model organisms in evolutionary research [33–35] and species belonging to Simochromis have been used as models in ethology [36–38]. Problems, however, remain in species delineation and a nuclear phylogeny [39] even showed Simochromis to be paraphyletic (see Historical account).
We aim at reassessing the taxonomy and interrelationships of the species currently classified under Simochromis and Pseudosimochromis following an integrative approach. For this, we combine morphometric, molecular and parasitological data and compare them with previously published phylogenetic reconstructions [39]. For the parasitological approach, we target monogenean flatworms, which are commonly used to improve the understanding of their host’s biogeography, phylogeny and taxonomy [18–19,40–47]. Monogeneans have a simple one-host lifecycle, high species diversity and a relatively high host-specificity. These traits make them suitable markers to investigate biodiversity and speciation in groups of closely related fishes [48]. The most species-rich genus parasitizing African cichlids is Cichlidogyrus Paperna, 1960 [49]. This monogenean genus is often considered to belong to the Ancyrocephalidae, although numerous studies suggest that Ancyrocephalidae does not form a monophyletic group and that its representatives should be considered members of Dactylogyridae [50–53]. Representatives of Cichlidogyrus are common on Lake Tanganyika cichlids [35] and 16 species have already been described [44,54–57], six of which infect tropheine cichlids [55,57]. Although no Cichlidogyrus species were hitherto formally identified from Simochromis and Pseudosimochromis, three representatives of the monogenean Gyrodactylus von Nordmann, 1832 have been described from Zambian S. diagramma [58]. We will describe the Cichlidogyrus fauna infecting Simochromis and Pseudosimochromis and compare this fauna with that found on related tropheine genera [39,55,57]. By characterising the morphology and host range of Cichlidogyrus species, we aim to shed extra light on the interrelationships of Simochromis and Pseudosimochromis through an additional line of evidence.
Historical account
Simochromis currently contains five nominal species [31]: S. diagramma (Günther, 1894), with junior synomym: Tilapia adolfi (Steindachner, 1909), S. babaulti Pellegrin, 1927, S. marginatus Poll, 1956, S. margaretae Axelrod & Harrison, 1978 and S. pleurospilus Nelissen, 1978. Pseudosimochromis curvifrons (Poll, 1942) was originally described as a Simochromis and Interochromis loocki (Poll, 1949) and Limnotilapia dardennii (Boulenger, 1899) at some point also belonged to this genus. Both Simochromis and Pseudosimochromis browse filamentous algae at the lake’s rocky shores although the species differ in their tolerance to sediment [59].
Boulenger [60] erected Simochromis together with eight other Lake Tanganyika cichlid genera and with S. diagramma as the only species. The description was very brief, with the single row of lateral conical teeth given as the sole character separating it from Tilapia. Later the same year, Boulenger [61] provided a more detailed genus description. Simochromis babaulti was described based on a single specimen from Uvira. Its colour pattern, less steep head profile, larger mouth and lower counts for teeth rows and gill rakers separated this species from its congener [62]. Pseudosimochromis curvifrons was originally described as a Simochromis [63] but the extreme curvature of the head inspired Nelissen [64] to place it into a separate genus. Five specimens from the Ubwari peninsula were used for the description of S. marginatus and Poll listed the number of dorsal soft rays, the colour pattern, the higher body, shorter mouth and larger eye as characters to distinguish this species from S. babaulti [65].
Although there is little doubt concerning the validity of the species listed above, confusion exists on two species described almost simultaneously in 1977 and 1978: S. margaretae and S. pleurospilus. Nelissen [66] described the latter based on an observation of P. Brichard that in the southern end of the lake S. babaulti occurs sympatrically with a highly similar species. Although some morphometric differences were found between S. babaulti and S. pleurospilus, all of them overlapped. Hence, the difference in colour pattern, especially the presence of lateral rows of red dots on the flanks in S. pleurospilus but absent in S. babaulti, was used to separate the species [66]. Konings [67] already suggested that S. pleurospilus could represent a southern morph of S. babaulti and observations along the western shore revealed populations with colour patterns intermediate between those of the typical S. babaulti and S. pleurospilus [68]. Moreover, recent molecular results also did not support the monophyly of both species [39]. Simochromis margaretae was described on four specimens from Kigoma that were compared with specimens of all congeners and with P. curvifrons, all of which, except for S. pleurospilus, were collected at the same locality [69]. Since then, S. margaretae has never been observed again, despite intensive sampling. This led Konings [67] to speculate that S. margaretae could be a junior synonym of S. marginatus.
Besides on the species level, problems also exist on the generic level. Although a cladistic approach showed Simochromis to be monophyletic [70], recent nuclear molecular results tell a different story [39]. In an AFLP-based phylogeny of the Tropheini, Simochromis was resolved as paraphyletic with S. diagramma being a separate clade and with S. babaulti, S. pleurospilus, S. marginatus and P. curvifrons (the latter being sister to the three previous ones) forming a monophyletic clade within the “sediment dwellers” [39]. This group also includes ‘Ctenochromis’ horei (Günther, 1894), ‘Gnathochromis’ pfefferi (Boulenger, 1898) and Limnotilapia dardennii.
Materials and Methods
Host morphology
In total, 114 specimens, including type material for all nominal species belonging to Simochromis and Pseudosimochromis, were examined using traditional morphometric techniques. Most specimens belong to the collections of the Royal Museum for Central Africa (Tervuren, Belgium) (RMCA) whereas additional material originates from the Royal Belgian Institute for Natural Sciences (Brussels, Belgium) (RBINS), the Natural History Museum (London, United Kingdom) (BMNH), the Muséum National d’Histoire Naturelle (Paris, France) (MNHN) and the South African Institute for Aquatic Biodiversity (Grahamstown, Republic of South Africa) (SAIAB) (see appendix 1). Eight additional type specimens from the Naturhistorisches Museum (Vienna, Austria) (NMW) were studied on site; however, as they were examined by a different person, they were not included in the morphometric analysis.
Eighteen meristics and 21 measurements were collected. For the meristics, these are: ASp: the number of anal spines, ASR: the number of anal soft rays, DSp: the number of dorsal spines, DSR: the number of dorsal soft rays, Pect: the number of pectoral rays, ULL: the number of pored scales in the upper lateral line, MLL: the number of scales in the ‘middle’ lateral line (i.e. the part of the upper lateral line separated from the lower lateral line by only one scale row instead of two), LLL: the number of scales in the lower lateral line (when a minute lateral line scale, shorter than half of the previous scale, was present before the articulation of the caudal fin, this was counted as 0.5), LongL: the number of scales in the longitudinal line, CP: the number or scales around the caudal peduncle, IOS: inter-orbital scales, i.e. the smallest number of scales between the eyes, GRL: the number of gill rakers on the lower part of the first gill arch (not including the middle gill raker), GRU: the number of gill rakers on the upper part of the first gill arch (not including the middle gill raker), BTU: the total number of bicuspid teeth in the first row of the upper jaw, BTL: the total number of bicuspid teeth in the first row of the lower jaw, LatT: the total number of lateral (non bicuspid) teeth on both sides of the upper Jaw, AV: the number of abdominal vertebrae and CV: the number of caudal vertebrae, the first caudal vertebra is the one to which the first caudal pterygiophore is pointed [71]. X rays were made using the Visix equipment (Medex Loncin SA), which includes a Gem X 160 X ray generator and a high resolution digital X ray detector, Dereo HR1. For the number of lower gill rakers, care was taken to count the reduced gill rakers (sensu [71]) as well. Sometimes, these could only be observed by the different refraction of the light when a concentrated shaft of light was pointed to the anteriormost part of the lower branchial arch [72]. Although not treated separately for the exploratory analyses, reduced and non-reduced gill rakers were recorded separately and listed as such in the character tables.
The 21 measurements are: TL: total length (not used in the analyses), SL: standard length, LaD: lacrimal bone depth, SnL: snouth length, LJL: lower jaw length, PPL: premaxillary processus length, ED: eye diameter, IOW: inter-orbital width, MW: mouth width (measured at the posterior teeth of the upper jaw), HL: head length, BD: body depth, PeL: the length of the longest pectoral ray, ASL: the length of the third anal spine, DFB: dorsal fin base length, AFB: anal fin base length, PrD: pre-dorsal distance, PrP: pre-pectoral distance, PrV: pre-ventral distance, PrA: pre-anal distance, CPL: caudal peduncle length, CPD: caudal peduncle depth. All measurements were taken with a dial calliper up to 0.1 mm. Special care was taken that all of them (except for those from the caudal peduncle) were measured between well-defined bony points. All lateral measurements and meristics were taken on the left side of the body. For the Simochromis pleurospilus allotype, the right side of the body was used for scale counts. For the paratype of S. margaretae for which the lower jaw was missing, the teeth count on the lower jaw was obtained from the original description [69]. For one S. babaulti specimen, the gill arches had been dissected and the number of lower gill rakers was treated as a missing value. The shape of gill rakers and frontal teeth, the latter based on Yamaoka [59], as well as colour patterns of preserved specimens were qualitatively recorded. Measurments and meristics are given as supporting information.
Meristics and measurements were explored separately using Principal Component Analysis (PCA). This was done on the correlation matrix of the raw meristics and on the covariance matrix of the log-transformed measurements. For the PCA conducted on the log-transformed measurements, the first PC is interpreted as describing growth [73]. Variable loadings are given as coefficients. Pair-wise Mann-Whitney U-tests were conducted on the percentages of the linear measurements as well as on the meristics. Percentages of measurements were expressed with respect to head (HL) and standard length (SL), for measurements on the head and the rest of the body respectively. As these measurements as well as the teeth counts could contain allometric variation, these tests were conducted on subsets of similar size class specimens (p>0.1 for SL). As there is discussion regarding the status of S. pleurospilus versus that of S. babaulti, different populations of S. babaulti were examined separately and compared with S. pleurospilus. For this, S. babaulti specimens were grouped according to their geographic origin: the north (including the type locality: Uvira, Kavimvira, Bujumbura, n = 6), the northeast (Segunga, Kalela, Kabwe, n = 8), the east (Mpimbwe Hills, Kasinde, Karema, ‘midway between Ikola and Mkangasi’, Msamba Bay Kalia, Ulwile Island and Kapele, n = 22) and the west (Mukamba and Kyanza, n = 11). We considered all specimens originating from the extreme southeastern end of the lake (Chaitika, Cap Kabeyeye, Cap Nundo, Kama Bay, n = 13) as belonging to S. pleurospilus. Bonferroni correction was performed for the Mann-Whitney U tests on individual variables.
Host genetics: barcoding
For 30 specimens (S. diagramma (n = 5), S. babaulti (n = 16), S. marginatus (n = 4), S. pleurospilus (n = 3) and P. curvifrons (n = 2)) encompassing all the nominal taxa except S. margaretae, the first subunit of the cytochrome c oxidase gene (COI) was sequenced following [74]. As these species do not form a monophylum, specimens from all other “sediment dwelling” tropheine species were included in the barcoding analysis: L. dardennii (n = 3), ‘C.’ horei (n = 3) and ‘G.’ pfefferi (n = 3). Sequences are available at NCBI GenBank under accession numbers KP336420-KP336458. Sequence analysis was carried out in MEGA v.5 [75]. Model selection performed using the same software indicated the Kimura-2-parameter [76] + Γ model (with gamma-shape parameter = 0.14) as optimal model of molecular evolution, based on the Bayesian information criterion. Pairwise genetic distances were calculated in MEGA according to this model.
Parasite morphology
Host cichlid fish were either retrieved from the RMCA collections, collected on site using gill nets or purchased from local fishermen and subsequently deposited in the RMCA collections. They were identified to species level on site by Christian Sturmbauer (Karl-Franzens University of Graz, Austria) or Donatien Muzumani Risasi (Centre de Recherche en Hydrobiologie-Uvira, DRC) and ex situ by the authors. Newly collected fish were kept alive in aerated tanks until they were sacrificed by severing the spinal cord or with an overdose of MS-222. The entire fish or the right branchial arches were stored in pure ethanol. Samples were collected under research permit no. 2007-258-CC-2006-151 from the Tanzania Commission for Science and Technology (COSTECH), under mission statement no. 013/MNRST/CRHU/2010 from the Congolese Ministère de la Recherche Scientifique et Technologique—CRH-Uvira and with permits from the Department of Fisheries, Zambian Ministry of Agriculture and Co-operatives. Gills were inspected for parasites under a Wild M5 (field), Olympus SZX12 or Wild M8 (laboratory) stereomicroscope. Monogenea were removed with a dissection needle. They were mounted on a slide under a cover-slip and fixed using ammonium picrate glycerine [77].
Pictures and measurements of the hard parts of haptor and male copulatory apparatus (MA) were taken based on [78] using a Leica DM2500 microscope at a magnification of 1000x (oil immersion, 10x ocular) with the software LAS v.3.6 and a DFC 425 Leica camera. The numbering of haptoral parts was adopted from ICOPA IV [79]; terminology follows [80] (i.e. “uncinuli” for marginal hooks) and the measurements taken are shown in Fig 1. Measurements are in micrometers and presented as the average + the standard deviation, with the range in parentheses and the number of measured specimens in superscript. Type material was deposited in the invertebrate collection of the RMCA, in the MNHN, and in the Iziko South African Museum (Cape Town, Republic of South Africa) (SAMCTA). When the slide containing the holotype of a species also contained other specimens, holotypes were individually marked. Symbiotypes [81] and host vouchers [82] were deposited in the RMCA.
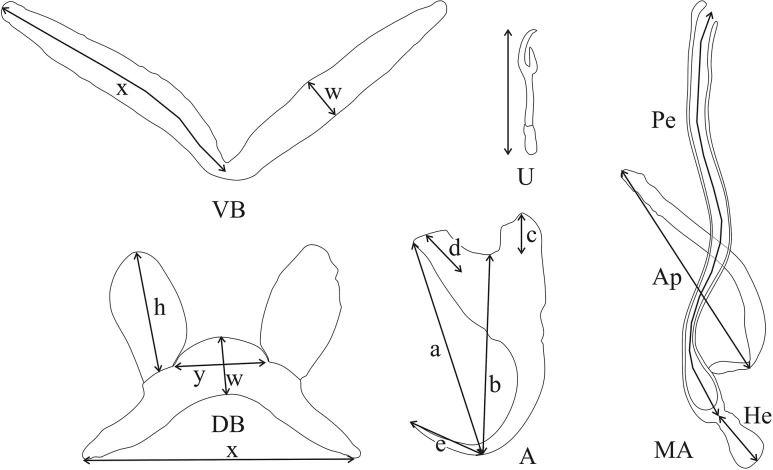
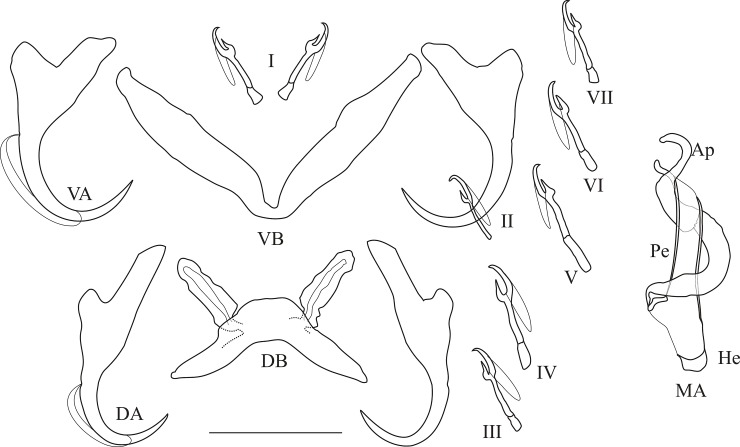
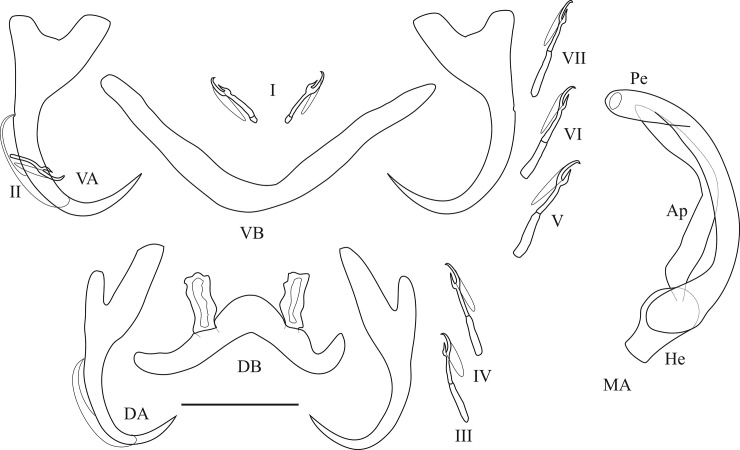
Fig 1. Measurements used to study the new Cichlidogyrus species.
(DB) dorsal transverse bar: (h) length of dorsal bar auricle, (w) dorsal bar maximum width, (x) dorsal bar total length, (y) distance between auricles. (A) anchor: (a) anchor total length, (b) anchor blade length, (c) anchor shaft length, (d) anchor guard length, (e) anchor point length (both dorsal and ventral anchors were examined). (MA) male apparatus: (Ap) accessory piece length, (Pe) penis total length, (He) heel length. (U) uncinuli length. (VB) ventral transverse bar: (w) ventral bar maximum width, (x) length of one ventral bar branch.
Nomenclatural acts
The electronic edition of this article conforms to the requirements of the amended International Code of Zoological Nomenclature, and hence the new names contained herein are available under that Code from the electronic edition of this article. This published work and the nomenclatural acts it contains have been registered in ZooBank, the online registration system for the ICZN. The ZooBank LSIDs (Life Science Identifiers) can be resolved and the associated information viewed through any standard web browser by appending the LSID to the prefix "http://zoobank.org/". The LSID for this publication is: urn:lsid:zoobank.org:pub:765EC2C4-3413-4379-B24A-E2D121BFC204. The electronic edition of this work was published in a journal with an ISSN, and has been archived and is available from the following digital repositories: PubMed Central, LOCKSS.
Results
Host morphology: inter-specific analysis of Simochromis and Pseudosimochromis
Meristics
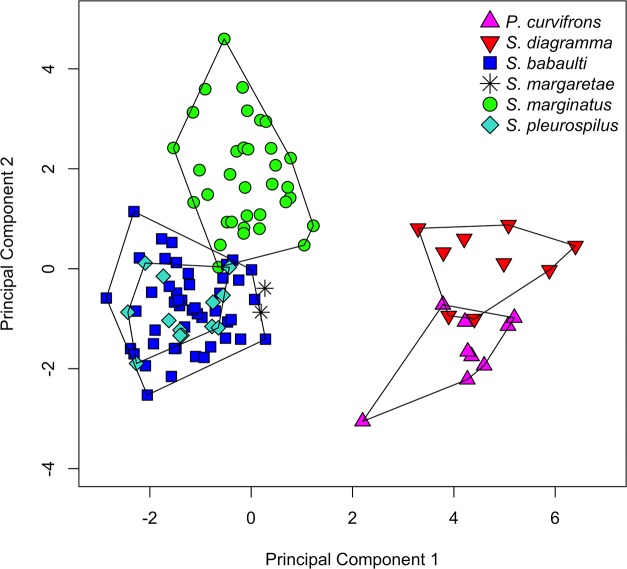
The 16 meristics that contained variation for all 144 specimens were analysed using PCA (Table 1). The number of scales around the caudal peduncle and the number of anal spines were constant (16, resp. 3) and thus omitted from the analysis. A scatter-plot of the second versus the first PC is presented in Fig 2. The first PC, explaining 28.39% of the variance, separated S. diagramma and P. curvifrons from S. babaulti, S. margaretae, S. marginatus and S. pleurospilus, further called the ‘small’ Simochromis. For this axis, values for both S. margaretae paratypes were higher than those for all but one of the S. babaulti and S. pleurospilus specimens. The most important variables in this PC were the number of anal soft rays (ASR), abdominal vertebrae (AV) and lower gill rakers (LGR). The second PC, explaining 14.21% of the variance, allowed for an almost complete separation between S. diagramma and P. curvifrons. This axis also separated S. marginatus from S. margaretae and, albeit with some overlap, from S. pleurospilus and S. babaulti. The main contributors to this axis were the number of bicuspid teeth on the upper and lower jaw (BTU, BTL) and the number of dorsal soft rays (DSR). A separation between P. curvifrons and S. diagramma was also obtained by axis 3 and 4 whereas axis 3 and 5 again separated S. marginatus from S. margaretae (results not shown). None of the PC could distinguish S. pleurospilus from S. babaulti and these species will be hence investigated later (see S. babaulti versus S. pleurospilus ).
Table 1. PCA on meristics of the inter-specific analysis.
| PC1 | PC2 | |
|---|---|---|
| Variance (%) | 28.391 | 14.207 |
| ASR | 0.4021 | -0.1524 |
| DSp | 0.2020 | 0.3169 |
| DSR | 0.1386 | -0.3505 |
| Pect | 0.2214 | 0.0052 |
| ULL | -0.0422 | -0.1119 |
| MLL | -0.2181 | -0.2666 |
| LLL | 0.3175 | 0.1874 |
| LongL | 0.3041 | 0.0758 |
| IOS | 0.2699 | -0.1208 |
| GRU | 0.0120 | 0.3265 |
| GRL | 0.3548 | 0.1434 |
| BTU | -0.1316 | 0.5220 |
| BTL | -0.2443 | 0.4458 |
| LatT | 0.1923 | 0.0233 |
| AV | 0.3896 | 0.1234 |
| CV | -0.1483 | -0.0144 |
Loadings and explained variance of the first four PC of a PCA conducted on the correlation matrix of 16 meristics taken on 114 specimens.
Fig 2. PCA on meristics of the inter-specific analysis.
PC2 vs. PC1 of a PCA on 16 meristics from 114 specimens belonging to the six Simochromis and Pseudosimochromis species.
A summary of the meristics for all species examined and the results of the Mann-Whitney U tests are presented in Table 2. Here, values for S. babaulti and S. pleurospilus were grouped together given that they could not be separated in the PCA. Although 56 comparisons of meristics were found to differ significantly between the species by using Mann-Whitney U tests, very few allowed for the separation of species. Indeed, although S. diagramma and P. curvifrons and the ‘small’ Simochromis species formed two distinct groups in the exploratory analysis, all meristics overlapped between them. In fact, only two meristics, the number of inter-orbital scales (IOS) and the number of lower biscuspid teeth (BTL), could be used to separate P. curvifrons from the ‘small’ Simochromis species (4–6 vs 2–3 for IOS and 20–22 vs. 24–40 for BTL). Between S. diagramma and the ‘small’ Simochromis species, all meristics overlapped. For one meristic: the number of lower lateral line scales (LLL) values for S. diagramma were significantly higher than for all the ‘small’ Simochromis species (12–14.5 vs. 8–13). Moreover, S. diagramma and P. curvifrons had eight or nine soft anal fin rays (ASR) whereas on all but one of the S. babaulti, S. pleurospilus and S. marginatus specimens seven or six were counted. The two S. margaretae paratypes had seven and eight soft anal rays respectively. The number of bicuspid teeth in the upper and lower jaw could be used to distinguish P. curvifrons from S. diagramma (21–28 vs. 31–39 for BTU and 20–22 vs. 23–30 for BTL).
Table 2. Summary of meristics of the different Simochromis and Pseudosimochromis species.
| S. diagramma | P. curvifrons | S. babaulti—S. pleurospilus | S. marginatus | S. mae | M-W U | |
|---|---|---|---|---|---|---|
| N | 9 | 9 | 60 | 34 | 2 | |
| DSp, DSR | XVI,10(1); XVII,9(1); XVII,11(2); XVIII,9(1); XVIII,10(2); XVIII,11(2) | XVI,10(1); XVII,9(2); XVII,10(1); XVIII,9(4); XVIII,10(1) | XVI,9(4); XVI,10(18); XVI,8(3); XVII,9(25); XVII,10(2); XVIII,9(1) | XVI,9(5); XVII,8(3); XVII,9(14); XVIII,8(10); XVIII,8(6) | XVII, 9 | d,c,m>b (DSp) d>b; c,b>m (DSR) |
| Asp, ASR | III, 8(8); III, 9(1) | III, 8(7); III, 9(2) | III, 7(60) | III, 6(1); III, 7(32); III, 8(1) | III, 7; III, 8 | d,c,me>b,m (ASR) |
| ULL: /—MLL | 22-0(2); 23-0(4); 24-0(1); 25-0(2) | 20-0(1); 21-0(1); 21-2(1); 22-0(3); 23-1(1); 24-0(2) | 20-3(1); 21-0(4); 21-1(3); 21-2(5); 22-0(8); 22-1(20); 22-2(5); 22-3(3); 23-0(4); 23-1(4); 23-2(2); 23-3(1) | 21-0(1); 22-0(16); 22-2(2); 22-3(1); 23-0(9); 23-1(3); 23-2(1); 24-0(1) | 22–0; 23–0 | b>d,m (MLL) |
| LLL | 12(2); 13(3); 14(2); 14.5(2) | 9(1); 10(2); 10.5(1); 11(2); 12(2); 13(1) | 8(7); 8.5(1); 9(20); 9.5(3); 10(19); 10.5(2); 11(6); 12(1); 12.5(1) | 8(1); 9(3); 10(9); 10.5(3); 11(10); 11.5(4); 12(3); 13(1) | 10; 11 | d>c,b,m,me d,c,m>b |
| LongL | 33(5); 33.5(1); 34(1); 34.5(1) | 32(4); 31.5(1); 33(4) | 30.5(2); 31(17); 31.5(5); 32(34); 33(2); 34(1) | 31(2); 31.5(4); 32(23); 32.5(3); 33(2) | 31; 32 | d>b,m |
| Pect | 15(1); 16(8) | 15(8); 16(1) | 14(1); 15(57); 16(2) | 14(1); 15(31); 16(2) | 13; 14 | d>c,b,m; b>me |
| LGR-1-UGR | 10-1-2(1); 11-1-2(1); 11-1-3(1); 11-1-4(1); 12-1-2(1); 12-1-3(1); 12-1-4(1); 13-1-3(2) | 10-1-3(4); 10-1-4(1); 11-1-4(1); 12-1-3(2); 12-1-4(1) | 4-4-1-3(1); 1-5-1-3(1); 2-5-1-3(3); 3-5-1-2(1); 3-5-1-3(21); 3-5-1-4(1); 4-5-1-2(1); 4-5-1-3(4); 4-5-1-4(1); 5-5-1-3(1); 2-6-1-3(7); 2-6-1-4(1); 3-6-1-3(9); 2-6-1-4(1); 4-6-1-3(1); 2-7-1-3(4); 1-8-1-4(1); 2-8-1-3(1)* | 4-5-1-3(6); 4-5-1-4(3); 5-5-1-3(4); 5-5-1-5(1); 3-6-1-3(4); 3-6-1-4(2); 3-6-1-5(1); 4-6-1-4(5); 4-6-1-5(1); 5-6-1-3(2); 5-6-1-4(2); 2-7-1-4(1); 3-7-1-4(2) | 2-7-1-2 | d,c>m>b (LGR) m>b; b>me (UGR) |
| BTU | 31(2); 34(3); 35(1); 36(1); 39(2) | 21(1); 22(1); 26(3); 27(2); 28(2) | 26(1); 28(3); 29(4); 30(7); 31(6); 32(11); 33(6); 34(11); 35(3); 37(2); 38(5); 43(1) | 34(5); 35(4); 36(5); 37(5); 38(4); 39(3); 40(3); 41(3); 42(2) | 32; 36 | m>b>c; d>c |
| BTL | 23(1); 24(2); 25(1); 26(3); 29(1); 30(1) | 20(3); 21(2); 22(4) | 24(3); 25(1); 26(5); 27(2); 28(7); 29(3); 30(11); 31(7); 32(6); 33(4); 34(9); 35(1); 37(1) | 25(1); 26(2); 30(3); 31(2); 32(4); 34(9); 35(1); 36(3); 38(3); 39(1); 40(5) | 30; 24 | m>b>d>c |
| LatT | 12(2); 15(4); 16(1); 17(1); 18 | 11(1); 12(1); 13(2); 15(1); 16(1); 17(1); 18(2) | 4(1); 6(2); 7(1); 8(4); 9(5); 10(7); 11(5); 12(9); 13(6); 14(7); 15(4); 16(5); 17(3); 18(1) | 8(1); 9(1); 10(4); 11(5); 12(5); 13(6); 14(7); 15(2); 16(1); 17(1); 18(1) | 16; 20 | |
| IOS | 2(4); 3(3); 4(2) | 4(3); 5(5); 6(1) | 2(46); 3(14) | 2(20); 3(14) | 2; 3 | c>d,b,m |
| AV + CV | 14+17(1); 15+17(5); 15+18(2); 16+17(1) | 14+16(1); 14+17(1); 15+15(2); 15+16(1); 15+17(4) | 13+16(2); 13+17(17); 13+18(18); 14+16(1); 14+17(19); 14+18(3) | 13+18(1); 14+16(1); 14+ 17(32) | 14+16 | b>m>c; m>me (CV) c,d>m>b (AV) |
Values for S. pleurospilus and S. babaulti are grouped together. Mann-Whitney U tests are summarised with d: S. diagramma, c: P. curvifrons, b: S. babaulti and S. pleurospilus, m: S. marginatus and me: S. margaretae (S. mae). ULL is the sum of the two values, reduced and non-reduced gill rakers are listed separately for S. babaulti, S. pleurospilus, S. marginatus and S. margaretae.
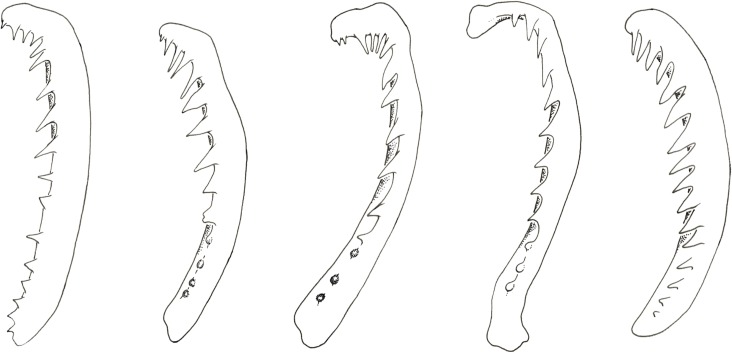
When the shape of the gill rakers was taken into account, the two main groups identified by PC1 could be separated. Indeed, whereas in S. marginatus, S. babaulti, S. pleurospilus and S. margaretae there was a clear transition between the blunt reduced and the sharp non-reduced gill rakers, this was not the case in S. diagramma or in P. curvifrons (Fig 3). In the latter species, reduced gill rakers were never observed and sharp gill rakers were present up until the anteriormost part of the first lower gill arch. In S. babaulti and S. pleurospilus the difference between the non-reduced and the reduced gill rakers was the most striking and the reduced gill rakers could only be observed by their different refraction of light [72]. The anteriormost of the non-reduced gill rakers, however, was always visible as protruding out of the fleshy tissue. A similar situation was encountered in S. marginatus although, in this species, some of the reduced gill rakers protruded slightly, but were still blunt. In the two S. margaretae paratypes, the two anteriormost lower gill rakers differed from the seven other gill rakers both by their smaller size and by their blunt shape. Yet, they were more developed than in the other ‘small’ Simochromis species and clearly visible. In S. diagramma, the anteriormost lower gill rakers were small but there was a gradual transition between the long villiform lower gill rakers dorsally and the short gill rakers ventrally. Also, all gill rakers clearly protruded from the fleshy tissue. In P. curvifrons, a similar gradual reduction in size was encountered although with less difference in shape as all gill rakers were relatively short. Hence, the number of sharp non-reduced lower gill rakers (4–8 vs. 10–13) separated the small Simochromis species from S. diagramma and P. curvifrons.
Fig 3. Gill arches of Simochromis and Pseudosimochromis species.
From left to right: P. curvifrons, S. babaulti, S. marginatus, S. margaretae and S. diagramma. Specimens illustrated are listed in Appendix.
As was the case for the gill rakers, the shape and arrangement of the frontal teeth also differed between the species. In P. curvifrons, frontal teeth were densely set and had a nearly symmetrical shape, with the medial and lateral cusp being nearly equally long and with only a small notch between them. A similar situation was observed in S. margaretae although here the notch between the cusps was relatively deep. In S. babaulti, S. pleurospilus and S. marginatus teeth were also densely set. In these species, the two cusps were asymmetrical with the median cusp being larger than the lateral. In S. diagramma teeth were not as densely set and there was a gap between individual teeth. In this species, the medial cusp, which extended over the gap between the teeth, was much larger than the lateral cusp (see [59]). Another criterion mentioned by Yamaoka [59] to separate Simochromis from Pseudosimochromis was the distance between the outer row of bicuspid teeth and the first row of tricuspid teeth. While this distance was small in P. curvifrons, a relatively large distance (i.e. larger than the distance between the consecutive rows of tricuspid teeth) was observed in S. diagramma, S. babaulti, S. pleurospilus and S. marginatus. In S. margaretae, however, this distance was also small.
Finally, in most S. babaulti and S. pleurospilus specimens, the upper lateral line ran over two adjacent longitudinal scale rows. Hence, the anterior section of the upper lateral line was separated from the lower lateral line by two transverse scales and the posterior section by only one transverse scale. As the upper lateral line was always confluent, the pore on the posterior most scale of the anterior section of the upper lateral line was bended down to connect to the pore on the first scale of the posterior section. This posterior section was here named the ‘middle’ lateral line (MLL). When no such ‘middle’ lateral line was observed, the pore of the posteriormost scale of the upper lateral line was also bended down in S. babaulti and S. pleurospilus. In S. marginatus, P. curvifrons and S. margaretae, a ‘middle’ lateral line was sometimes present. For the latter species, this was not observed on the paratypes examined, although it was visible in the illustration of the holotype [69]. In the cases where in these three species no ‘middle’ lateral line was present, the last pore of the upper later line was, however, not bended down. In S. diagramma, a ‘middle’ lateral line was never observed and the pore on the last scale of the upper lateral line was always straight.
Measurements
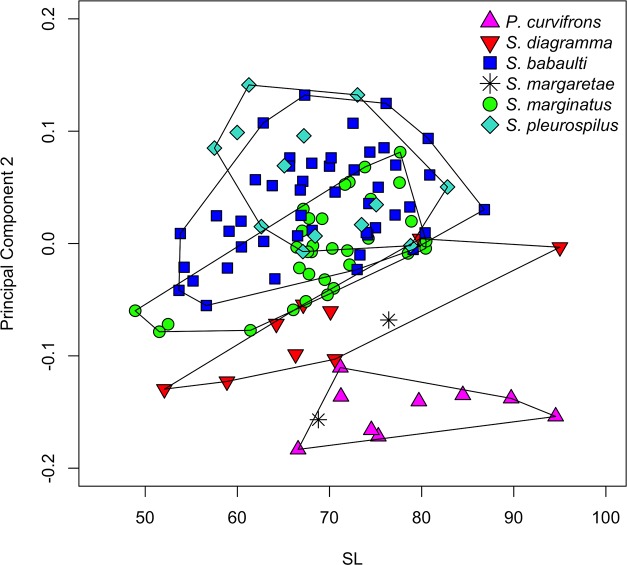
A PCA was performed on 20 log-transformed measurements of 114 specimens (Table 3). For the paratype of S. margaretae of which the lower jaw wa s missing, the lower jaw length was interpolated by assuming that its length relative to the head length is identical to that of the other paratype. The first PC, explaining 82.60% of the variance, explained growth and was not investigated [73]. Although the second principal component included an allometric effect, with lower within-group values for smaller specimens than for larger, it did separate different groups (Fig 4). Pseudosimochromis curvifrons specimens had the smallest values for PC2, S. diagramma was intermediate and S. marginatus, S. babaulti and S. pleurospilus had the highest values. Within the latter group, values for S. babaulti and S. pleurospilus were, on average, higher than those for S. marginatus. Values for the two S. margaretae specimens overlapped with those of S. diagramma and P. curvifrons. The second PC explained 5.92% of the variance and had the inter-orbital width (IOW) as its main contributor. Some separation was also observed on PC3 to PC5 (not shown). The third PC only allowed for a separation between P. curvifrons and S. diagramma. PC4 separated S. margaretae, with the highest values for this axis, from all other species. Finally, PC5 was the first that separated S. babaulti and S. pleurospilus from S. marginatus, although with a considerable amount of overlap. Even when a subsequent PCA, restricted to the specimens of S. babaulti, S. pleurospilus and S. marginatus was performed, the latter species could only be partially separated (not shown). None of the PCs separated S. babaulti from S. pleurospilus and measurements of these species will be investigated later (see S. babaulti versus S. pleurospilus ).
Table 3. PCA on measurements of the inter-specific analysis.
| PC1 | PC2 | PC3 | PC4 | PC5 | |
|---|---|---|---|---|---|
| VAR (%) | 82.6090 | 5.9182 | 2.4578 | 2.2426 | 1.7082 |
| SL | 0.2014 | 0.0383 | 0.0868 | 0.1341 | 0.0318 |
| LaD | 0.2896 | 0.1078 | 0.0133 | -0.3102 | -0.0260 |
| SnL | 0.2775 | 0.1465 | -0.0470 | -0.2431 | 0.0112 |
| LJL | 0.2025 | 0.3823 | 0.0777 | -0.4347 | -0.1361 |
| PPL | 0.2279 | -0.2269 | 0.1024 | -0.4303 | -0.1345 |
| ED | 0.1484 | 0.2124 | -0.0897 | 0.0724 | 0.2817 |
| IOW | 0.3244 | -0.6834 | -0.3457 | -0.0446 | -0.0669 |
| MW | 0.2720 | 0.3373 | -0.2728 | 0.3122 | -0.4225 |
| HL | 0.2092 | 0.1073 | -0.0775 | -0.1547 | 0.0222 |
| BD | 0.2313 | -0.1361 | 0.0751 | -0.0220 | 0.1890 |
| PeL | 0.2377 | -0.0667 | 0.0029 | 0.0670 | 0.6384 |
| ASL | 0.1332 | 0.0836 | 0.2477 | 0.1187 | 0.0269 |
| DFB | 0.2170 | -0.1084 | 0.2758 | 0.1063 | -0.0432 |
| AFB | 0.2243 | -0.1103 | 0.3294 | 0.1788 | -0.3463 |
| PrD | 0.2105 | 0.0817 | 0.0880 | -0.1304 | 0.1239 |
| PrP | 0.1926 | 0.1728 | -0.2777 | 0.1032 | 0.0126 |
| PrV | 0.1917 | 0.0969 | -0.3588 | 0.2725 | 0.1276 |
| PrA | 0.1995 | 0.0541 | -0.1118 | 0.1716 | 0.0667 |
| CPL | 0.1707 | 0.0570 | 0.5292 | 0.2701 | 0.1584 |
| CPD | 0.2201 | -0.1564 | 0.0885 | 0.2395 | -0.2677 |
Loadings and explained variance of the first five PC of a PCA conducted on the covariance matrix of 20 log-transformed measurements taken on 114 specimens.
Fig 4. PCA on measurements of the inter-specific analysis.
PC2 of a PCA on the 20 measurements from 114 specimens belonging to the six Simochromis and Pseudosimochromis species, plotted vs. SL.
The relative measurements of all specimens and the results of the Mann-Whithey U tests are summarised in Table 4. Here as well, values for S. pleurospilus were lumped with those of S. babaulti. Forty-six relative measurements differed significantly between the species. In spite of the absence of significant differences between S. margaretae and the other species, due to the limited number of specimens available, the species’ distinction was obtained directly from the measurements. Indeed, the measurements for the length of the lower jaw (LJL) were smaller and those of the depth of the caudal peduncle (CPD) larger than those measured for all other species. Moreover, while S. margaretae clustered somewhat with P. curvifrons, five other measurements; i.e. the lacrimal depth (LaD), snout length (SnL), premaxillary processus length (PPL), eye diameter (ED) and pre-ventral length (PrV) did not overlap between both species. Simochromis babaulti, S. pleurospilus and S. marginatus could be separated from the other species by their smaller relative inter-orbital with (IOW) (17.3–28.3 vs. 29.1–37.0). The pre-ventral distance separated P. curvifrons from S. diagramma (33.6–37.4 vs. 39.2–43.5). Finally, the caudal peduncle was always longer than deep in S. babaulti, S. pleurospilus and S. marginatus (64.2–97.1%) whereas it was always deeper than long in S. margaretae (108.4–110.9%).
Table 4. Summary of measurements of the different Simochromis and Pseudosimochromis species.
| S. diagramma | P. curvifrons | S. babaulti | S. marginatus | S. margaretae | MWU | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | 9 | 9 | 60 | 34 | 2 | |||||||||||
| min | mean | max | min | mean | max | min | mean | max | min | mean | max | min | mean | max | ||
| SL (mm) | 52.1 | 69.4 | 95.0 | 66.6 | 78.6 | 94.6 | 53.7 | 68.7 | 86.8 | 48.9 | 69.7 | 80.5 | 68.8 | 72.6 | 76.4 | |
| LaD° | 20.1 | 23.0 | 25.3 | 23.5 | 25.5 | 28.8 | 19.9 | 23.0 | 25.7 | 19.9 | 23.0 | 26.5 | 19.5 | 19.8 | 20.1 | c>b,m |
| SnL° | 33.1 | 38.7 | 41.6 | 38.7 | 40.5 | 41.9 | 34.1 | 38.7 | 43.6 | 32.5 | 39.2 | 45.2 | 36.0 | 36.3 | 36.6 | |
| LJL° | 26.5 | 30.1 | 31.2 | 25.2 | 27.5 | 29.9 | 26.0 | 31.4 | 34.3 | 25.4 | 30.1 | 34.9 | 23.2 | 23.2 | 23.2 | d>c; b>m>c |
| PPL° | 25.0 | 28.5 | 30.8 | 30.4 | 32.3 | 34.3 | 21.4 | 26.5 | 29.1 | 23.9 | 26.6 | 29.9 | 24.6 | 25.2 | 25.8 | c>m; c>d >b |
| ED° | 32.2 | 34.3 | 35.5 | 29.7 | 31.0 | 32.4 | 33.5 | 36.0 | 39.9 | 33.1 | 37.4 | 42.7 | 36.4 | 36.8 | 37.2 | m>b>d>c |
| IOW° | 29.1 | 31.2 | 33.7 | 29.2 | 33.5 | 37.0 | 17.3 | 22.8 | 27.5 | 23.0 | 25.7 | 28.3 | 29.3 | 31.6 | 33.9 | d,c>m>b |
| MW° | 29.8 | 34.1 | 39.7 | 26.2 | 29.6 | 34.2 | 27.4 | 33.9 | 41.0 | 28.2 | 32.8 | 40.9 | 32.4 | 33.3 | 34.1 | b,m>c |
| HL* | 30.2 | 31.5 | 33.0 | 28.8 | 31.2 | 33.2 | 29.0 | 30.9 | 35.3 | 28.9 | 30.7 | 34.0 | 31.5 | 31.7 | 31.9 | |
| BD* | 35.4 | 37.5 | 41.5 | 36.7 | 39.3 | 41.8 | 31.0 | 35.9 | 39.5 | 32.5 | 37.7 | 41.0 | 37.7 | 37.7 | 37.8 | c,m>b |
| PectL* | 29.5 | 31.2 | 33.5 | 28.8 | 32.6 | 36.8 | 25.5 | 30.4 | 34.1 | 31.2 | 33.7 | 36.3 | 29.4 | 30.4 | 31.3 | m>d,b |
| ASp* | 13.9 | 15.6 | 17.0 | 12.6 | 14.3 | 15.9 | 13.8 | 15.9 | 18.4 | 13.8 | 15.3 | 17.6 | 16.0 | 16.6 | 17.2 | b>m |
| DFB* | 56.7 | 58.8 | 61.0 | 60.2 | 62.4 | 65.3 | 52.6 | 58.5 | 62.3 | 54.3 | 57.7 | 60.2 | 58.9 | 60.8 | 62.6 | c>d,b,m |
| AFB* | 16.2 | 18.1 | 19.8 | 18.4 | 19.5 | 21.3 | 15.6 | 18.2 | 20.1 | 15.6 | 17.2 | 18.7 | 18.2 | 18.8 | 19.5 | c>b,m |
| PrD* | 34.1 | 36.0 | 37.6 | 34.7 | 36.6 | 39.0 | 32.7 | 36.4 | 40.4 | 33.4 | 37.0 | 40.7 | 36.2 | 36.8 | 37.5 | |
| PrP* | 31.8 | 32.6 | 32.8 | 28.1 | 29.7 | 32.3 | 28.5 | 31.1 | 36.8 | 28.0 | 31.0 | 33.8 | 31.8 | 32.4 | 33.0 | d>c |
| PrV* | 39.2 | 41.5 | 43.5 | 33.6 | 35.6 | 37.4 | 34.2 | 37.5 | 43.8 | 35.2 | 38.9 | 43.9 | 40.1 | 41.6 | 43.1 | d,m>c,b |
| PrA* | 68.1 | 70.4 | 71.2 | 62.9 | 65.7 | 68.7 | 63.4 | 66.7 | 70.7 | 64.6 | 67.9 | 70.7 | 66.7 | 68.9 | 71.2 | d>m>c,b |
| CPL* | 12.2 | 13.8 | 15.5 | 11.6 | 14.1 | 16.1 | 12.8 | 14.5 | 16.1 | 12.1 | 14.2 | 16.7 | 12.3 | 12.3 | 12.4 | |
| CPD* | 11.7 | 12.2 | 12.9 | 11.1 | 12.1 | 12.9 | 9.9 | 11.3 | 12.8 | 10.1 | 11.1 | 12.0 | 13.3 | 13.5 | 13.7 | d>b,m; c>m |
Measurements are indicated as percentage of head ° or standard length *, for the 114 specimens studied. Values for S. pleurospilus are grouped with those of S. babaulti. Results of Mann-Whitney U tests are summarised with d: S. diagramma, c: P. curvifrons, b: S. babaulti and S. pleurospilus and m: S. marginatus.
Colour patterns
In about half of the S. babaulti and S. pleurospilus specimens, one to three anal ocelli were present, both in males and, albeit less frequently, in females. A dark spot on the anteriormost part of the dorsal fin was observed in almost all specimens. This spot was black and clearly visible in most of the males, whereas it ranged from back to light brown in the females. On specimens from the northern and the northeastern shores, the spot measured about one third of the height of the dorsal fin and was equidistant from its proximal and its distal end. In specimens from all other areas this spot was higher and covered the distal two thirds of the anteriormost part of the dorsal fin. Eight to nine lateral bands were visible on the flanks of most specimens.
On S. marginatus, a single ocellus was observed in five of the 34 specimens, all of which were males. The dark edge on the dorsal fin, which gave the species its name [65] was visible as a very narrow edge in specimens from the Kavala Islands (Musinwa), from the extreme north (Luhanga) and from the Burton Bay (Lubumba and Kisokwe) whereas specimens caught at the northern and the eastern side of the Ubwari peninsula (Manga, Cap Banza and Ubwari ‘East’ and ‘West’) had a broad dorsal band that covered the distal third of the dorsal fin and that broadened posteriorly. In the specimens from the eastern shore, a broad band was observed on the male whereas a narrow band was present on the two females. Up to seven lateral bands were counted on the dorsal half of the flanks of some specimens, although, on others, none were visible. The two S. margaretae specimens had a broad band on the distal half of the dorsal fin. Seven vertical bars and two horizontal bands following the lateral lines were visible on the flanks. No ocelli were observed. In P. curvifrons and in S. diagramma, lateral bands and anal ocelli were present on some specimens. Dark spots were observed on the membranes between the dorsal spines but a clear band on the dorsal fin was lacking.
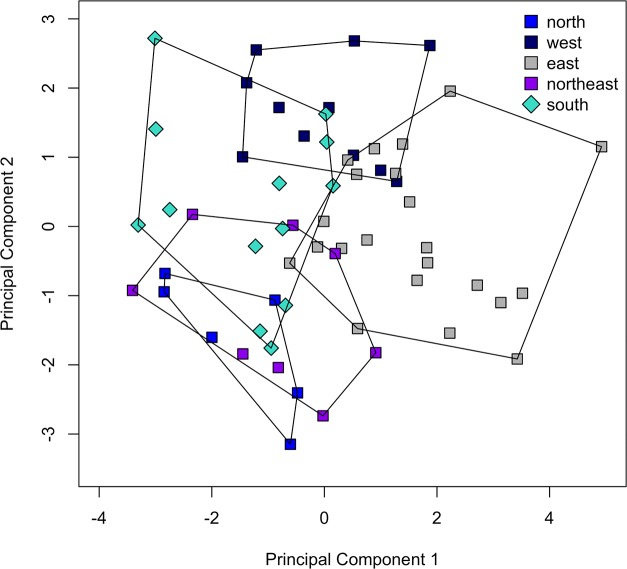
Simochromis babaulti versus S. pleurospilus
Meristics
As no separation could be obtained between S. pleurospilus and S. babaulti in the previous analyses, a subsequent PCA was performed on the two species separately, split up in geographical units (Table 5). The number of soft anal rays was always seven and this variable was omitted from the analysis. On a scatter-plot of the first versus the second PC four partially overlapping groups were identified (Fig 5): a combined northern and northeastern group, a western group, a southern group (S. pleurospilus) and an eastern group. The first two groups were separated by PC2, the latter two, incompletely, by PC1. No separation was observed on PC3, whereas PC4 separated northern and northeastern S. babaulti (not shown). The main contributors for PC1 were the number of lower gill rakes (LGR) and upper lateral line scales (ULL); for PC2, these were the number of dorsal soft rays (DSR), longitudinal line scales (LongL) and caudal vertebrae (CV) and for PC4 these were the number of abdominal vertebrae (AV) and mid-lateral line scales (MLL).
Table 5. PCA on meristics of S. babaulti and S. pleurospilus.
| PC1 | PC2 | PC3 | PC4 | |
|---|---|---|---|---|
| Variance (%) | 21.5600 | 13.4960 | 11.9570 | 9.6323 |
| DSp | 0.3487 | -0.1932 | -0.3144 | 0.0601 |
| DSR | -0.1702 | 0.4863 | 0.0638 | 0.1935 |
| Pect | -0.0918 | -0.1769 | 0.2166 | 0.3860 |
| ULL | 0.4348 | 0.0231 | 0.1153 | -0.2255 |
| MLL | 0.3142 | -0.1228 | 0.1641 | -0.4263 |
| LLL | -0.2332 | 0.3035 | -0.0703 | 0.1341 |
| LongL | 0.2406 | 0.4751 | -0.1916 | 0.1457 |
| IOS | 0.2078 | -0.0618 | 0.3064 | 0.2438 |
| GRU | 0.1943 | 0.1292 | 0.0065 | 0.2848 |
| GRL | -0.3577 | -0.0827 | -0.0106 | -0.2308 |
| BTU | 0.2273 | 0.2512 | 0.3778 | -0.1726 |
| BTL | 0.0246 | 0.2484 | 0.6020 | -0.0574 |
| LatT | -0.2892 | -0.0766 | 0.2854 | 0.0552 |
| AV | 0.3071 | -0.0890 | -0.0186 | 0.5012 |
| CV | 0.0432 | 0.4422 | -0.2955 | -0.2388 |
Loadings and explained variance of the first four PC of a PCA conducted on the correlation matrix of 15 meristics taken on 60 specimens.
Fig 5. PCA on meristics of S. babaulti and S. pleurospilus.
PC2 vs. PC1 of a PCA on the 15 meristics for 60 specimens belonging to four geographical groups of S. babaulti and to S. pleurospilus (the southern group).
Pairwise Mann-Whitney U tests showed that the different geographical groups differed significantly in meristics for 23 pair wise comparisons (Table 6). For all but two of the comparisons between the groups, the northeastern versus the northern and the northeastern versus the southern group, significant differences were found. Only one meristic, the number of upper bicuspid teeth (BTU), differed between the northern and the southern group: i.e. the groups that include the type localities of S. babaulti and S. pleurospilus respectively, whereas all comparisons including the western and the eastern groups differed in at least two of the meristics. Meristic values overlapped between any of the five geographical groups except for the number of scales in the upper lateral line (ULL) (21–22 in the northern vs. 23–24 in the western group), the number of scales in the longitudinal line (LongL) (30.5–31.5 in the northern and the northeastern vs. 32–34 in the western group) and the number of upper bicuspid teeth (BTU) (26–30 in the northern vs. 31–38 in the western group) (not shown). All of the meristics of S. pleurospilus overlapped with those of S. babaulti.
Table 6. Summary of pairwise Mann-Whithey U tests between 5 groups of S. babaulti and S. pleurospilus.
| > | N | NE | E | W | S |
|---|---|---|---|---|---|
| N | / | PeL* | ULL**, MLL*, LongL***, BTU*, BD**, PeL** | ULL**, LongL**, BTU* | BTU*, LaD*, PeL** |
| NE | / | / | DSp*, LongL*** | DSR*, LongL**, CV* | SnL* |
| E | / | LGR* | / | DSR**, PrP*, PrV* | LGR* |
| W | / | BD* | DSp**, PPL*, BD**, DFB** | / | LJL* |
| S | / | ASL* | DSp**, ULL***, LongL*, AV** | ULL*, LongL* | / |
Test were performed on 15 meristics and 20 percentages of measurements (bold) with; N: north, NE: northeast, E: east, W: west and S: south (S. pleurospilus). Values are significantly larger in groups listed in the columns than in the rows, *, ** and *** denote significance at the 0.05, 0.001 and 0.0001 level after Bonferroni correction.
Measurements
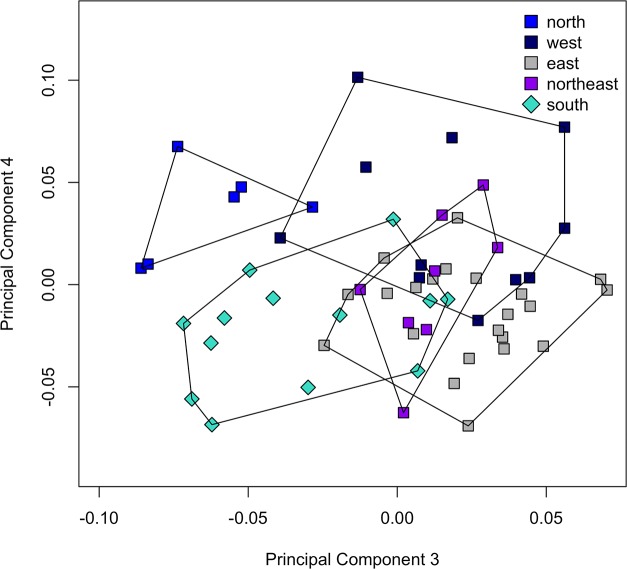
A PCA was performed on the covariance matrix of log-transformed measurements taken on S. babaulti and S. pleurospilus specimens (Table 7). Here, the second PC did not show any pattern (not shown) and a scatter-plot of the fourth versus the third PC was presented (Fig 6). The third PC incompletely separated the northern S. babaulti (including the holotype) from all other S. babaulti groups. Values from the southern group, i.e. S. pleurospilus, overlapped with all of the other groups. The fourth PC incompletely separated S. pleurospilus from the northern and western S. babaulti. Specimens from the northern and western shores had, on average, higher values for this axis than specimens from the eastern and northeastern groups. In spite of what was observed for the meristics, none of the PC allowed for a separation between S. babaulti specimens from the central eastern and northeastern shores. For PC3, explaining 2.23% of the variance, the mouth (MW) and inter-orbital width (IOW) were the main contributors whereas for PC 4, explaining 1.79%, these were the length of the caudal peduncle (CPL) and of the premaxillary processus (PPL). The differences observed in the exploratory analysis were reflected by pair-wise Mann-Whitney U tests (Table 6). These revealed 14 measurements to differ significantly. Measurements differed significantly between all groups except between the northeastern and the eastern groups. Nevertheless, only one measurement, the length of the pectoral fin, could be used to separate any of the groups (25.39–27.75% in the northern vs. 27.45–34.13%SL in all other groups, with values for only one western specimen overlapping). As for the meristics, all measurements (as %) overlapped between S. pleurospilus and S. babaulti.
Table 7. PCA on the measurements of S. babaulti and S. pleurospilus.
| PC1 | PC2 | PC3 | PC4 | |
|---|---|---|---|---|
| VAR (%) | 86.4450 | 4.5187 | 2.2275 | 1.7855 |
| SL | 0.2044 | 0.0546 | 0.0509 | 0.1799 |
| LaD | 0.2853 | -0.2767 | 0.0844 | -0.1820 |
| SnL | 0.2685 | -0.2555 | -0.0200 | -0.0454 |
| LJL | 0.2116 | -0.2855 | 0.0279 | -0.3451 |
| PPL | 0.2013 | 0.0571 | 0.1120 | -0.4067 |
| ED | 0.1600 | -0.1143 | 0.1756 | -0.0152 |
| IOW | 0.2846 | 0.3370 | -0.3976 | -0.1814 |
| MW | 0.3017 | -0.1808 | -0.6190 | 0.1903 |
| HL | 0.2033 | -0.2071 | 0.0971 | -0.0590 |
| BD | 0.2289 | 0.2350 | 0.0674 | -0.3280 |
| PeL | 0.2533 | 0.1329 | 0.3486 | -0.0536 |
| ASL | 0.1383 | 0.1654 | 0.3422 | 0.0870 |
| DFB | 0.2099 | 0.3223 | 0.0250 | 0.0723 |
| AFB | 0.2178 | 0.3144 | -0.1370 | 0.0705 |
| PrD | 0.2053 | -0.0332 | 0.1016 | -0.1620 |
| PrP | 0.2017 | -0.3307 | 0.0501 | 0.2804 |
| PrV | 0.2088 | -0.2270 | 0.0587 | 0.2986 |
| PrA | 0.2007 | -0.0612 | 0.0643 | 0.2257 |
| CPL | 0.1884 | 0.2446 | 0.2814 | 0.4368 |
| CPD | 0.2262 | 0.1991 | -0.1815 | 0.1049 |
Loadings and explained variance of the first four PC of a PCA conducted on the covariance matrix of 20 log-transformed measurements taken on 60 specimens.
Fig 6. PCA on measurements of S. babaulti and S. pleurospilus.
PC4 vs. PC3 of a PCA on the 20 measurements belonging to four geographical groups of S. babaulti and to S. pleurospilus (the southern group).
Host genetics: barcoding
Inter- and intra-specific distances for Simochromis, Pseudosimochromis and other “sediment dwelling” tropheine species are compared in Table 8. Sequences of the commonly used barcoding gene COI show a clear distinction between the within-species and the between-species level for all analyzed cichlids. Simochromis babaulti displays remarkably higher intra-specific genetic variation. Its difference with S. pleurospilus is of the same order of magnitude as the genetic distances within S. babaulti. As mitochondrial markers are not reliable to infer interspecific historical relationships in these fishes [39], these barcoding data are only used to compare genetic distances within and between species, without an attempt at phylogenetic reconstruction.
Table 8. Barcoding results for Pseudosimochromis, Simochromis and the other “sediment dwelling” tropheine genera.
| intraspecific | ‘C.’ horei | ‘G.’ pfefferi | L. dardennii | P. curvifrons | S. babaulti | S. diagramma | S. marginatus | |
|---|---|---|---|---|---|---|---|---|
| ‘C.’ horei | 0.6–1.4 | |||||||
| ‘G.’ pfefferi | 0–1.3 | 7.1–10.1 | ||||||
| L. dardennii | 0.3 | 1.9–2.7 | 5.9–7.1 | |||||
| P. curvifrons | 0.2 | 3.4–4.2 | 7.5–9.2 | 3.2–3.5 | ||||
| S. babaulti | 0–3.8 | 3.5–5.5 | 7.6–9.8 | 3.3–4.4 | 2.9–4.4 | |||
| S. diagramma | 0.2–0.8 | 4.4–5.7 | 8.5–10.1 | 3.9–5.2 | 5.3–6.4 | 4.3–6.3 | ||
| S. marginatus | 0–0.5 | 4.6–6.9 | 9.4–11.3 | 4.7–5.7 | 6.1–7.0 | 5.7–7.6 | 6.3–7.9 | |
| S. pleurospilus | 0 | 4.9–5.8 | 9.7–10.2 | 4.7 | 4.4–4.7 | 0.7–4.0 | 5.8–6.4 | 7.3–7.9 |
Comparison of intra- (left column) and inter-specific (minimum-maximum) gamma-corrected pairwise genetic distances (in %) for COI.
Parasitology
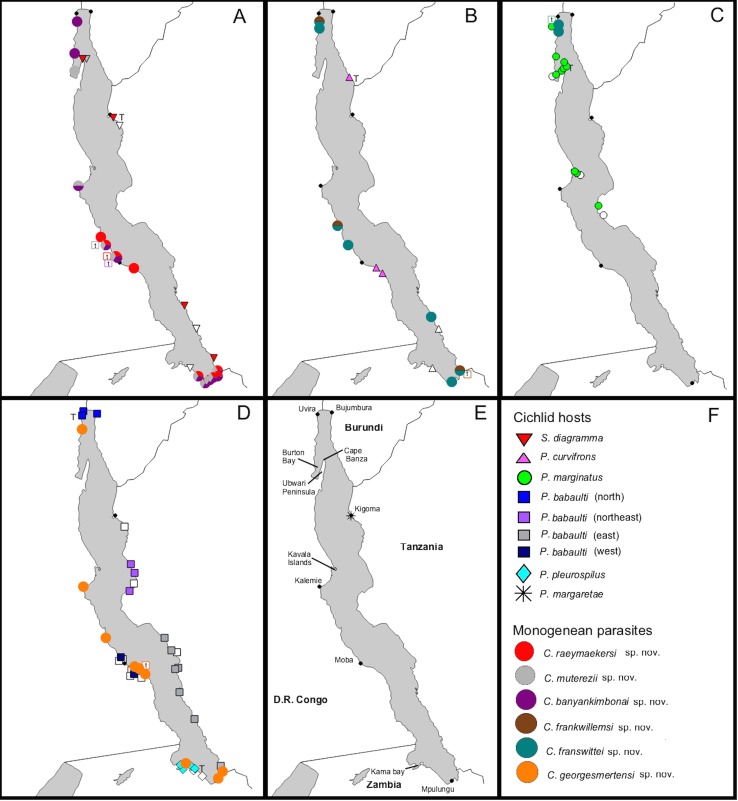
Monogeneans were collected from the gills of S. diagramma, S. babaulti, S. pleurospilus, S. marginatus and P. curvifrons. Investigation of the two S. margaretae paratypes did not yield any monogenean gill parasites. All monogeneans belong to Cichlidogyrus Paperna, 1960 (sensu [83] and [84]): Ancyrocephalidae (but see Introduction), and are new to science; six species are described below (Figs 7 and 8; Table 9). Note that the authors of the new taxa are different from the authors of this paper; Article 50.1 and Recommendation 50A of the International Code of Zoological Nomenclature.
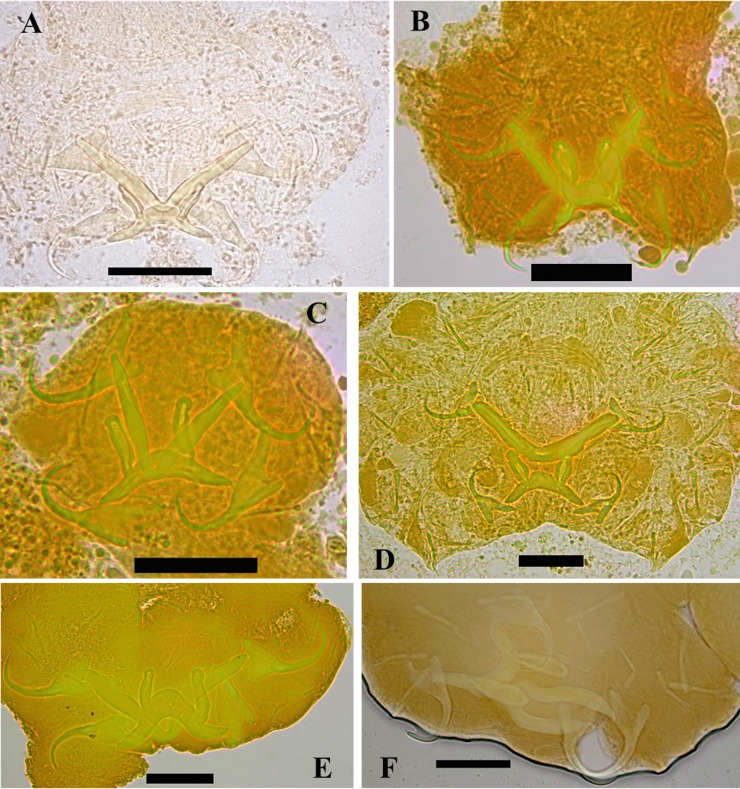
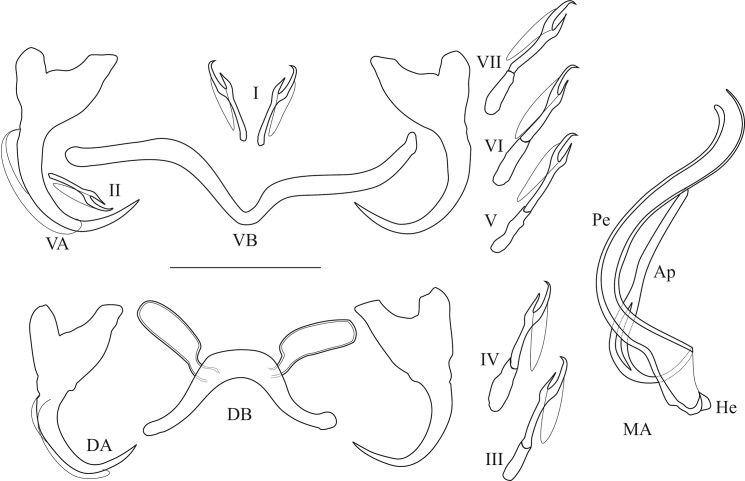
Fig 7. Haptoral morphology of the newly described Cichlidogyrus species infecting Simochromis and Pseudosimochromis cichlids.
Micrographs depict the attachment organ of A. C. raeymaekersi sp. nov. B. C. muterezii sp. nov. C. C. banyankimbonai sp. nov. D. C. georgesmertensi sp. nov. E. C. franswittei sp. nov. F. C. frankwillemsi sp. nov. Scale bar = 20 μm (A, F) or 30 μm (B, C, D, E).
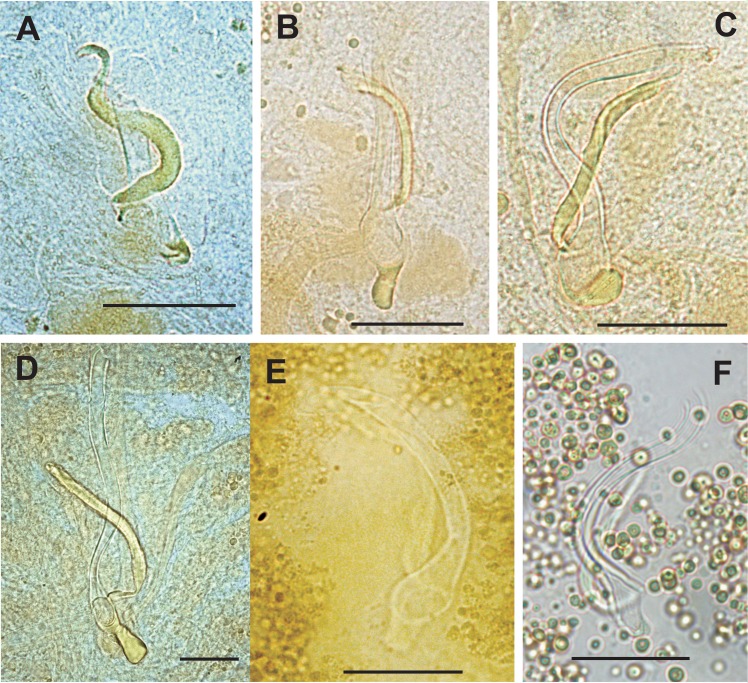
Fig 8. Male genital morphology of the newly described Cichlidogyrus species infecting Simochromis and Pseudosimochromis cichlids.
Micrographs depict the male copulatory organ of A. C. raeymaekersi sp. nov. B. C. muterezii sp. nov. C. C. banyankimbonai sp. nov. D. C. georgesmertensi sp. nov. E. C. franswittei sp. nov. F. C. frankwillemsi sp. nov. Scale bar = 20 μm.
Table 9. Measurements of Cichlidogyrus species infecting Simochromis and Pseudosimochromis species.
| C. raeymaekersi sp. nov. | C. muterezii sp. nov. | C. banyankimbonai sp. nov. | C. georgesmertensi sp. nov. | C. franswittei sp. nov. | C. frankwillemsi sp. nov. | ||
|---|---|---|---|---|---|---|---|
| AS | L | 628 ± 86.8 (430.1–812.9) 25 | 536 ± 149.4 (354.2–777.4) 11 | 516 ± 150.6 (304.1–686.1) 9 | 1111 ± 202.6 (744.3–1520.2) 25 | 388 ± 47.0 (307.5–509.9) 20 | 307 ± 66.9 (171.3–406.8) 13 |
| W | 124.4 ± 17.5 (91.4–156.9) 25 | 122.8 ± 25.5 (84.6–159.9) 11 | 121.8 ± 36.4 (67.6–164.3) 10 | 231.8 ± 40.6 (165.0–309.2) 25 | 84.2 ± 19.4 (55.4–140.4) 20 | 100.4 ± 33.4 (68.0–182.4) 13 | |
| Ph | W | 38.4 ± 4.2 (30.8–47.1) 25 | 59.3 ± 7.7 (47.3–71.4) 12 | 42.8 ± 6.3 (32.3–49.3) 11 | 72.9 ± 13.0 (37.3–92.0) 25 | 26.5 ± 4.3 (20.8–36.9) 20 | 28.1 ± 7.8 (20.3–47.1) 11 |
| DA | a | 39.1 ± 5.7 (29.9–46.4) 50 | 27.5 ± 1.6 (24.2–30.6) 22 | 30.4 ± 2.5 (25.9–35.1) 22 | 28.3 ± 2.2 (19.1–32.7) 49 | 35.1 ± 2.5 (30.8–39.5) 30 | 22.1 ± 0.7 (20.5–23.4) 27 |
| b | 27.7 ± 3.9 (20.3–33.1) 49 | 22.0 ± 1.2 (19.4–24.1) 22 | 22.7 ± 2.1 (18.8–26.6) 22 | 20.2 ± 1.2 (17.7–22.9) 48 | 24.7 ± 1.5 (22.2–28.7) 30 | 18.8 ± 0.7 (17.4–20.0) 27 | |
| c | 3.4 ± 0.9 (1.7–5.1) 49 | 3.8 ± 1.1 (2.1–5.8) 22 | 3.3 ± 0.6 (2.4–4.7) 22 | 5.2 ± 0.8 (3.6–7.0) 48 | 4.0 ± 0.7 (2.5–5.2) 30 | 4.0 ± 0.6 (3.2–5.1) 27 | |
| d | 14.3 ± 2.1 (10.2–18.0) 49 | 8.9 ± 1.2 (6.7–11.8) 22 | 11.0 ± 1.3 (9.2–13.9) 22 | 11.3 ± 1.4 (8.6–15.4) 49 | 14.2 ± 1.9 (9.3–17.2) 30 | 7.5 ± 0.6 (6.2–8.8) 27 | |
| e | 10.7 ± 1.1 (8.4–13.4) 50 | 7.8 ± 0.9 (6.2–10.1) 22 | 9.0 ± 0.8 (6.4–10.1) 22 | 7.5 ± 0.7 (6.2–9.5) 49 | 10.1 ± 1.2 (7.8–12.2) 30 | 7.7 ± 0.8 (5.9–10.2) 27 | |
| DB | x | 37.6 ± 5.1 (29.3–46.0) 25 | 30.0 ± 3.8 (23.7–36.6) 12 | 35.3 ± 3.3 (30.1–41.5) 12 | 36.5 ± 3.3 (30.6–44.4) 25 | 33.9 ± 4.5 (23.2–40.8) 20 | 23.9 ± 3.6 (19.7–29.1) 8 |
| y | 12.7 ± 2.6 (9.0–19.0) 25 | 11.0 ± 1.5 (9.5–14.8) 12 | 10.7 ± 1.6 (8.8–13.5) 12 | 12.5 ± 1.2 (10.4–15.0) 24 | 12.3 ± 2.4 (7.9–16.9) 20 | 8.8 ± 1.6 (7.0–11.0) 10 | |
| w | 8.8 ± 2.1 (5.0–12.2) 25 | 6.4 ± 1.0 (4.1–7.7) 12 | 7.1 ± 1.1 (5.7–9.3) 12 | 6.6 ± 0.9 (5.0–8.0) 25 | 6.4 ± 1.1 (4.1–8.9) 20 | 4.1 ± 0.9 (3.0–5.4) 10 | |
| h | 13.8 ± 2.2 (9.6–18.5) 49 | 16.0 ± 2.5 (12.5–22.9) 24 | 15.5 ± 2.9 (10.5–20.9) 24 | 15.2 ± 2.1 (8.5–20.1) 48 | 12.3 ± 1.3 (9.5–15.0) 33 | 11.8 ± 1.5 (8.8–14.0) 19 | |
| VA | a | 31.8 ± 3.2 (26.1–36.2) 50 | 29.5 ± 1.0 (27.3–31.5) 20 | 31.2 ± 2.2 (28.0–35.6) 24 | 27.9 ± 1.4 (24.5–30.5) 50 | 32.0 ± 1.7 (28.3–35.5) 31 | 23.7 ± 0.7 (22.0–24.6) 26 |
| b | 30.5 ± 3.3 (24.3–35.4) 49 | 25.8 ± 1.0 (23.5–28.3) 20 | 25.6 ± 1.9 (22.7–28.9) 24 | 23.9 ± 1.4 (20.8–27.2) 50 | 28.9 ± 1.4 (26.5–31.5) 31 | 21.5 ± 0.8 (19.6–23.3) 26 | |
| c | 3.7 ± 1.1 (1.8–6.2) 49 | 4.0 ± 0.9 (2.4–5.6) 20 | 3.6 ± 0.8 (2.1–5.0) 24 | 5.7 ± 0.9 (3.4–7.7) 50 | 4.3 ± 0.7 (2.8–5.5) 31 | 3.4 ± 0.5 (2.6–4.2) 26 | |
| d | 9.3 ± 1.6 (5.7–13.9) 49 | 8.2 ± 1.2 (5.9–10.7) 20 | 9.9 ± 1.3 (7.7–13.0) 24 | 9.1 ± 1.1 (6.4–11.5) 50 | 10.1 ± 1.3 (8.0–12.7) 31 | 7.5 ± 0.7 (6.1–9.1) 26 | |
| e | 11.2 ± 0.9 (9.5–13.3) 50 | 9.7 ± 0.9 (7.4–11.0) 20 | 10.6 ± 0.7 (8.7–11.7) 24 | 8.7 ± 0.8 (6.7–10.1) 50 | 12.0 ± 1.2 (9.2–14.4) 31 | 8.9 ± 1.1 (4.5–10.1) 26 | |
| VB | x | 38.8 ± 5.0 (30.0–47.2) 50 | 32.7 ± 2.7 (27.8–38.0) 24 | 33.9 ± 3.0 (30.1–40.5) 24 | 40.4 ± 2.9 (33.6–46.3) 48 | 33.5 ± 3.0 (28.9–42.4) 34 | 26.4 ± 2.4 (22.2–29.6) 17 |
| w | 7.5 ± 1.4 (4.9–10.1) 25 | 5.7 ± 0.9 (3.8–6.7) 12 | 5.8 ± 0.9 (4.8–7.4) 12 | 6.4 ± 0.9 (5.1–8.8) 25 | 5.6 ± 0.6 (4.5–6.9) 18 | 3.1 ± 0.6 (2.5–4.0) 9 | |
| U | I | 11.9 ± 0.4 (11.2–12.9) 50 | 12.5 ± 0.6 (10.7–13.2) 20 | 12.3 ± 0.5 (11.6–13.7) 23 | 11.9 ± 0.9 (10.1–14.4) 47 | 11.9 ± 0.6 (11.0–13.6) 36 | 11.4 ± 0.6 (10.2–12.8) 19 |
| II | 10.7 ± 0.3 (10.1–11.9) 47 | 11.7 ± 0.4 (10.8–12.4) 16 | 11.5 ± 0.3 (11.0–12.1) 17 | 11.2 ± 0.7 (9.4–12.6) 44 | 10.6 ± 1.0 (8.9–11.7) 11 | 10.7 ± 0.4 (10.0–11.3) 16 | |
| III-VII | 16.3 ± 2.0 (12.1–20.9) 229 | 22.5 ± 1.6 (19.2–27.1) 101 | 15.9 ± 2.3 (12.1–20.8) 101 | 21.7 ± 2.1 (15.6–27.2) 205 | 18.2 ± 2.6 (13.4–25.0) 95 | 18.1 ± 1.4 (15.2–22.4) 103 | |
| MA | He | 2.3 ± 0.6 (1.3–4.0) 25 | 7.0 ± 1.2 (5.1–8.4) 12 | 4.1 ± 1.0 (2.1–5.7) 12 | 11.3 ± 2.6 (7.1–15.8) 25 | 5.4 ± 1.5 (3.1–9.1) 20 | 0.9 ± 0.7 (0.0–1.8) 16 |
| Pe | 29.3 ± 1.4 (27.2–32.4) 25 | 42.4 ± 4.6 (37.5–50.3) 12 | 58.7 ± 3.5 (54.1–65.1) 12 | 92.2 ± 2.6 (88.0–96.9) 25 | 53.5 ± 2.4 (47.1–57.0) 20 | 49.2 ± 3.1 (43.7–54.6) 16 | |
| Ap | 25.5 ± 1.5 (23.2–30.0) 25 | 29.5 ± 3.0 (24.4–33.3) 12 | 33.5 ± 1.5 (30.7–35.5) 12 | 60.7 ± 2.7 (53.1–64.8) 25 | 36.0 ± 3.6 (30.7–46.1) 20 | 31.3 ± 1.7 (28.2–34.6) 16 |
Summary of measurments [average ± standard deviation (minimum, maximum) all in μm], with AS: Adult size, L: length, W: width, Ph: Pharynx, DA: dorsal anchor, DB: dorsal bar, VA: ventral anchor, VB: ventral bar, U: uniculi and MA: male apparatus, other abbrevations as in Fig 1, subscripts denote the number of specimens examined.
Species Descriptions
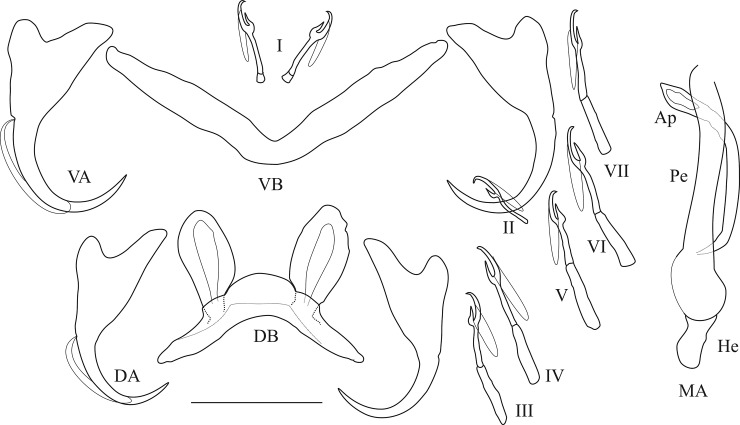
Cichlidogyrus raeymaekersi Pariselle & Vanhove sp. nov. (Figs 7A, 8A and 9; Table 9)
Fig 9. Haptoral and genital hard parts of Cichlidogyrus raeymaekersi sp. nov.
(Ap) accessory piece (DB) dorsal transverse bar, (DA) dorsal anchor, (He) heel, (MA) male apparatus, (Pe) penis, (VB) = ventral transverse bar, (VA) ventral anchor, (I) to (VII) uncinuli, scale bar = 20 μm.
urn:lsid:zoobank.org:act:DF18D90F-7103-496C-ADF8-3C23AE5922C2
Type host: Simochromis diagramma (Günther, 1894)
Infection site: Gills.
Type locality: Mukamba, D.R.Congo (6° 57’ S 29° 43’ E) (April 16th, 2010, on MRAC B0-12-P-659 and -662)
Other localities: Kabulu, D.R.Congo (6° 40’ S, 29° 30’ E) (April 21st, 2010); Kalambo Lodge, Zambia (8° 37’ S 31° 12’ E) (April 18th, 2008, on B2-4-P-58-60 (1), B2-4-P-61-64 (2), August 29th— 31st, 2011, on B1-23-P-376-410); Kapakwe, D.R.Congo (6° 58’ S 29° 44’ E) (April 17th, 2010, on MRAC B0-12-P-674 and -677); Katoto, Zambia (08° 48’ S 31° 01' E) (September 12th, 15th, 2011, on MRAC B1-23-P-479-496); Kyanza, D.R.Congo (7° 7’ S 29° 59’) (April 19th, 2010); Mbita Island, Zambia (08° 45’ S 31° 05’ E) (April 10th, 2008, on B2-4-P-114-115; September 9th, 15th, 2011, on B1-23-P-444-460); Mugayo, D.R.Congo (6° 47’ S 29° 34’ E) (April 11th, 2010, on MRAC B0-12-P-357); Muzumwa, Zambia (08° 42’ S 31° 12’ E) (September 3rd, 2011, on B1-23-411-443); Tumbi, Zambia (08° 42’ S 30° 55’ E) (August 25th, 2011 B1-23-P-324-375); Wonzye Point, Zambia (8° 43’ S 31° 08’ E) (August 23rd, 2011, on MRAC B1-23-P-517-549).
Material studied: 25 individuals (21 from Mukamba, 2 from Kapakwe and 2 from Mugayo).
Type material: holotype: MRAC 37757 (1, Mukamba); paratypes: MRAC 37757 (4, Mukamba); MRAC 37756 (5, Mukamba), MNHN HEL423 (4, Mukamba), SAMCTA 61807 (2, Kapakwe).
Etymology: The name C. raeymaekersi sp. nov. is given in honour of Dr. Joost A.M. Raeymaekers (Belgium) for his research on the fish hosts and his help in our work.
Description: Haptor: dorsal anchor with short shaft, long guard and arched blade; dorsal transverse bar thick and arched; ventral anchor with marked shaft and guard; ventral transverse bar V-shaped and thick, uncinuli I small (sensu [49], i.e. relative to the length of uncinuli II, the pair which retains its larval length [85]), uncinuli III to VII short (sensu [85]). Penis, beginning in an elongated bulb, with short heel, is a short, straight and wide tube with a bevelled ending. Accessory piece simple and spirally coiled, winds around penis (1.5 turns) and attached by thin filament to distal extremity of basal bulb. No sclerotised vagina observed.
Comments: Cichlidogyrus raeymaekersi sp. nov. belongs to the group with short uncinuli I and III to VII (sensu [49]). This group includes: C. acerbus Dossou, 1982; C. amieti Birgi & Euzet, 1983; C. amphoratus Pariselle & Euzet, 1995; C. berrebii Pariselle & Euzet, 1994; C. bifurcatus Paperna, 1960; C. cirratus Paperna, 1964; C. cubitus Dossou, 1982; C. fontanai Pariselle & Euzet, 1997; C. gillardinae Muterezi Bukinga, Vanhove, Van Steenberge & Pariselle, 2012; C. giostrai Pariselle, Bilong Bilong & Euzet, 2003; C. gistelincki Gillardin, Vanhove, Pariselle, Huyse & Volckaert, 2011; C. haplochromii Paperna & Thurston, 1969; C. irenae Gillardin, Vanhove, Pariselle, Huyse & Volckaert, 2011; C. karibae Douëllou, 1993; C. lagoonaris Paperna, 1969; C. levequei Pariselle & Euzet, 1996; C. longipenis Paperna & Thurston, 1969; C. louipaysani Pariselle & Euzet, 1994; C. makasai Vanhove, Volckaert & Pariselle, 2011; C. mbirizei Muterezi Bukinga, Vanhove, Van Steenberge & Pariselle, 2012; C. mulimbwai Muterezi Bukinga, Vanhove, Van Steenberge & Pariselle, 2012; C. nageus Řehulková, Mendlová & Šimková, 2013; C. njinei Pariselle, Bilong Bilong & Euzet, 2003; C. nyongensis Pariselle, Bitja Nyom & Bilong Bilong, 2013; C. ornatus Pariselle & Euzet, 1995; C. pouyaudi Pariselle & Euzet, 1994; C. rognoni Pariselle, Bilong Bilong & Euzet, 2003; C. sanjeani Pariselle & Euzet, 1997; C. sclerosus Paperna & Thurston, 1969; C. slembroucki Pariselle & Euzet, 1998; C. steenbergei Gillardin, Vanhove, Pariselle, Huyse & Volckaert, 2011; C. sturmbaueri Vanhove, Volckaert & Pariselle, 2011; C. tilapiae Paperna, 1960, C. vandekerkhovei Vanhove, Volckaert & Pariselle and C. zambezensis Douëllou, 1993. Cichlidogyrus raeymaekersi sp. nov. can easily be distinguished from all these species by the shape of its penis and associated accessory piece (short, straight and wide; spirally coiled (1.5 turns), winds around the penis, attached to the basal bulb, respectively). Only C. reversati Pariselle & Euzet, 2003 resembles C. raeymaekersi sp. nov. by the S-shaped accessory piece (coiled, winds around the penis, attached), but they can be distinguished by the shape of the ending of the penis (bevelled in C. raeymaekersi sp. nov. versus folded in C. reversati) and the size of the uncinuli pair I (short in C. raeymaekersi sp. nov. versus large in C. reversati). Cichlidogyrus raeymaekersi sp. nov. was previously recorded as Cichlidogyrus sp. 1 in [86].
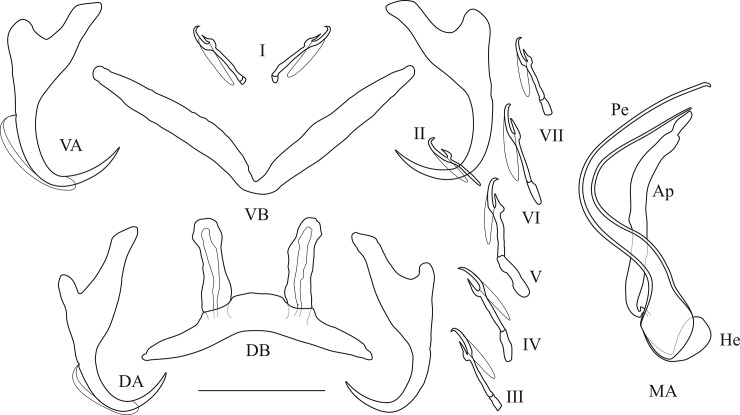
Cichlidogyrus muterezii Pariselle & Vanhove sp. nov. (Figs 7B, 8B and 10; Table 9)
Fig 10. Haptoral and genital hard parts of Cichlidogyrus muterezii sp. nov.
(Ap) accessory piece (DB) dorsal transverse bar, (DA) dorsal anchor, (He) heel, (MA) male apparatus, (Pe) penis, (VB) = ventral transverse bar, (VA) ventral anchor, (I) to (VII) uncinuli, scale bar = 20 μm.
urn:lsid:zoobank.org:act:155B5110-BB2E-4314-9F3C-EA47444DAFC9
Type host: Simochromis diagramma (Günther, 1894).
Infection site: Gills.
Type locality: Mugayo, D.R.Congo (6° 47’ S 29° 33’ E) (April 11th, 2010, on MRAC B0-12-P-357).
Other localities: Wonzye Point, Zambia (8° 43’ S 31° 08’ E) (August 23rd, 2011, on MRAC B1-23-P-517-549); Mbita Island, Zambia (08° 45’ S 31° 05’ E) (September 9th, 15th, 2011, on B1-23-P-444-460); Kalemie, D.R.Congo (5° 55’ S 29° 12’ E) (April 23rd, 2010, on MRAC B1-12-P-1096); Kisokwe, D.R.Congo (4° 15’ S, 29° 11’ E) (March 23rd, 2010, on B0-12-P-966-967 (1)); Mukamba, D.R.Congo (6° 57’ S 29° 43’ E) (April 16th, 2010, on B0-12-P-659); Kapakwe, D.R.Congo (6° 53’ S 29° 44’ E) (April 17th, 2010, on MRAC B0-12-P-674); Kalambo Lodge, Zambia (8° 37’ S 31° 12’ E) (April 18th, 2008, on B2-4-P-58-60 (1), August 30th, 2011, on B1-23-P-376-410); Katoto, Zambia (08° 48’ S 31° 01' E) (September 12th, 15th, 2011, MRAC B1-23-P-479-496); Tumbi, Zambia (08° 42’ S 30° 55’ E) (August 25th, 2011 on B1-23-P-342-375); Muzumwa, Zambia (08° 42’ S 31° 12’ E) (September 3rd, 2011, on B1-23-411-443).
Material studied: 12 individuals (1 from Mugayo, 2 from Mukamba, 2 from Wonzye, 1 from Kalambo, 2 from Katoto and 4 from Muzumwa).
Type material: holotype: MRAC 37755 (1, Mugayo); paratypes: MRAC 37754 (1, Katoto); MNHN HEL422 (1, Mukamba).
Etymology: The name is given in honour of biologist Fidel Muterezi Bukinga (D.R. Congo), who studies the monogenean fauna of Lake Tanganyika cichlids, for his help in our research.
Description: Haptor: dorsal anchor with marked shaft, long guard and arched blade; dorsal transverse bar regularly arched with large auricles; ventral anchor with marked shaft and guard; ventral transverse bar V-shaped; uncinuli I small (sensu [49]), uncinuli III to VII of medium size. Penis, beginning in an oval bulb, with well-developed heel, is a straight and large tube. Accessory piece simple, curved and attached to the basal bulb. No sclerotised vagina observed.
Comments: Cichlidogyrus muterezii sp. nov. belongs to the same group as C. raeymaekersi sp. nov. The former can be distinguished from all the species within this group by the short C-shaped accessory piece and the well-developed heel associated with a straight and large penis. Cichlidogyrus muterezii sp. nov. was previously recorded as Cichlidogyrus sp. 2 in [86].
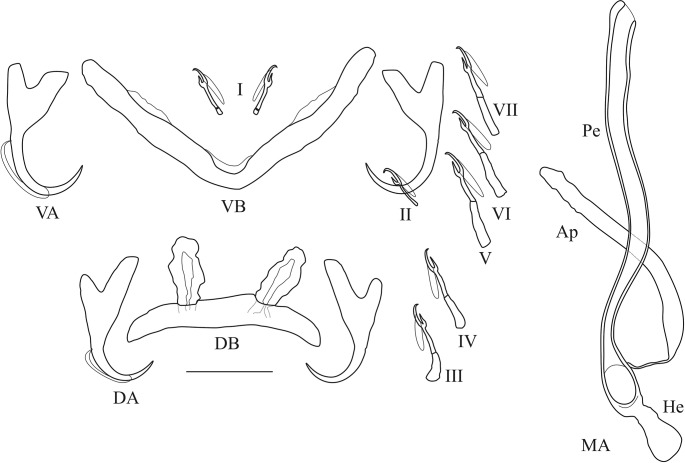
Cichlidogyrus banyankimbonai Pariselle & Vanhove sp. nov. (Figs 7C, 8C and 11; Table 9)
Fig 11. Haptoral and genital hard parts of Cichlidogyrus banyankimbonai sp. nov.
(Ap) accessory piece (DB) dorsal transverse bar, (DA) dorsal anchor, (He) heel, (MA) male apparatus, (Pe) penis, (VB) = ventral transverse bar, (VA) ventral anchor, (I) to (VII) uncinuli, scale bar = 20 μm.
urn:lsid:zoobank.org:act:E9C78A9A-7390-4904-81EE-D2B45AEFABEB
Type host: Simochromis diagramma (Günther, 1894).
Infection site: Gills.
Type locality: Mukamba, D.R.Congo (6° 57’ S 29° 43’ E) (April 16th, 2010, on MRAC B0-12-P-659 and -662).
Other localities: Kalemie, D.R.Congo (5° 55’ S 29° 12’ E) (April 23rd, 2010, on B0-12-P-1096); Lubumba, D.R.Congo (3° 59’ S, 29° 7’ E) (March 24th, 2010, on B0-12-P-621-625 (1)); Luhanga, D.R.Congo (3° 31’ S, 29° 9’ E) (March 27th, 2010, on B0-12-P-970); Mukamba, D.R.Congo (6° 57’ S 29° 43’ E) (April 16th, 2010, on MRAC B0-12-P-659); Mugayo, D.R.Congo (6° 47’ S 29° 33’ E) (April 11th, 2010, on MRAC B0-12-P-356); Kalambo Lodge, Zambia (8° 37’ S 31° 12’ E) (August 29th— 31st, 2011 on B1-23-P-376-410); Katoto, Zambia (08° 48’ S 31° 01' E) (September 12th, 15th, 2011, MRAC B1-23-P-479-496); Mbita Island, Zambia (08° 45’ S 31° 05’ E) (September 9th, 15th, 2011, on B1-23-P-444-460); Muzumwa, Zambia (08° 42’ S 31° 12’ E) (September 3rd, 2011, on B1-23-411-443); Tumbi, Zambia (08° 42’ S 30° 55’ E) (August 25th, 2011 on B1-23-P-342-375); Wonzye Point, Zambia (8° 43’ S 31° 08’ E) (August 23rd, 2011, on MRAC B1-23-P-517-549).
Material studied: 12 individuals, (3 from Mukamba, 2 from Katoto, 2 from Mbita, 1 from Wonzye, 2 from Tumbi and 2 from Kalambo Lodge).
Type material: holotype: MRAC 37753 (1, Mukamba); paratypes: MRAC 37752 (1, Mukamba); MNHN HEL421 (1, Mukamba).
Etymology: The name is given in honour of biologist Dr. Gaspard Caporal Banyankimbona (Burundi), a former office mate of the authors in Tervuren and Leuven, for his contributions to our research and to Burundese and African ichthyology.
Description: Haptor: dorsal anchor with marked shaft, long guard and arched blade; dorsal transverse bar slightly arched; ventral anchor with marked shaft and guard; ventral transverse bar V-shaped; uncinuli I small (sensu [85]), uncinuli III to VII short (sensu [49]). Penis, beginning in an oval bulb, the proximal end of which is covered by wide and short heel, is a large C-shaped tube with thick walls and constant diameter. Accessory piece long, simple and slightly curved, shorter than penis and ending in slightly constricted portion. Accessory piece attached by its thin beginning to the basal bulb. No sclerotized vagina observed.
Comments: Cichlidogyrus banyankimbonai sp. nov. belongs to the same group as C. raeymaekersi sp. nov. and C. muterezii sp. nov. It can be distinguished from the other species in this group by the C-shaped and large penis, the short heel covering the basal bulb’s proximal extremity, and the simple accessory piece. It resembles C. muterezii sp. nov., but can be distinguished by the length of the penis (59 versus 42), the length of uncinuli pairs III to VII (16 versus 23) and the length and shape of the heel (4 versus 7, well-developed versus only covering the bulb’s extremity). The only other Cichlidogyrus species in this group that also have a wide and C-shaped penis and a simple accessory piece that is shorter than the penis and narrows down towards the distal extremity are C. gillardinae, C. irenae and C. steenbergei. These species infect other tropheine (C. irenae and C. steenbergei) or haplochromine (C. gillardinae) cichlids of Lake Tanganyika. Cichlidogyrus banyankimbonai sp. nov. can be distinguished from C. gillardinae by the length and the thickness of the wall of the penis (59 and thick in C. banyankimbonai sp. nov. versus 47 and thin in C. gillardinae), from C. irenae by the length and the diameter of the penis (59 and wide in C. banyankimbonai sp. nov. versus 69.5 and very large with a swollen portion in C. irenae) and from C. steenbergei by the length, the thickness of the wall and the diameter of the penis and the surface of its basal bulb (59, thick, wide and smooth in C. banyankimbonai sp. nov. versus 63, thin, very large and striated in C. steenbergei).
Cichlidogyrus banyankimbonai sp. nov. was previously recorded as Cichlidogyrus sp. 3 in [86].
Cichlidogyrus georgesmertensi Pariselle & Vanhove sp. nov. (Figs 7D, 8D and 12; Table 9)
Fig 12. Haptoral and genital hard parts of Cichlidogyrus georgesmertensi sp. nov.
(Ap) accessory piece (DB) dorsal transverse bar, (DA) dorsal anchor, (He) heel, (MA) male apparatus, (Pe) penis, (VB) = ventral transverse bar, (VA) ventral anchor, (I) to (VII) uncinuli, scale bar = 20 μm.
urn:lsid:zoobank.org:act:A8C8782C-F8F5-4003-A076-460B0897B3B3
Type host: Simochromis babaulti (Pellegrin, 1927).
Other host: Simochromis pleurospilus Nelissen, 1978.
Infection site: Gills.
Type locality: Kyanza, D.R.Congo (7° 07’ S 29° 59’ E) (April 19th, 2010, on MRAC B0-12-P-409, S. babaulti)
Other localities: On S. babaulti: Bemba, D.R.Congo (3° 37’ S 29° 09’ E) (March 26th, 2010, on B0-12-P-846); Lukuga outflow, Kalemie, D.R.Congo (5° 55’ S 29° 11’ E) (April 24th, 2010, on B0-12-P-1089); Kabulu, D.R.Congo (6° 40’ S, 29° 30’ E) (April 21st, 2010, on B0-12-P-849-850); Kikoti, D.R.Congo (7° 11’ S 30° 04’ E) (April 20th, 2010, on B0-12-P-427); Mufazi, D.R.Congo (7° 05’ S 29° 55’ E) (April 13th, 2010, on B0-12-P-816-829 (1)). On S. pleurospilus: Kalambo Lodge, Zambia (8°37’ S 31° 12’ E) (April 16th, 2008 on B2-04-P-70-79 (1)); Kama Bay, Zambia (8° 30’ S 30° 40’ E) (January 6th, 1976, on MRAC 76-4-P-464-465, paratypes of S. pleurospilus); Wonzye Point, Zambia (08° 44’ S 31° 08’ E) (April 12th, 2008 on B2-04-P-69).
Material studied: 25 individuals, all from type locality.
Type material: holotype: MRAC 37751 (1, Kyanza); paratypes: MRAC 37751 (4, Kyanza), MRAC 37750 (5, Kyanza), MNHN HEL420 (5, Kyanza), SAMCTA 61808 (5, Kyanza).
Etymology: The species epithet, georgesmertensi, honours linguist Georges Mertens (Belgium), former Kiswahili lecturer to M.P.M.V. and M.V.S., for his contributions to spreading the knowledge of this language.
Description: Haptor: dorsal anchor with marked shaft, long guard and arched blade; dorsal transverse bar slightly arched; ventral anchor with marked shaft and guard; ventral transverse bar V-shaped; uncinuli I small (sensu [85]), uncinuli III to VII of medium size (sensu [85]). Penis, beginning in an oval bulb, with well-developed and club-shaped heel is a long and slightly sinuous tube, of which diameter is larger at the extremity than at the beginning and with thick walls. Accessory piece simple and slightly curved and attached by a filament to basal bulb. No sclerotized vagina observed.
Comments: Cichlidogyrus georgesmertensi sp. nov. belongs to the same group as C. raeymaekersi sp. nov., C. muterezii sp. nov. and C. banyankimbonai sp. nov. It can be distinguished from all the species in this group by the length and diameter of the penis (long and thick walled) and that of the heel (club-shaped and long). The host specimen investigated from Kama Bay also harboured other monogenean gill parasites not identified to species level (belonging to Gyrodactylus and Cichlidogyrus). Its C. georgesmertensi sp. nov. displayed a MA and haptoral connective bars that are larger than in the type series. Both anomalies are not further considered here since the host individual, which was collected by Brichard [66] could have been kept in captivity together with other fish species, a condition that could have influenced monogenean development and host range (e.g. [87]).
Cichlidogyrus franswittei Pariselle and Vanhove sp. nov. (Figs 7E, 8F and 13; Table 9)
Fig 13. Haptoral and genital hard parts of Cichlidogyrus franswittei sp. nov.
(Ap) accessory piece (DB) dorsal transverse bar, (DA) dorsal anchor, (He) heel, (MA) male apparatus, (Pe) penis, (VB) = ventral transverse bar, (VA) ventral anchor, (I) to (VII) uncinuli, scale bar = 20 µm.
urn:lsid:zoobank.org:act:293F7CC7-70B8-469A-B2AA-F22AB1E02131
Type host: Simochromis marginatus (Poll, 1956).
Other host: Pseudosimochromis curvifrons (Poll, 1942).
Infection site: Gills.
Type locality: Luhanga, D.R.Congo (3° 31’ S 29° 9’ E) (October 30th, 1957, on MRAC 129678 and 129680), on type host and (March 27th, 2010, on B0-12-P-748) on P. curvifrons.
Other localities: On S. marginatus: Bemba, D.R.Congo (3° 37’ S 29° 09’ E) (March 26th, 2010, on B0-12-P-429). On P. curvifrons: Bemba, D.R.Congo (3° 37’ S 29° 09’ E) (March 26th, 2010, on B0-12-P-430); Cape Tembwe, D.R. Congo (6° 30’ S, 29° 25’ E) (April 10th, 2010, on B0-12-P-722); Kalambo Lodge, Zambia (8°37’ S 31° 12’ E) (April 18th, 2008, on B2-04-P-98-110(1)); Kasakalawe/Chanzimu, Zambia (8° 47’ S 31° 5’ E) (April 13th, 2008, on B2-04-P-97); Mugayo North, D.R.Congo (6° 47’ S 29° 34’ E) (April 11th, 2010, on B0-12-P-750); Musamba, Tanzania (7° 50’S 30° 47’E) (April 25th, 2008).
Material studied: 20 individuals, all from type locality.
Type material: holotype: MRAC 37749 (1, Luhanga); paratypes: MRAC 37748 (1, Luhanga), MNHN HEL419 (1, Luhanga), SAMCTA 61812 (1, Luhanga).
Etymology: the name C. franswittei sp. nov. is given in honour of the late Dr. Frans Witte (1950–2013), biologist (The Netherlands), for his enormous contributions to (Lake Victoria) cichlid research and the kindness and enthusiasm in his teaching and research.
Description: Thick and striated tegument. Haptor: dorsal anchor with marked shaft, long guard and arched blade; dorsal transverse bar thick and arched, sometimes W-shaped; ventral anchor with marked guard and shaft; ventral transverse bar V-shaped; uncinuli I small (sensu [85]), uncinuli III to VII short (sensu [85]). Penis, beginning in a marked bulb, with developed heel is a long, wide and curved tube with a thin wall (often creased) and a sub-terminal aperture. Accessory piece simple and C-shaped and attached to middle of basal bulb. No sclerotized vagina observed.
Comments: C. franswittei sp. nov. belongs to the same group as C. raeymaekersi sp. nov., C. muterezii sp. nov., C. banyankimbonai sp. nov. and C. georgesmertensi sp. nov. It is the only Cichlidogyrus species in this group with a long and large penis that has a sub-terminal opening.
Cichlidogyrus frankwillemsi Pariselle and Vanhove sp. nov. (Figs 7F, 8E and 14; Table 9)
Fig 14. Haptoral and genital hard parts of Cichlidogyrus frankwillemsi sp. nov.
(Ap) accessory piece (DB) dorsal transverse bar, (DA) dorsal anchor, (He) heel, (MA) male apparatus, (Pe) penis, (VB) = ventral transverse bar, (VA) ventral anchor, (I) to (VII) uncinuli, scale bar = 20 µm.
urn:lsid:zoobank.org:act:864DD4A0-58A0-4255-BDD1-34316D2D83D5
Type host: Pseudosimochromis curvifrons (Poll, 1942).
Infection site: Gills.
Type locality: Kalambo Lodge, Zambia (08°37’ S 31° 12’ E) (April 18th, 2008, on B2-04-P-98-110).
Other localities: Cape Tembwe, D.R. Congo (6° 30’ S, 29° 25’ E) (April 10th, 2010, on B0-12-P-722); Luhanga, D.R.Congo (3° 31’ S 29° 09’ E) (March 27th, 2010, on B0-12-P-748)
Material studied: 19 individuals, 16 from Kalambo lodge and three from Cape Tembwe.
Type material: holotype: MRAC 37747 (1, Kalambo Lodge); paratypes: MRAC 37747 (1, Kalambo Lodge), MRAC 37746 (2, Kalambo Lodge).
Etymology: the species epithet, frankwillemsi, honours biologist Frank Willems (Zambia / The Netherlands), of the Kasanka Trust for his contributions to community-based conservation and nature education in Africa, in gratitude for his help in our research and travels.
Description: Tegument thick and striated. Ovoid pharynx. Haptor: dorsal anchor small with marked shaft and guard and arched blade; dorsal transverse bar thin and slightly sclerotised (often hard to see), arched with thin and well-developed auricles; ventral anchor with marked guard and shaft, slightly larger than dorsal anchor; ventral transverse bar V-shaped, thin and slightly sclerotised (often hard to see); uncinuli I small (sensu [85]), uniculi III to VII short (sensu [85]). Penis, beginning in a slightly marked bulb, with reduced or without heel, is a S-shaped tube with almost constant diameter, its wall thickened at the middle. Accessory piece simple, flattened in the middle, attached to basal bulb and crossing penis proximally. No sclerotized vagina observed.
Comments: Cichlidogyrus frankwillemsi sp. nov. belongs to the same group as C. raeymaekersi sp. nov., C. muterezii sp. nov., C. banyankimbonai sp. nov., C. georgesmertensi sp. nov. and C. franswittei sp. nov. It can be distinguished from all other species in this group, except for C. gistelincki, by the shape of the penis: S-shaped and thick walled. It differs from C. gistelincki in the length of the penis (49 in C. frankwillemsi sp. nov. versus 35 in C. gistelincki) and the shape of the accessory piece (simple and flattened in the middle in C. frankwillemsi sp. nov. versus slender, twisted and with distal end covered by a pointed cap in C. gistelincki).
Discussion
We followed an integrative approach to revise the taxonomy of the species currently assigned to Simochromis and Pseudosimochromis, using both intrinsic (fish morphology and genetics) and extrinsic traits (distribution patterns of monogenean parasitic flatworms). The results presented above confirm the distinction of S. diagramma from P. curvifrons and from the ‘small’ Simochromis species (S. babaulti, S. margaretae, S. marginatus and S. pleurospilus). Within the latter group, the specific status of S. pleurospilus versus that of S. babaulti is not confirmed (see below) whereas S. margaretae and S. marginatus were shown to well-defined species, even though only two specimens of the former species were available for study.
Many quantitative as well as qualitative morphological traits separate P. curvifrons from S. babaulti, S. pleurospilus and S. marginatus. Yet, for most of these features, S. margaretae is intermediate between these species and P. curvifrons. This is the case for the shape of the gill rakers and of the oral teeth, for the distance between the outer and inner teeth and for some meristics, such as the number of anal soft rays. The two S. margaretae specimens also had values intermediate between those of the other ‘small’ Simochromis species and P. curvifrons in the PCAs of meristics and measurements (Figs 2 and 3). Moreover, S. margaretae had a steep, highly convex and narrow head as typical for P. curvifrons. Although some of the traits in which S. diagramma differs from the ‘small’ Simochromis species are also shared by P. curvifrons, the most clear-cut of them; the absence of reduced gill rakers, can be interpreted as a symplesiomorphism. Indeed, these structures are also absent in other “sediment dwelling” tropheines. Furthermore, S. diagramma differs clearly in dental morphology from P. curvifrons and from the ‘small’ Simochromis species.
To conclude, qualitative morphological data suggest a closer affinity between P. curvifrons and the ‘small’ Simochromis species than between the ‘small’ Simochromis species and S. diagramma. This is in agreement with a nuclear phylogeny of the Tropheini [39] in which the ‘small’ Simochromis species and P. curvifrons from a monophylum. Unfortunately, S. margaretae has only been collected once, and no tissue samples, suitable for molecular analysis, are available. Therefore, the intermediate position of this species between S. curvifrons and the other ‘small’ Simochromis species has not been assessed genetically.
The distribution of their Cichlidogyrus parasite fauna provided additional evidence for the interrelationships between these cichlids. The haptoral morphology, which was shown to be systematically informative on the scale of Cichlidogyrus lineages, is important for the classification of Cichlidogyrus species. Details of the genital structures, on the other hand, are mostly useful in distinguishing between closely related Cichlidogyrus species [88–89]. Several haptoral features (pronounced asymmetry between shaft and guard in the dorsal anchor, only small uncinuli), as well as some more general characteristics of the genitals (relatively simple accessory piece and copulatory tube, absence of sclerotized vagina) clearly suggest an affinity between the Cichlidogyrus species described above with species described from other tropheine cichlids [55,57].
When looking at the haptoral morphology of the Cichlidogyrus species infecting Simochromis and Pseudosimochromis, species infecting S. diagramma were shown to belong to a distinct morphological group. Indeed, C. raeymaekersi sp. nov., C. muterezii sp. nov. and C. banyankimbonai sp. nov., found on S. diagramma , all displayed substantial length differences between their ventral anchor guards and shafts. Their dorsal transverse bars were either quite thick or had comparatively large auricles. This clearly sets these three species apart from the species infecting P. curvifrons and the ‘small’ Simochromis species: C. georgesmertensi sp. nov., C. franswittei sp. nov. and C. frankwillemsi sp. nov. These species had less asymmetrical ventral anchor roots, and either thinner dorsal bars or dorsal bars with relatively small auricles. Likewise, the species infecting the other “sediment-dwelling” tropheines ‘C.’ horei, ‘G.’ pfefferi and L. dardennii also displayed less asymmetry between ventral anchor shaft and guard than the parasites of S. diagramma. This is in concurrence with S. diagramma stemming from an early offshoot of this clade. Given the high host specificity in Cichlidogyrus infecting tropheine cichlids [45], sharing a Cichlidogyrus species may signal a shared ancestry of the cichlid hosts. Cichlidogyrus franswittei sp. nov. is shared between P. curvifrons and S. marginatus whereas Simochromis babaulti and S. pleurospilus share C. georgesmertensi sp. nov. Cichlidogyrus frankwillemsi sp. nov., finally, has only been recorded from P. curvifrons (Fig 15).
Fig 15. Distribution of Cichlidogyrus gill parasites on their tropheine hosts.
Phylogenetic reconstruction of the “sediment dwellers” clade within the Tropheini and S. diagramma is derived from [39]. Cichlidogyrus species are visualised by their haptoral ventral anchors, which is a systematically informative trait. Six Cichlidogyrus species are described in this paper, three others in [55]. For P. margaretae, no tissue samples suitable for genetic analysis exist, neither were gill parasites recovered from the available specimens.
The distribution of their Cichlidogyrus parasite fauna, combined with the differences in haptoral morphology between the parasites of S. diagramma and its congeners, provided an additional line of evidence, next to the nuclear phylogeny [39] and the morphology of these cichlids, to justify the generic placement of P. curvifrons as well as the transfer of all ‘small’ Simochromis species: S. babaulti, S. pleurospilus, S. marginatus and S. margaretae to Pseudosimochromis. This renders Simochromis, only containing S. diagramma, monotypic.
The morphological results and barcoding data presented above, however, did not support the specific status of P. pleurospilus with regard to P. babaulti. Both species also hosted the same Cichlidogyrus species: C. georgesmertensi sp. nov. This also suggested that they are conspecific or at the very least closely related. It is therefore important to consider whether there are genetic or morphological differences between P. pleurospilus and P. babaulti warranting their status as two separate species. Although specimens belonging to P. pleurospilus differed significantly in morphology from geographically separated groups of P. babaulti populations, these differences were not larger than those observed between the different P. babaulti groups. This was corroborated by our barcoding results (Table 8). Therefore, P. babaulti should be considered a Lake Tanganyika species harbouring a considerable amount of geographical variation, with P. pleurospilus as a junior synonym. A large amount of geographic variation was also observed in different populations of Neolamprologus niger (Poll, 1956) [90] and of Tropheus duboisi [74]. Finally, this synonymy is in agreement with recent molecular findings. Notwithstanding that a deep split was found in the mitochrondrial phylogeny between P. babaulti and P. pleurospilus, AFLP data did not support the monophyly of both species [39].
The reason P. pleurospilus was originally considered a separate species was that Brichard mentioned the presence of two sympatric but differently coloured S. babaulti-like species occurring in sympatry at the southern end of Lake Tanganyika [66]. Nevertheless, although Nelissen [66] compared the 13 S. pleurospilus type specimens with 72 S. babaulti specimens, none of these S. babaulti specimens was collected at the same locality as the S. pleurospilus types. Moreover, according to Brichard’s communications [66], P. babaulti occurs in the crevices between the rocks whereas P. pleurospilus can be found in the open (i.e. deeper) water. Taborsky [38] observed that P. babaulti specimens move to deeper water when they become sexually active, whereas juveniles are found closer to shore. Given that, for P. babaulti, the colouration pattern changes depending on the sexual or aggressive behaviour of the individual [37], the difference in colour pattern observed by Brichard could be attributed to differences in development or activity and not to the presence of two species. Moreover, in a recent survey of the western lakeshore, populations of mixed colour pattern, encompassing specimens with a colouration typical either to P. babaulti or to P. pleurospilus, were found [68].
Pseudosimochromis, containing P. curvifrons, P. babaulti, P. marginatus and P. margaretae can be distinguished from all other tropheine genera by the unique combination of the following characters: three anal spines (versus 4–7 in Tropheus), bicuspid firmly set outer anterior oral teeth (versus tricuspid loosly set teeth in Petrochromis Boulenger, 1898, bicuspid loosly set teeth in Interochromis Yamaoka, Hori & Kuwamura, 1988 and conical or rounded teeth in adult ‘Ctenochromis’ horei, ‘Gnathochromis’ pfefferi and Lobochilotes Boulenger, 1915) which are densely set (versus gaps present between adjacent anterior oral teeth in Limnotilapia Regan, 1920 and in Simochromis).
As the largest of the three S. diagramma syntypes (BMNH 1889–30.9–11) corresponded in size (70. 6mm SL; 90.7mm TL) and in meristics to the specimen illustrated by Günther [91], this specimen is hereby designated as the lectotype, following recommendation ICZN 1999: 74.4. Finally, the synonymy of Tilapia adolfi Steindachner, 1909 with S. diagramma [92] was verified by inspection of six syntypes (NMW 24777–81, of which lot 24777 contains two specimens). Steindachner [93], however, mentioned seven specimens in the species’ original description, whereas only six could be located in the Vienna Museum. The identification of these specimens as S. diagramma was confirmed and the largest of the six specimens examined: NMW 24777–1 (79.3 mm SL) is hereby designated as the lectotype of Tilapia adolfi Steindachner, 1909. It should be noted, however, that Tilapia adolfi Steindachner, 1916, is a different species, with replacement name Tilapia hornorum Trewavas, 1966, currently a subspecies of Oreochromis urolepis (Norman, 1922) [94].
Lake Tanganyika cichlid tribes can be defined on anatomical characters [95] and are supported by molecular studies [22,26]. Within Tropheini, genera are mostly defined by oral morphology and teeth shape, characters linked to feeding habits and ecology. In Tropheus and Pseudosimochromis, the single row of closely set outer bicuspid teeth (with Tropheus having a much broader jaw than Pseudosimochromis) reflects their specialisation as browsers. Simochromis and Limnotilapia, in which large spaces are present between the bicuspid teeth, have a broader ecological niche [59]. The transfer of S. babaulti, S. marginatus and S. margaretae to Pseudosimochromis and the fact that its sister clade, consisting of ‘Gnathochromis’ pfefferi and ‘Ctenochromis’ horei [39] should change generic position renders six tropheine genera monotypic: Interochromis, Simochromis, Limnotilapia, Lobochilotes and ‘Gnathochromis’ pfefferi and ‘Ctenochromis’ horei. Remarkably, three of these genera: Simochromis, Lobochilotes and ‘Ctenochromis’ were shown to have a hybrid origin [39]. As, in tropheine cichlids, generic definitions are strongly linked to ecological niche; this suggests that the evolutionary potential to occupy a certain niche was aided by hybridisation. The emergence of evolutionary novelties through hybridisation was suggested by Seehausen [96] and already shown for cichlids from Camerounian Crater Lakes [97]. It could have played a role in the success of Tropheini, as it was suggested that the ancestors of this tribe colonised Lake Tanganyika when other endemic cichlid lineages had already diversified ([32] but see [98]).
The three non-monotypic genera: Tropheus, Pseudosimochromis and Petrochromis all show a considerable amount of morphological variation; the former two were shown to be monophyletic using nuclear markers. Using mitochondrial markers, monophyly could not be obtained for Tropheus and Pseudosimochromis as the inclusion of T. duboisi Marlier, 1958 and P. curvifrons in their genus was not supported [39]. Also from a morphological and an ecological point of view, these two species represent the odd one out within their genus. Tropheus duboisi is considered a primitive Tropheus species, with a less specialised oral morphology. Previously, it was suggested that P. curvifrons was a highly specialised algal browser, restricted to the rocky shore [69], whereas its congeners have a more general cichlid bauplan [99] and are less stenotypic. Muschick et al. [26], however, found vegetal (aufwuchs) as well as animal (insects and crustaceans) remains in the guts of P. curvifrons, which contradicts a specialised life style. Moreover, the species’ stable isotope profile [26] also showed P. curvifrons to be a generalist. For P. babaulti, however, guts contained almost 100% vegetal material, showing a specialised ecology. In contrast to Tropheus, where there is no intermediate species between the ‘primitive’ T. duboisi and the specialised other species, an intermediate phenotype exists between that of P. curvifrons and that of P. babaulti and P. marginatus: P. margaretae. This species was collected in a well-vegetated area [69], which deviates from the rocky shore at which P. curvifrons is found and which corresponds more with the preferred diet of P. babaulti.
This study is an example of how Cichlidogyrus species may serve as complementary markers to investigate the taxonomy of their hosts (Fig 15). It also shows the potential of parasitology as an additional discipline in integrative taxonomy. Although many factors might obscur patterns in parasite assemblages [100–101], a certain degree of congruence between host and parasite interrelationships is often apparent [102]. The utility of Cichlidogyrus as additional markers in tropheine cichlids, including Simochromis and Pseudosimochromis (Figs 15 and 16), is no surprise as high host specificity has been shown in this system before [45]. Yet, P. margaretae was only collected once, and the Cichlidogyrus fauna infecting Simochromis and Pseudosimochromis consisted of species which were all new to science. This shows that even for well-studied models such as Lake Tanganyika cichlids, our basic understanding of biological diversity still depends on new discoveries.
Fig 16. Map of Lake Tanganyika with an overview of specimens examined.
Collection localities of hosts and parasites with: filled symbols: cichlids used for morphology, empty symbols: cichlids used for barcoding, pie charts: collection sites of Cichlidogyrus parasites, T: cichlid type locality and t: Cichlidogyrus type locality, for A. Simochromis diagramma, B. Pseudosimochromis curvifrons, C. P. marginatus, D. P. babaulti (including its junior synonym P. pleurospilus), E. P. margaretae (including localities mentioned in text) and F. symbols and colours used to denote collection sites of cichlids and of Cichlidogyrus specimens.
Appendix: Specimens Examined
Gill arches were drawn for specimens denoted with * (Fig 3)
Simochromis diagramma
Morphology: BMNH 1889.1.30.9 (1), lectotype, 70.59 mm SL, Ujiji, Tanzania, coll. Coode-Hore; BMNH 1889.1.30.10–11 (2), paralectotypes, 52.08, 58.87mm SL, Ujiji, Tanzania, coll. Coode-Hore; MRAC 106503 (1), 79.84 mm SL, Kasanga, plage au sud de la rivière Kawa, Tanzania, 8° 28’ S 31° 9’ E, coll. M. Poll; MRAC 95-96-P-1377-90 (2), 66.32*, 67.15 mm SL, Ninde, Tanzania, 7° 40’ S 30° 43’ E, coll. exp. 95; MRAC B0-12-P-354-355 (2), 64.23, 95.01 mm SL, Cap Banza, Ubwari Peninsula, D.R.Congo, 4° 04’ S 29° 14’ E, coll. exp. 2010; MRAC B0-12-P-356-357 (1), Mugayo, D.R.Congo, 6° 47’ S 29° 33’ E, coll. exp. 2010; DNA barcoding: MRAC T95-24 (1), MRAC 95-96-P-2949 (1), Wampembwe, Tanzania, 8° 0’ S 30° 53’ E, coll. exp. 95; T95-1767 (1), Chaitika Point, Zambia, 8° 34’ S 30° 48’ E, coll. exp. 95; MRAC 92-81-P-1354 (1), Masaka Point, Tanzania, 5° 2’ S 29° 46’ E, coll. exp. 92; MRAC B0-12-P-354 (1), Cap Banza, Ubwari, D.R.Congo, 4° 4’ S 29° 14’ E, coll. exp. 2010; not included in analysis: NMW 24777–1 (1), lectotype of Tilapia adolfi Steindachner, 1909, 79.3 mm SL, Lake Tanganyika, coll. unknown; NMW 24777–2, 24778–81 (5), paralectotypes of Tilapia adolfi Steindachner, 1909, 56.4–67.6 mm SL, Lake Tanganyika, coll. unknown.
Pseudosimochromis curvifrons
Morphology: MRAC 53101, holotype, 89.72 mm SL, Nyanza, Burundi, coll. A. Lestrade; MRAC 53102–53103 (2), paratypes, 84.47, 94.55 mm SL, Nyanza, Burundi, coll. A. Lestrade; MRAC B0-12-P-441-444 (3), 71.18, 74.54, 75.3 mm SL, Kyanza, D.R.Congo, 7° 7’ S 29° 59’ E, coll. exp. 2010; MRAC B0-12-P-445-448 (3), 66.59, 71.24, 79.70* mm SL, Kikoti, D.R.Congo, 7° 11’ S 30° 4’ E, coll. exp. 2010; DNA barcoding: MRAC 95-96-P-652 (1), Chaitika Point, Zambia, 8° 34’ S 30° 48’ E, coll. exp. 95; MRAC T95-1349 (1) Wampembwe, Tanzania, 8° 0’ S 30° 53’ E, coll. exp. 95.
Pseudosimochromis babaulti
Morphology: MNHN 1927–318, holotype, 62.81 mm SL, Ouvira, D.R. Congo, 3° 24’ S 29° 8’ E, coll. G. Babault; MRAC 86560 (1), 60.4 mm SL, Kamvimvira, D.R.Congo, 3°21’ S 29° 9’ E, coll. Miss. Pisc. Katanga; MRAC 114401 (1), 114410 (1), 55.19, 57.73 mm SL, Uvira, D.R.Congo, 3° 24’ S 29° 8’ E, coll. G. Marlier; MRAC 74-19-P-8-9 (2), 64.07–78.72 mm SL, dans les environs de Bujumbura, 3° 23’ S 29° 22’ E, coll. RUCA; MRAC 92-81-P-200 (1), 79.08 mm SL, Ulwile Island, northern shore, Tanzania, 7° 28’ S 30° 34’ E, coll. exp. 92; MRAC 92-81-P-613 (1), 86.81 mm SL, Mpimbwe Hills, northern part of Shashete bay, Tanzania, 7° 7’ S 30° 30’ E, coll. exp. 92; MRAC 92-81-P-641-642 (2), 70.00–80.38 mm SL, just south of Kasinde, Tanzania, 7° 6’ S 30° 33’ E, coll. exp. 92; MRAC 92-81-P-716-719, 721–723, 727–728 (9), 53.81, 54.22, 56.81, 58.92, 59.10, 60.44, 66.54, 73.00, 76.15 mm SL, just south of Karema, Tanzania, 6° 51’ S 30° 27’ E, coll. exp. 92; MRAC 92-81-P-794-796 (3), 92-81-P-794-799 (1), 74.27, 77.12, 77.19, 80.88 mm SL, halfway between Ikola and Mkangasi, Tanzania, 6° 40’ S 30° 21’E, coll. exp. 92; MRAC 92-81-P-904 (1), 63.79 mm SL, Kalia, bay at mouth of Lugonesi River, Tanzania, 6° 27’ S 30° 8’ E, coll. exp. 92; MRAC 92-81-P-1150, 1053–1055, 1059 (5), 53.66, 62.86, 65.67, 68.08, 73.99 mm SL, Segunga, south of Segunga bay, Tanzania, 5°35’ S 29° 51’ E, coll. exp. 92; MRAC 92-81-P-1222 (1), 70.18 mm SL, Kalela, Tanzania, 5° 59’ S 29° 50’ E, coll. exp. 92; MRAC 92-81-P-1228 (1), 66.83 mm SL, 3 km north of Kabwe, Tanzania, 5°43’ S 29° 54’ E, coll. exp. 92; MRAC 95-96-P-643-645 (3), 95-96-P-3315 (1), 73.30, 75.29, 75.92, 80.71 mm SL, Msamba bay, Tanzania, 7° 51’ S 30° 47’ E, coll. exp. 95; MRAC 95-96-P-3317 (1), 66.91 mm SL, Kapele, Zambia, 8° 35’ S 31° 10’ E, coll. exp. 95; MRAC B0-12-P-424-425 (2), B0-12-P-394-398 (4), 61.95, 65.69, 67.10, 67.31, 74.15, 74.99 mm SL, Kyanza, D.R.Congo, 7° 07’ S 29° 58’ E; MRAC B0-12-P-411-417 (5), 68.12, 70.61, 72.55, 72.68, 74.38* mm SL, Mukamba, D.R.Congo, 6° 57’ S 29° 43’ E, coll. exp. 2010; MRAC 77-35-P-117 (1), holotype of P. pleurospilus, 78.80 mm SL, Chaitika, Zambia, 8° 34’ S 30° 48’ E, coll. P. Brichard; MRAC 77-35-P-121 (1), allotype of P. pleurospilus, 62.60 mm SL, Chaitika, Zambia, 8° 34’ S 30° 48’ E, coll. P. Brichard; MRAC 77-35-P-118-120 (3), paratypes of P. pleurospilus, 67.11, 75.07, 82.81 mm SL, Chaitika, Zambia, 8° 34’ S 30° 48’ E, coll. P. Brichard; MRAC 76-4-P-464, 465, 480 (3) paratypes of P. pleurospilus, 57.51, 65.10, 67.22 mm SL, Kama bay, Zambia, coll. P. Brichard; MRAC 76-4-P-95-96 (2), paratypes of P. pleurospilus, 68.44, 73.48 mm SL, Cap Kabayeye, à l’est de Kasaba bay, Zambia, coll. P. Brichard; MRAC 76-4-98-99 (2), paratypes of P. pleurospilus, 59.98, 61.25 mm SL, Cap Nundo, Zambia, 8° 31’S 30° 38’ E, coll. P. Brichard, MRAC 78-25-P-703-704 (1), 73.04 mm SL, Chaitika, Zambia, 8° 34’ S 30° 48’ E, coll. P. Brichard; DNA barcoding: MRAC 92-81-P-1355, 56 (2), Masaka Point, Tanzania, 5° 2’ S 29° 46’ E, coll. exp. 92; MRAC 92-81-P-1228 (1), 3 km north of Kabwe, Tanzania, 5°43’ S 29° 54’ E, coll. exp. 92; MRAC 92-81-P-726, 27 (2), just south of Karema, Tanzania, 6° 51’ S 30° 27’ E, coll. exp. 92; MRAC B0-12-P-812, 73 (2), Mufazi, D.R.Congo, 7° 05’ S 29° 55’ E, coll. exp. 2010; MRAC B0-12-P-420 (1), Mukamba, D.R.Congo, 6° 57’ S 29° 43’ E, coll. exp. 2010; MRAC B0-12-P-1094, 95 (2), Mtoto, D.R.Congo, 6° 58’ S 29° 44’ E, coll. exp. 2010; MRAC B0-12-P-841, 42 (2), Kapakwe, D.R.Congo, 6° 58’ S 29° 44’ E, coll. exp. 2010; MRAC B0-12-P-424, 25 (2), Kyanza, D.R.Congo, 7° 7’ S 29° 59’, coll. exp. 2010; MRAC B0-12-P-427, 28 (2), Kikoti, D.R.Congo, 7° 11’ S 30° 04’ E, coll. exp. 2010; as P. pleurospilus: MRAC T95-1314, 15 (2), Nakaku Village, Zambia, 8° 41’ S 30° 54’ E, coll. exp. 95; MRAC T95-1771 (1), Chaitika Point, Zambia, 8° 34’ S 30° 48’ E, coll. exp. 95.
Pseudosimochromis margaretae
Morphology: SAIAB 966 (2), paratypes, 68.79*, 76.43 mm SL, Kigoma Harbour, Tanzania, coll. G. S. Axelrod, 4° 52’ S 29° 37’ E
Pseudosimochromis marginatus
Morphology: MRAC 106523, holotype, 74.22 mm SL, Manga (Ubwari), plage et rive rocheuse, D.R.Congo, 4° 9’ S 29° 13’ E, coll. M. Poll; INRS 360 (2), paratypes, 51.53, 67.77 mm SL, Manga (Ubwari), plage et rive rocheuse, D.R.Congo, 4° 9’ S 29° 13’ E, coll. M. Poll; MRAC 106524–25 (2), paratypes, 67.03, 70.46 mm SL, Manga (Ubwari), plage et rive rocheuse, D.R.Congo, 4° 9’ S 29° 13’ E, coll. M. Poll; MRAC 129674–87 (5), 68.20, 71.68, 72.18, 77.67, 80.44 mm SL, Luhanga, D.R.Congo, 3° 31’ S 29° 9’ E, coll. G. Matthes; MRAC 82-12-P-157-158 (2), 67.78, 69.48 mm SL, Ubwari, face ouest, D.R.Congo, between 4° 07’ S 29° 15’E and 4° 31’ S 29° 13’ E, coll. M. Schreyen; MRAC 92-81-P-998, 1007, 1014* (3), 61.42, 74.48, 78.52 mm SL, 1/3 distance Bulu Point—Luagala Point, Tanzania, 6° 10’ S 29° 40’ E, coll. exp. 92; MRAC B0-12-P-375-379 (5), 67.44, 69.20, 72.15, 73.84, 80.34 mm SL, Lubumba, D.R.Congo, 3° 59’ S 29° 7’ E, coll. exp. 2010; MRAC B0-12-P-360 (2), 68.05, 71.94 mm SL, Cap Banza, Ubwari, D.R.Congo, 4° 4’ S 29° 14’ E, coll. exp. 2010; MRAC B0-12-P-361 (1), 67.16 mm SL, Kisokwe, D.R.Congo, 4° 15’ S 29° 11’ E, coll. exp. 2010; MRAC B0-12-P-382 (1), 80.49 mm SL, Magogoro, Kavala Islands, D.R.Congo, 5° 39’ S 29° 12’ E, coll. exp. 2010; MRAC B0-12-P-384-385 (2), 48.91, 52.48 mm SL, Mukindu, D.R.Congo, 5° 36’ S 29° 23’ E, coll. exp. 2010; MRAC B0-12-P-386-393 (8), 66.33, 66.07, 78.89, 77.60, 70.30, 69.78, 66.73, 67.65, Musinwa, D.R.Congo, 5° 41’ S 29° 25’ E, coll. exp. 2010; DNA barcoding: MRAC B0-12-P-361 (1), Kisokwe, D.R.Congo, 4° 15’ S 29° 11’ E, coll. exp. 2010; MRAC B0-12-P-384 (1), Mukindu, D.R.Congo, 5° 36’ S 29° 23’ E, coll. exp. 2010; MRAC B0-12-P-390 (1), Musinwa, D.R.Congo, 5° 41’ S 29° 25’ E, coll. exp. 2010; MRAC 92-81-P-998 (1), 1/3 distance Bulu Point—Luagala Point, Tanzania, 6° 10’ S 29° 40’ E, coll. exp. 92.
Additional specimens used for DNA-barcoding
Limnotilapia dardennii: MRAC 92-81-P-285, Ulwile Island, northern shore, Tanzania, 7° 28’ S 30° 34’ E, coll. exp. 92; MRAC 95-96-P-2867, Wampembwe, Tanzania, 8° 0’ S 30° 53’ E, coll. exp. 95; 95-96-P-2866, Punda Point, Tanzania, 7° 27’ S 30° 36’ E, coll. exp. 95; ‘Ctenochromis’ horei: MRAC 92-81-P-618, Mpimbwe Hills, northern part of Shashete bay, Tanzania, 7° 7’ S 30° 30’ E, coll. exp. 92; MRAC 92-81-P-680, north of Mkombe, Tanzania, 6° 58’ E 30° 34’ S, coll. exp. 92; MRAC 92-81-P-1357, Masaka Point, Tanzania, 5° 2’ S 29° 46’ E, coll. exp. 92; ‘Gnathochromis’ pfefferi: MRAC 95-96-P-869, Chaitika Point, Zambia, 8° 34’ S 30° 48’ E, coll. exp. 95; MRAC 95-96-P-2817, Wampembwe, Tanzania, 8° 0’ S 30° 53’ E, coll. exp. 95; MRAC 92-81-P-1315, Kibwe Bay, Tanzania, 5° 24’ E 29° 46’ S, coll. exp. 92.
Supporting Information
Measurements and meristics taken on 114 specimens belonging to Simochromis and Pseudosimochromis and measurements taken on the specimens of Cichlidogyrus raeymaekersi sp. nov., C. muterezii sp. nov., C. banyankimbonai sp. nov., Cichlidogyrus georgesmertensi sp. nov., C. franswittei sp. nov. and C. frankwillemsi sp. nov.
(XLSX)
Acknowledgments
This is the sixth publication in a series on the ancyrocephalids of Lake Tanganyika. P.I. Hablützel, J.A.M. Raeymaekers, A.F. Grégoir, J. Bamps, D. Muzumani Risasi, T. Mulimbwa N’sibula, F. Muterezi Bukinga, V. Muderhwa Nshombo, J. Mbirize Ndalozibwa, V. Lumami Kapepula, C. Gillardin, G. Banyankimbona, C. Sturmbauer, L. Makasa, J.K. Zimba, T. Veall, O.R. Mangwangwa, A. Avenant-Oldewage and S. Risch are gratefully acknowledged for their help in collecting the material for this study, A. Konings and R. Blažek for pictures of cichlids, A. Reygel for providing Fig 3, G. Cael for providing Fig 15 and E.J. Vreven for the inspection of the NMW specimens.
This is publication ISE-M 2015–046.
Data Availability
Morphological data is contained within the paper and and its Supporting Information files. Sequences are available from NCBI GenBank under accession numbers KP336420-KP336458. Nomenclatural acts are registered in ZooBank. Type and vouchers specimens are deposited in the Royal Museum for Central Africa, the Muséum national d'Histoire naturelle and the Iziko South African Museum.
Funding Statement
At the time of conducting this research, M.V.S. and M.P.M.V. were Ph.D. fellows, and T.H. a post-doctoral fellow of the Research Foundation – Flanders (FWO-Vlaanderen). Field work was funded by two travel grants from the Research Foundation – Flanders to M.P.M.V. and two grants from the King Leopold III Fund for Nature Exploration and Conservation to M.V.S. and M.P.M.V. Currently, M.P.M.V. is supported by the Czech Science Foundation, Project no. P505/12/G112 (European Centre of Ichthyoparasitology (ECIP) – Centre of excellence). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Schlick-Steiner BC, Steiner FM, Seifert B, Stauffer C, Christian E, Crozier RH. Integrative taxonomy: a multisource approach to exploring biodiversity. Annu Rev Entomol. 2010; 55: 421–438. 10.1146/annurev-ento-112408-085432 [DOI] [PubMed] [Google Scholar]
- 2. Dayrat B. Towards integrative taxonomy. Biol J Linn Soc. 2005; 85: 407–415. [Google Scholar]
- 3. Padial JM, Miralles A, De la Riva I, Vences M. The integrative future of taxonomy. Front Zool. 2010; 7: 16 10.1186/1742-9994-7-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gomez A, Wright PJ, Lunt DH, Cancino JM, Carvalho GR, Hughes RN. Mating trials validate the use of DNA barcoding to reveal cryptic speciation of a marine bryozoan taxon. P Roy Soc Lond B Bio. 2007; 274: 199–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Arthofer W, Rauch H, Thaler-Knoflach B, Moder K, Muster C, Schlick-Steiner BC, et al. How diverse is Mitopus morio? Integrative taxonomy detects cryptic species in a small-scale sample of widespread harvestman. Mol Ecol. 2013; 14: 3850–3863. 10.1111/mec.12340 [DOI] [PubMed] [Google Scholar]
- 6. Sueur J, Puissant S. Similar look but different song: a new Cicadetta species in the montana complex (Insecta, Hemiptera, Cicadidae). Zootaxa. 2007; 1442: 55–68. [Google Scholar]
- 7. Paidal JM, Köhler J, Muños A, De La Riva I. Assessing the taxonomic status of tropical frogs through bioacoustics: geographical variation in the advertisment calls in the Eleutherodactylus discoidalis species group (Anura). Zool J Linn Soc. 2008; 152: 353–365. [Google Scholar]
- 8. Lavoué S, Sullivan JP, Arnegard ME. African weakly electric fishes of the genus Petrocephalus (Osteoglossomorpha: Mormyridae) of Odzala National Park, Republic of the Congo (Lékoli River, Congo River basin) with description of five new species. Zootaxa. 2010; 2600: 1–52. [Google Scholar]
- 9. Haverty MI, Forschler BT, Nelson LJ. An assessment of the taxonomy of Reticulitermes (Isoptera: Rhinotermitidae) from the southeastern United States based on cuticular hydrocarbons. Sociobiology. 1996; 28: 218–318. [Google Scholar]
- 10. Tsigenopoulos CS, Ráb P, Naran D, Berrebi P. Multiple origins of polyploidy in the phylogeny of southern African barbs (Cyprinidae) as inferred from mtDNA markers. Heredity. 2002; 88: 466–473. [DOI] [PubMed] [Google Scholar]
- 11. Hawlitschek O, Porch N, Hendrich L, Balke M. Ecological niche modelling and nDNA sequencing support a new, morphologically cryptic beetle species unveiled by DNA barcoding. PLoS ONE. 2011; 6: e16662 10.1371/journal.pone.0016662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ahmadzadeh F, Flecks M, Carretero MA, Mozaffari O, Böhme W, Harris JD, et al. Cryptic species patterns in Iranian rock lizards uncovered by integrative taxonomy. PLoS ONE. 2013; 12: e80563 10.1371/journal.pone.0080563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ramírez B. A new classification of Ficus . Ann Mo Bot Gard. 1977; 64: 296–310. [Google Scholar]
- 14. Krylova EM, Sahling H. Vesicomydae (Bivalvia): Current taxonomy and distribution. PLoS ONE. 2010; 5: e9957 10.1371/journal.pone.0009957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Combes C. Parasitism The ecology and evolution of intimate interactions. Chicago: University of Chicago Press; 2001. [Google Scholar]
- 16. de Meeûs T, Michalakis Y, Renaud F. Santa Rosalia revisited: or why are there so many kinds of parasites in ‘the garden of early delights’? Parasitol Today. 1998; 14: 10–13. [DOI] [PubMed] [Google Scholar]
- 17. Smith AM, Rodriguez JJ, Whitfield JB, Deans AR, Janzen DH, Hallwachs W, et al. Extreme diversity of tropical parasitoid wasps exposed by iterative integration of natural history, DNA barcoding, morphology, and collections. P Natl Acad Sci USA. 2008; 105: 12359–12364. 10.1073/pnas.0805319105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Paugy D, Guégan JF, Agnèse JF. Three simultaneous and independent approaches to the characterization of a new species of Labeo (Teleostei, Cyprinidae) from West Africa. Can J Zool. 1990; 68: 1124–1131. [Google Scholar]
- 19. Boeger WA, Kritsky DC. Parasites, fossils and geologic history: historical biogeography of the South American freshwater croakers, Plagioscion spp. (Teleostei, Sciaenidae). Zool Scr. 2003; 32: 3–11. [Google Scholar]
- 20. Nieberding C, Morand S, Libois R, Michaux JR. A parasite reveals cryptic phylogeographic history of its host. P Roy Soc Lond B Bio. 2004; 271: 2559–2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kocher TD. Explosive speciation: the cichlid fish model. Nat Rev Genet. 2004; 5: 288–298. [DOI] [PubMed] [Google Scholar]
- 22. Koblmüller S, Sefc KM, Sturmbauer C. The Lake Tanganyika cichlid species assemblage: recent advances in molecular phylogenetics. Hydrobiologia. 2008; 615: 5–20. [Google Scholar]
- 23. Seehausen O. African cichlid fish: a model system in adaptive radiation research. P Roy Soc Lond B Bio. 2012; 273: 1987–1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Whitfield JB, Lockhart PJ. Deciphering ancient rapid radiations. Trends Ecol Evol. 2007; 22: 258–265. [DOI] [PubMed] [Google Scholar]
- 25. Wagner CE, McCune AR, Lovette IJ. Recent speciation between sympatric Tanganyikan cichlid colour morphs. Mol Ecol. 2012; 21: 3283–3292. 10.1111/j.1365-294X.2012.05607.x [DOI] [PubMed] [Google Scholar]
- 26. Muschick M, Indermauer A, Salzburger W. Convergent evolution within an adaptive radiation of cichlid fishes. Curr Biol. 2012; 22: 1–7. 10.1016/j.cub.2011.12.009 [DOI] [PubMed] [Google Scholar]
- 27. Snoeks J. Cichlid diversity, speciation and systematics: examples from the Great African Lakes. Journal of Aquaculture and Aquatic Sciences. 2001; 9: 150–166. [Google Scholar]
- 28. Cohen AS, Lezzar KE, Tiercelin JJ, Soregan M. New paleogeographic and lake-level reconstructions of Lake Tanganyika: implications for tectonics, climatic and biological evolution in a rift lake. Basin Res. 1997; 9: 107–132. [Google Scholar]
- 29. Snoeks J. How well known is the ichthyofauna of the large East African lakes? Adv Ecol Res. 2000; 31: 17–38. [Google Scholar]
- 30. Takahashi T, Koblmüller S. A new species of Petrochromis (Perciformes: Cichlidae) from Lake Tanganyika. Ichthyol Res. 2014; 61: 252–264. [Google Scholar]
- 31. Poll M. Classification des Cichlidae du Lac Tanganyika. Tribus, genres et espèces. Acad R Belg Mém Cl Sci. 1986; 45: 1–163. [Google Scholar]
- 32. Salzburger W, Mack T, Verheyen E, Meyer A. Out of Tanganyika: Genesis, explosive speciation and phylogeny of the haplochromine cichlid fishes. BMC Evol Biol. 2005; 5: 17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sturmbauer C, Meyer A. Genetic divergence, speciation and morphological stasis in a lineage of African cichlid fishes. Nature. 1992; 358: 578–581. [DOI] [PubMed] [Google Scholar]
- 34. Koblmüller S, Salzburger W, Obermüller B, Eigner E, Sturmbauer C, Sefc K. Separated by sand, fused by dropping water: habitat barriers and fluctuating water levels steer the evolution of rock-dwelling cichlid populations in Lake Tanganyika. Mol Ecol. 2011; 20: 2272–2290. 10.1111/j.1365-294X.2011.05088.x [DOI] [PubMed] [Google Scholar]
- 35. Raeymaekers JAM, Hablützel PI, Grégoir AF, Bamps J, Roose AK, Vanhove MPM, et al. Contrasting parasite communities among allopatric colour morphs of the Lake Tanganyika cichlid Tropheus . BMC Evol Biol. 2013; 13: 41 10.1186/1471-2148-13-41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Nelissen MHJ. Contribution to the ethology of Simochromis diagramma (Günther) (Pisces, Cichlidae). Acta Zool Pathol Ant. 1974; 61: 31–46. [PubMed] [Google Scholar]
- 37. Nelissen MHJ. Contribution to the ethology of Simochromis babaulti Pellegrin (Pisces, Cichlidae). Ann Soc Roy Zool Bel. 1976; 106: 167–175. [Google Scholar]
- 38. Taborsky B. Mothers determine offspring size in response to own juvenile growth conditions. Biol Letters. 2006; 2: 225–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Koblmüller S, Egger B, Sturmbauer C, Sefc KM. Rapid radiation, ancient incomplete lineage sorting and ancient hybridization in the endemic Lake Tanganyika cichlid tribe Tropheini. Mol Phylogenet Evol. 2010; 55: 318–334. 10.1016/j.ympev.2009.09.032 [DOI] [PubMed] [Google Scholar]
- 40. Guégan JF, Lambert A. Twelve new species of dactylogyrids (Platyhelminthes, Monogenea) from West African barbels (Teleostei, Cyprinidae), with some biogeographical implications. Syst Parasitol. 1990; 17: 153–181. [Google Scholar]
- 41.Pariselle A. Diversité, spéciation et évolution des Monogènes branchiaux de Cichlidae en Afrique de l’Ouest. Ph.D. thesis, Université de Perpignan. 1996.
- 42. Barson M, Přikrylová I, Vanhove MPM, Huyse T. Parasite hybridization in African Macrogyrodactylus spp. (Monogenea, Platyhelminthes) signals historical host distribution. Parasitology. 2010; 137: 1585–1595. 10.1017/S0031182010000302 [DOI] [PubMed] [Google Scholar]
- 43. Pariselle A, Boeger WA, Snoeks J, Bilong Bilong CF, Morand S, Vanhove M. The monogenean parasite fauna of cichlids: a potential tool for host biogeography. Int J Evol Biol 2011: 471480 10.4061/2011/471480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Muterezi Bukinga F, Vanhove MPM, Van Steenberge M, Pariselle A. Ancyrocephalidae (Monogenea) of Lake Tanganyika: III: Cichlidogyrus infecting the world’s biggest cichlid and the non-endemic tribes Haplochromini, Oreochromini and Tylochromini (Teleostei, Cichlidae). Parasitol Res. 2012; 111: 2049–2061. 10.1007/s00436-012-3052-1 [DOI] [PubMed] [Google Scholar]
- 45.Vanhove MPM. Species flocks and parasite evolution. Towards a co-phylogenetic analysis of monogenean flatworms of cichlids and gobies. Ph.D. thesis, KU Leuven. 2012.
- 46. Vanhove MPM, Van Steenberge M, Dessein S, Volckaert FAM, Snoeks J, Huyse T, et al. Biogeographical implications of Zambezian Cichlidogyrus species (Platyhelminthes: Monogenea: Ancyrocephalidae) parasitizing Congolian cichlids. Zootaxa. 2013; 3608: 398–400. 10.11646/zootaxa.3608.5.8 [DOI] [PubMed] [Google Scholar]
- 47. Vanhove MPM, Economou AN, Zogaris S, Giakoumi S, Zanella D, Volckaert FAM, et al. The Gyrodactylus (Monogenea, Gyrodactylidae) parasite fauna of freshwater sand gobies (Teleostei, Gobioidei) in their centre of endemism, with description of seven new species. Parasitol Res. 2014; 113: 653–668. 10.1007/s00436-013-3693-8 [DOI] [PubMed] [Google Scholar]
- 48. Pariselle A, Morand S, Deveney M, Pouyaud L. Parasite species richness of closely related hosts: historical scenario and “genetic” hypothesis In: Combes C, Jourdan J, editors. Hommage à Louis Euzet—Taxonomie, écologie et évolution des métazoaires parasites. Taxonomy, ecology and evolution of metazoan parasites Perpignan: Presses Universitaires de Perpignan; 2003. pp. 147–166. [Google Scholar]
- 49. Pariselle A, Euzet L. Systematic revision of dactylogyridean parasites (Monogenea) from cichlid fishes in Africa, the Levant and Madagascar. Zoosystema. 2009; 31: 849–898. [Google Scholar]
- 50. Kritsky DC, Boeger WA. The phylogenetic status of the Ancyrocephalinae Bychowsky, 1937 (Monogenea: Dactylogyroidea). J Parasitol. 1989; 75: 207–211. [PubMed] [Google Scholar]
- 51. Šimková A, Plaisance L, Matějusová I, Morand S, Verneau O. Phylogenetic relationships of the Dactylogyridae Bychowsky, 1933 (Monogenea: Dactylogyridea): the need for the systematic revision of the Ancyrocephalinae Bychowsky, 1937. Syst Parasitol. 2003; 54: 1–11. [DOI] [PubMed] [Google Scholar]
- 52. Šimková A, Matějusová I, Cunningham CO. A molecular phylogeny of the Dactylogyridae sensu Kritsky & Boeger (1989) (Monogenea) based on the D1-D3 domains of large subunit rDNA. Parasitology. 2006; 133: 43–54. [DOI] [PubMed] [Google Scholar]
- 53. Plaisance L, Littlewood DTJ, Olson PD, Morand S. Molecular phylogeny of gill monogeneans (Platyhelminthes, Monogenea, Dactylogyridae) and colonization of Indo‐West Pacific butterflyfish hosts (Perciformes, Chaetodontidae). Zool Scr. 2005; 34: 425–436. [Google Scholar]
- 54. Vanhove MPM, Volckaert FAM, Pariselle A. Ancyrocephalidae (Monogenea) of Lake Tanganyika: I: Four new species of Cichlidogyrus from Ophthalmotilapia ventralis (Teleostei, Cichlidae), the first record of this family in the basin. Zoologia (Curitiba, Impr). 2011; 28: 253–263. [Google Scholar]
- 55. Gillardin C, Vanhove MPM, Pariselle A, Huyse T, Volckaert FAM. Ancyrocephalidae (Monogenea) of Lake Tanganyika: II: description of the first Cichlidogyrus spp. parasites from Tropheini fish hosts (Teleostei, Cichlidae). Parasitol Res. 2012; 110: 305–313. 10.1007/s00436-011-2490-5 [DOI] [PubMed] [Google Scholar]
- 56. Pariselle A, Muterezi Bukinga F, Van Steenberge M, Vanhove MPM. Ancyrocephalidae (Monogenea) of Lake Tanganyika: IV: Cichlidogyrus parasitizing species of Bathybatini (Teleostei, Cichlidae): reduced host-specificity in the deepwater realm? Hydrobiologia. 2015; 748: 99–119. [Google Scholar]
- 57. Pariselle A, Van Steenberge M, Snoeks J, Volckaert FAM, Huyse T, Vanhove MPM. Ancyrocephalidae (Monogenea) of Lake Tanganyika: does the Cichlidogyrus parasite fauna of Interochromis loocki (Teleostei, Cichlidae) reflect its host’s phylogenetic affinities? Contrib Zool. 2015; 84: 25–38. [Google Scholar]
- 58. Vanhove MPM, Snoeks J, Volckaert FAM, Huyse T. First description of monogenean parasites in Lake Tanganyika: the cichlid Simochromis diagramma (Teleostei, Cichlidae) harbours a high diversity of Gyrodactylus species (Platyhelminthes, Monogenea). Parasitology. 2011; 138: 364–380. 10.1017/S0031182010001356 [DOI] [PubMed] [Google Scholar]
- 59. Yamaoka K. Trophic ecomorphology of Tanganyikan cichlids In: Kawanabe H, Hori M, Nagoshi M, editors. Fish communities in Lake Tanganyika. Kyoto: Kyoto University Press; 1997: pp. 25–56. [Google Scholar]
- 60. Boulenger GA. Report on the fishes recently obtained by Mr. J.E.S. Moore in Lake Tanganyika. P Zool Soc Lond. 1898; 3: 494–497. [Google Scholar]
- 61. Boulenger GA. Report on the collection of fishes made by Mr. J.E.S. Moore in Lake Tanganyika during his expedition, 1895–1896. With an Appendix by J.E.S. Moore, A.R.C.S. Trans Zool Soc London. 1898; 15: 1–30. [Google Scholar]
- 62. Pellegrin J. Mission Guy Babault, poisons du lac Tanganyika. B Mus Natl Hist Nat Série 1. 1927; 33: 499–501. 10.1007/s00436-010-2144-z [DOI] [PubMed] [Google Scholar]
- 63. Poll M. Cichlidae nouveaux du Lac Tanganyika appartenant aux collections du Musée du Congo. Revue de Zoologie et de Botanique Africaines. 1942; 36: 343–360. [Google Scholar]
- 64. Nelissen MHJ. Pseudosimochromis, a new genus of the family Cichlidae (Pisces) from Lake Tanganyika. Revue de Zoologie Africaine. 1977; 91: 1–54. [Google Scholar]
- 65. Poll M. Poissons Cichlidae. Exploration Hydrobiologique du Lac Tanganika (1946–1947). Résultats scientifiques. 1956; 3: 1–619. [Google Scholar]
- 66. Nelissen MHJ. Description of Simochromis pleurospilus sp. nov. a sibling species of S. babaulti from Lake Tanganyika (Pisces, Cichlidae). Revue de Zoologie Africaine. 1978; 92: 3 [Google Scholar]
- 67. Konings A. Tanganyika cichlids in their natural habitat El Paso: Cichlid Press; 1998. [Google Scholar]
- 68. Van Steenberge M, Vanhove MPM, Muzumani Risasi D, Mulimbwa N’simbula T, Muterezi Bukinga F, Pariselle A, et al. A recent inventory of the fishes of the north-western and central western coast of Lake Tanganyika (Democratic Republic Congo). Acta Ichthyol Piscat. 2011; 41: 201–214. [Google Scholar]
- 69. Axelrod GS, Harrison JA. Simochromis margaretae, a new species of cichlid fish from Lake Tanganyika. Special publication of the J.L.B. Smith institute of ichthyology. 1978; 9: 1–16. [Google Scholar]
- 70. Nelissen MHJ. A taxonomic revision of the genera Simochromis, Pseudosimochromis and Tropheus (Pisces, Cichlidae). Annales du Musée Royal de l’Afrique Centrale. Sciences Zoologiques, Série 8. 1979; 229: 1–54. [Google Scholar]
- 71. Barel CDN, van Oijen MJP, Witte F, Witte-Maas E. An introduction to the taxonomy and morphology of the haplochromine cichlidae of Lake Victoria. Neth J Zool. 1977; 27: 333–389. [Google Scholar]
- 72. Snoeks J. The cichlid diversity of Lake Malawi/Nyasa/Niassa: identification, distribution and taxonomy El Paso: Cichlid Press; 2004. [Google Scholar]
- 73. Jolicoeur P. The multivariate generalisation of the allometry equation. Biometrics. 1963; 19: 497–499. [Google Scholar]
- 74. Van Steenberge M, Vanhove MPM, Breman FC, Snoeks J. Complex geographical variation patterns in Tropheus duboisi Marlier, 1959 (Perciformes, Cichlidae) from Lake Tanganyika. Hydrobiologia. 2015; 748: 39–60. [Google Scholar]
- 75. Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol. 2011; 28: 2731–2739. 10.1093/molbev/msr121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Kimura M. A simple method for estimating evolutionary rate of base substitutions through comparative studies of nucleotide sequences. J Mol Evol. 1980; 16: 111–120. [DOI] [PubMed] [Google Scholar]
- 77. Malmberg G. On the occurence of Gyrodactylus on Swedish fishes. Skr Söd Sver Fiskför Asskr 1956. 1957; 19–76. [in Swedish] [Google Scholar]
- 78.Gussev AV. Order Dactylogyridea. In: Bychovskaya-Pavlovskaya IE, Gussev AV, Dubinina MN, Izymova NA, Smirnova TS, Sokolovskaya IL, Shtein GA, Shul’man SS, Epsthein VM, editors. Key to the parasites of freshwater fish of the USSR. Jerusalem: Israel Program for Scientific Translations; 1962 (1964). pp. 204–342. [Russian original: Opredelitel’ parazitov presnovohnyh ryb SSSR. Moscow-Leningrad: Izadtel’stovo Akademii Nauk SSSR]
- 79. Euzet L, Prost M. Report of the meeting on Monogenea: problems of systematics, biology and ecology In: Slusarski W, editor. Review of advances in parasitology. Warsaw: P.W.N. Polish Scientific Publishers; 1981: pp. 1003–1004. [Google Scholar]
- 80. Pariselle A, Euzet L. Gill parasites of the genus Cichlidogyrus Paperna, 1960 (Monogenea, Ancyrocephalidae) from Tilapia guineensis (Bleeker, 1862), with descriptions of six new species. Syst Parasitol. 1995; 30: 187–198. [Google Scholar]
- 81. Frey JK, Yates TL, Duszynski DW, Gannon WL, Gardner SL. Designation and curatorial management of type host specimens (symbiotypes) for new parasite species. J Parasitol. 1992; 78: 930–932. [Google Scholar]
- 82. Brooks DR. Extending the symbiotype concept to host voucher specimens. J Parasitol. 1993; 79: 631–633. [Google Scholar]
- 83. Paperna I. Studies on monogenetic trematodes in Israel. 2 Monogenetic trematodes of cichlids. Bamidgeh. 1960; 12: 20–33. [Google Scholar]
- 84. Pariselle A, Bilong Bilong C, Euzet L. Four new species of Cichlidogyrus Paperna, 1960 (Monogenea, Ancyrocephalidae), all gill parasites from African mouthbreeder tilapias of the genera Sarotherodon and Oreochromis (Pisces, Cichlidae), with a re-description of C. thurstonae Ergens, 1981. Syst Parasitol. 2003; 56: 201–210. [DOI] [PubMed] [Google Scholar]
- 85. Pariselle A, Euzet L. Four new species of Cichlidogyrus (Monogenea: Ancyrocephalidae), gill parasites of Tilapia cabrae (Teleostei: Cichlidae), with discussion on relative length of haptoral sclerites. Folia Parasit. 2003; 50: 195–201. [DOI] [PubMed] [Google Scholar]
- 86. Grégoir AF, Hablützel PI, Vanhove MPM, Pariselle A, Bamps J, Volckaert FAM, et al. Evidence for a link between host dispersal and parasite diversity in two sympatric cichlid fishes of Lake Tanganyika. Freshwater Biol. 2015; 60: 323–335. [Google Scholar]
- 87. Justine J-L. A redescription of Pseudorhabdosynochus epinepheli (Yamaguti, 1938), the type-species of Pseudorhabdosynochus Yamaguti, 1958 (Monogenea: Diplectanidae), and the description of P. satyui n. sp. from Epinephelus akaara off Japan. Syst Parasitol. 2009; 72: 27–55. 10.1007/s11230-008-9171-5 [DOI] [PubMed] [Google Scholar]
- 88. Pouyaud L, Desmarais E, Deveney M, Pariselle A. Phylogenetic relationships among monogenean gill parasites (Dactylogyridea, Ancyrocephalidae) infesting tilapiine hosts (Cichlidae): systematic and evolutionary implications. Mol Phylogenet Evol. 2006; 38: 241–249. [DOI] [PubMed] [Google Scholar]
- 89. Vignon M, Pariselle A, Vanhove MPM. Modularity in attachment organs of African Cichlidogyrus (Platyhelminthes, Monogenea, Ancyrocephalidae) reflects phylogeny rather than host specificity or geographic distribution. Biol J Linn Soc. 2011; 102: 694–706. [Google Scholar]
- 90. Risch S, Snoeks J. Geographic variation in Neolamprologus niger (Poll, 1956) (Perciformes, Cichlidae) from Lake Tanganyika (Africa). Zootaxa. 2008; 1857: 21–32. [Google Scholar]
- 91. Günther A. Descriptions of the reptiles and fishes collected by Mr. E. Coode-Hore on Lake Tanganyika. Proc Zool Soc Lond. 1894; 1893: 628–632. [Google Scholar]
- 92. Trewavas E. The name and natural distribution of the Tilapia from Zanzibar (Pisces, Cichlidae) World symposium on warm-water pond fish culture. Rome: FAO; 1966. [Google Scholar]
- 93. Steindachner F. Sitzung der mathematisch-naturwissenschaftligen klasse vom 9. December 1909. Kaiserliche Akademie der Wissenschaften in Wien. 1909; 26: 443–445. [Google Scholar]
- 94. Trewavas E. Tilapiine fishes of the genera Sarotherodon, Oreochromis and Danakilia London: British Museum (Natural History); 1983. [Google Scholar]
- 95. Takahashi T. Systematics of Tanganyikan cichlid fishes (Teleostei: Perciformes). Ichthyol Res. 2003; 50: 367–382. [Google Scholar]
- 96. Seehausen O. Hybridisation and adaptive radiation. Trends Ecol Evol. 2004; 19: 198–2007. [DOI] [PubMed] [Google Scholar]
- 97. Schliewen UK, Klee B. Reticulate speciation in Camerounian crater lake cichlids. Front Zool. 2004; 1: 5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Koblmüller S, Schliewen UK, Duftner N, Sefc KM, Katongo C, Sturmbauer C. Age and spread of the haplochromine cichlid fishes in Africa. Mol Phylogenet Evol. 2008; 49: 153–169. 10.1016/j.ympev.2008.05.045 [DOI] [PubMed] [Google Scholar]
- 99. Brichard P. Pierre Brichard’s book of cichlids and all the other fishes of Lake Tanganyika Neptune City: TFH Publishers; 1989. [Google Scholar]
- 100. Agosta SJ, Janz N, Brooks DR. How specialists can be generalists: resolving the "parasite paradox" and implications for emerging infectious disease. Zoologia (Curitiba, Impr). 2010; 27: 151–162. [Google Scholar]
- 101. Huyse T, Volckaert FAM. Comparing host and parasite phylogenies: Gyrodactylus flatworms jumping from goby to goby. Syst Biol. 2005; 54: 710–718 [DOI] [PubMed] [Google Scholar]
- 102. Brooks DR. Testing the context and extent of host-parasite coevolution. Syst Biol. 1979; 28: 299–307. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Measurements and meristics taken on 114 specimens belonging to Simochromis and Pseudosimochromis and measurements taken on the specimens of Cichlidogyrus raeymaekersi sp. nov., C. muterezii sp. nov., C. banyankimbonai sp. nov., Cichlidogyrus georgesmertensi sp. nov., C. franswittei sp. nov. and C. frankwillemsi sp. nov.
(XLSX)
Data Availability Statement
Morphological data is contained within the paper and and its Supporting Information files. Sequences are available from NCBI GenBank under accession numbers KP336420-KP336458. Nomenclatural acts are registered in ZooBank. Type and vouchers specimens are deposited in the Royal Museum for Central Africa, the Muséum national d'Histoire naturelle and the Iziko South African Museum.