Abstract
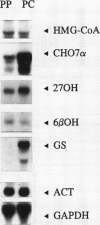
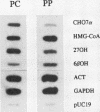
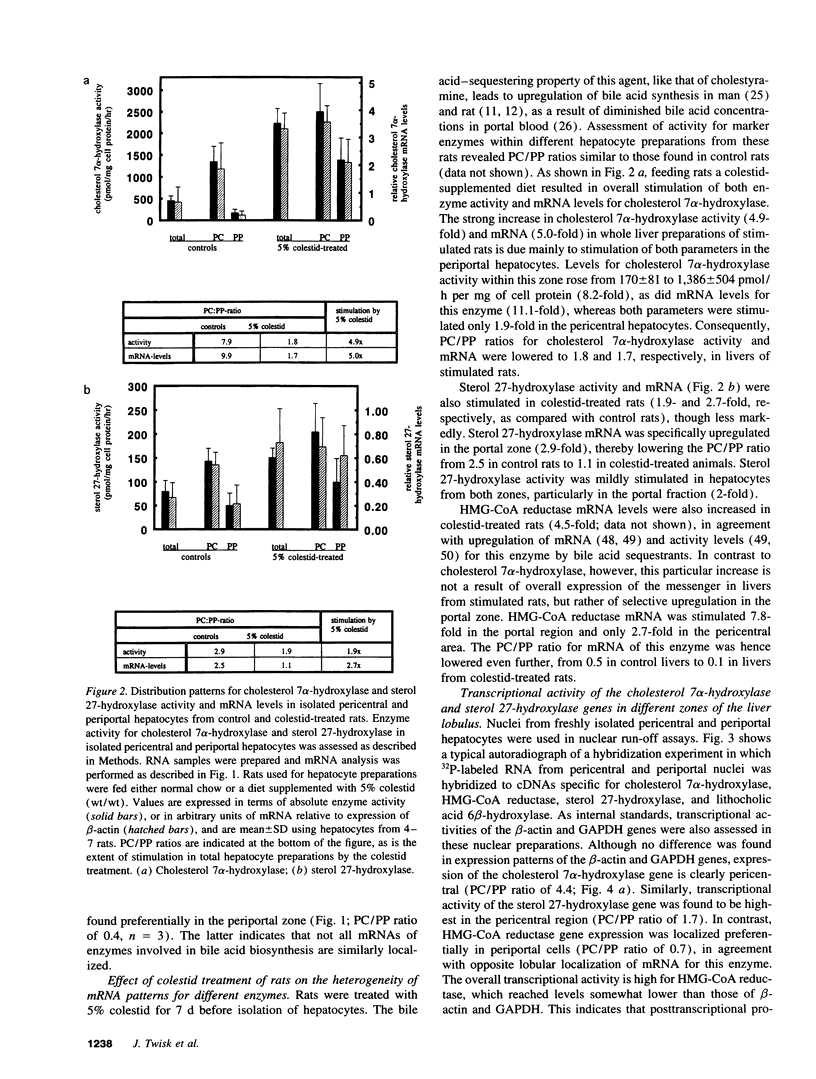
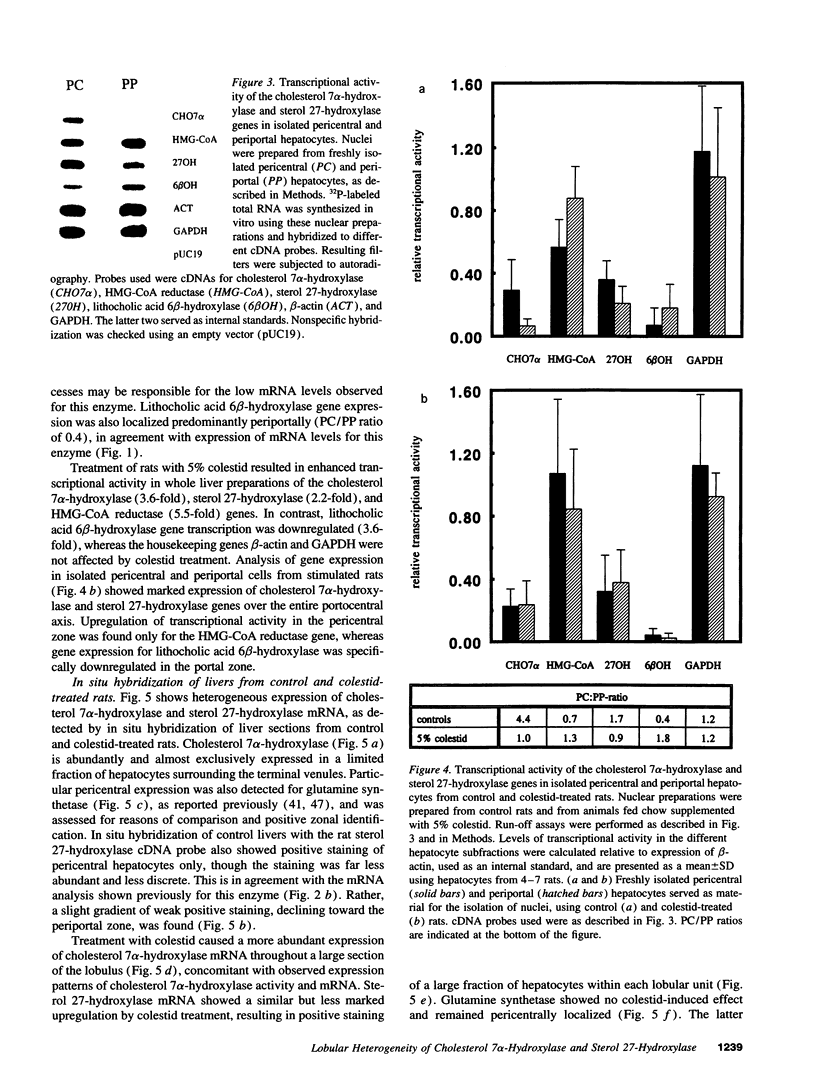
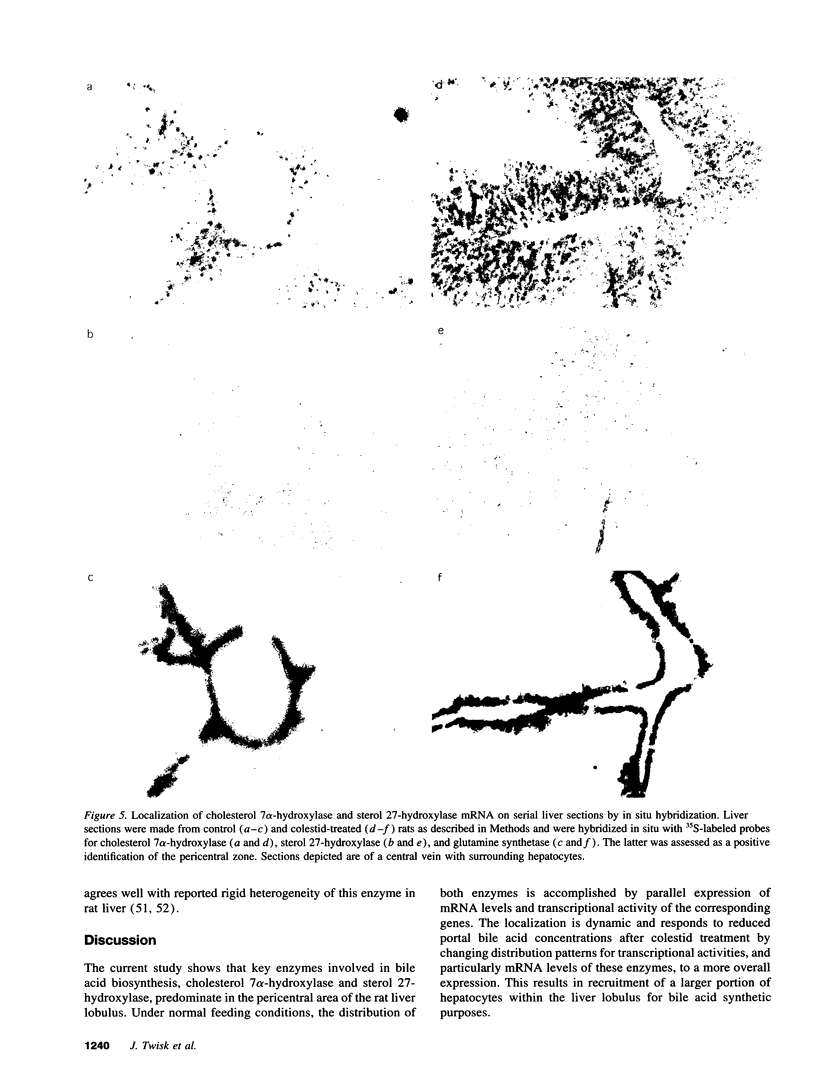
We investigated the lobular localization and molecular level of expression of cholesterol 7 alpha-hydroxylase and sterol 27-hydroxylase, two key enzymes in bile acid synthesis, in isolated periportal and pericentral hepatocytes and by in situ hybridization of rat liver. Enzyme activity, mRNA, and gene transcription of cholesterol 7 alpha-hydroxylase were predominant in pericentral hepatocytes of control rats, being 7.9-, 9.9-, and 4.4-fold higher than in periportal hepatocytes, respectively. Similar localization was found for sterol 27-hydroxylase: 2.9-, 2.5-, and 1.7-fold higher enzyme activity, mRNA, and gene transcription, respectively, was found in pericentral hepatocytes. Interruption of the enterohepatic circulation with colestid resulted in upregulation of these parameters for both enzymes, as a consequence of stimulated gene expression mainly in the periportal zone. In contrast, mRNA levels and gene transcription of 3-hydroxy-3-methylglutaryl CoA reductase showed opposite lobular distribution. Selective periportal expression for the latter was enhanced, but remained local, after colestid treatment. In situ hybridization showed unambiguously that cholesterol 7 alpha-hydroxylase mRNA is localized exclusively in the pericentral zone and that sterol 27-hydroxylase mRNA is expressed preferentially in the pericentral region, though less pronounced. Administration of colestid led to expression of both genes within a larger area of the liver lobulus. In conclusion, we suggest that cholesterol 7 alpha-hydroxylase and sterol 27-hydroxylase are coordinately regulated by the bile acid gradient over the lobulus, resulting in predominant expression in the pericentral zone. Opposite lobular localization of cholesterol and bile acid synthesis provides an alternative view to interregulation of these metabolic pathways.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson S., Davis D. L., Dahlbäck H., Jörnvall H., Russell D. W. Cloning, structure, and expression of the mitochondrial cytochrome P-450 sterol 26-hydroxylase, a bile acid biosynthetic enzyme. J Biol Chem. 1989 May 15;264(14):8222–8229. [PubMed] [Google Scholar]
- Axelson M., Sjövall J. Potential bile acid precursors in plasma--possible indicators of biosynthetic pathways to cholic and chenodeoxycholic acids in man. J Steroid Biochem. 1990 Aug 28;36(6):631–640. doi: 10.1016/0022-4731(90)90182-r. [DOI] [PubMed] [Google Scholar]
- Balasubramaniam S., Mitropoulos K. A., Myant N. B. Evidence for the compartmentation of cholesterol in rat-liver microsomes. Eur J Biochem. 1973 Apr 2;34(1):77–83. doi: 10.1111/j.1432-1033.1973.tb02730.x. [DOI] [PubMed] [Google Scholar]
- Baumgartner U., Schölmerich J., Karsch J., Gerok W., Farthmann E. H. Loss of zonal heterogeneity and cell polarity in rat liver with respect to bile acid secretion after bile drainage. Gastroenterology. 1991 Apr;100(4):1054–1061. doi: 10.1016/0016-5085(91)90282-p. [DOI] [PubMed] [Google Scholar]
- Björkhem I., Lewenhaupt A. Preferential utilization of newly synthesized cholesterol as substrate for bile acid biosynthesis. An in vivo study using 18O2-inhalation technique. J Biol Chem. 1979 Jun 25;254(12):5252–5256. [PubMed] [Google Scholar]
- Björkhem I. Mechanism of degradation of the steroid side chain in the formation of bile acids. J Lipid Res. 1992 Apr;33(4):455–471. [PubMed] [Google Scholar]
- Botham K. M., Lawson M. E., Beckett G. J., Percy-Robb I. W., Boyd G. S. Portal blood concentrations of conjugated cholic and chenodeoxycholic acids. Relationships to bile salt synthesis in liver cells. Biochim Biophys Acta. 1981 Jul 24;665(1):81–87. doi: 10.1016/0005-2760(81)90235-6. [DOI] [PubMed] [Google Scholar]
- Burger H. J., Gebhardt R., Mayer C., Mecke D. Different capacities for amino acid transport in periportal and perivenous hepatocytes isolated by digitonin/collagenase perfusion. Hepatology. 1989 Jan;9(1):22–28. doi: 10.1002/hep.1840090105. [DOI] [PubMed] [Google Scholar]
- Chin D. J., Gil G., Russell D. W., Liscum L., Luskey K. L., Basu S. K., Okayama H., Berg P., Goldstein J. L., Brown M. S. Nucleotide sequence of 3-hydroxy-3-methyl-glutaryl coenzyme A reductase, a glycoprotein of endoplasmic reticulum. Nature. 1984 Apr 12;308(5960):613–617. doi: 10.1038/308613a0. [DOI] [PubMed] [Google Scholar]
- Clarke C. F., Edwards P. A., Lan S. F., Tanaka R. D., Fogelman A. M. Regulation of 3-hydroxy-3-methylglutaryl-coenzyme A reductase mRNA levels in rat liver. Proc Natl Acad Sci U S A. 1983 Jun;80(11):3305–3308. doi: 10.1073/pnas.80.11.3305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebhardt R., Ebert A., Bauer G. Heterogeneous expression of glutamine synthetase mRNA in rat liver parenchyma revealed by in situ hybridization and Northern blot analysis of RNA from periportal and perivenous hepatocytes. FEBS Lett. 1988 Dec 5;241(1-2):89–93. doi: 10.1016/0014-5793(88)81037-8. [DOI] [PubMed] [Google Scholar]
- Gebhardt R. Heterogeneous intrahepatic distribution of glutamine synthetase. Acta Histochem Suppl. 1990;40:23–28. [PubMed] [Google Scholar]
- Gebhardt R., Jung W. Biliary secretion of sodium fluorescein in primary monolayer cultures of adult rat hepatocytes and its stimulation by nicotinamide. J Cell Sci. 1982 Aug;56:233–244. doi: 10.1242/jcs.56.1.233. [DOI] [PubMed] [Google Scholar]
- Gebhardt R., Mecke D. Heterogeneous distribution of glutamine synthetase among rat liver parenchymal cells in situ and in primary culture. EMBO J. 1983;2(4):567–570. doi: 10.1002/j.1460-2075.1983.tb01464.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebhardt R. Metabolic zonation of the liver: regulation and implications for liver function. Pharmacol Ther. 1992;53(3):275–354. doi: 10.1016/0163-7258(92)90055-5. [DOI] [PubMed] [Google Scholar]
- Gebhardt R., Williams G. M. Amino acid transport in established adult rat liver epithelial cell lines. Cell Biol Toxicol. 1986 Mar;2(1):9–20. doi: 10.1007/BF00117703. [DOI] [PubMed] [Google Scholar]
- Goresky C. A. Uptake in the liver: the nature of the process. Int Rev Physiol. 1980;21:65–101. [PubMed] [Google Scholar]
- Groothuis G. M., Hardonk M. J., Keulemans K. P., Nieuwenhuis P., Meijer D. K. Autoradiographic and kinetic demonstration of acinar heterogeneity of taurocholate transport. Am J Physiol. 1982 Dec;243(6):G455–G462. doi: 10.1152/ajpgi.1982.243.6.G455. [DOI] [PubMed] [Google Scholar]
- Grundy S. M., Bilheimer D. W. Inhibition of 3-hydroxy-3-methylglutaryl-CoA reductase by mevinolin in familial hypercholesterolemia heterozygotes: effects on cholesterol balance. Proc Natl Acad Sci U S A. 1984 Apr;81(8):2538–2542. doi: 10.1073/pnas.81.8.2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoekman M. F., Rientjes J. M., Twisk J., Planta R. J., Princen H. M., Mager W. H. Transcriptional regulation of the gene encoding cholesterol 7 alpha-hydroxylase in the rat. Gene. 1993 Aug 25;130(2):217–223. doi: 10.1016/0378-1119(93)90422-y. [DOI] [PubMed] [Google Scholar]
- Jelinek D. F., Andersson S., Slaughter C. A., Russell D. W. Cloning and regulation of cholesterol 7 alpha-hydroxylase, the rate-limiting enzyme in bile acid biosynthesis. J Biol Chem. 1990 May 15;265(14):8190–8197. [PMC free article] [PubMed] [Google Scholar]
- Jones A. L., Hradek G. T., Renston R. H., Wong K. Y., Karlaganis G., Paumgartner G. Autoradiographic evidence for hepatic lobular concentration gradient of bile acid derivative. Am J Physiol. 1980 Mar;238(3):G233–G237. doi: 10.1152/ajpgi.1980.238.3.G233. [DOI] [PubMed] [Google Scholar]
- Jungermann K., Katz N. Functional hepatocellular heterogeneity. Hepatology. 1982 May-Jun;2(3):385–395. doi: 10.1002/hep.1840020316. [DOI] [PubMed] [Google Scholar]
- Jungermann K., Katz N. Functional specialization of different hepatocyte populations. Physiol Rev. 1989 Jul;69(3):708–764. doi: 10.1152/physrev.1989.69.3.708. [DOI] [PubMed] [Google Scholar]
- Kempen H. J., Vos-Van Holstein M. P., de Lange J. Bile acids and lipids in isolated rat hepatocytes: content, synthesis, and release, as affected by cholestyramine treatment of the donor rats. J Lipid Res. 1982 Aug;23(6):823–830. [PubMed] [Google Scholar]
- Kempen H. J., Vos-van Holstein M., de Lange J. Bile acids and lipids in isolated rat hepatocytes. II. Source of cholesterol used for bile acid formation, estimated by incorporation of tritium from tritiated water, and by the effect of ML-236B. J Lipid Res. 1983 Mar;24(3):316–323. [PubMed] [Google Scholar]
- Kwekkeboom J., Princen H. M., van Voorthuizen E. M., Kempen H. J. Bile acids exert negative feedback control on bile acid synthesis in cultured pig hepatocytes by suppression of cholesterol 7 alpha-hydroxylase activity. Hepatology. 1990 Nov;12(5):1209–1215. doi: 10.1002/hep.1840120522. [DOI] [PubMed] [Google Scholar]
- Lamers W. H., Hilberts A., Furt E., Smith J., Jonges G. N., van Noorden C. J., Janzen J. W., Charles R., Moorman A. F. Hepatic enzymic zonation: a reevaluation of the concept of the liver acinus. Hepatology. 1989 Jul;10(1):72–76. doi: 10.1002/hep.1840100115. [DOI] [PubMed] [Google Scholar]
- Li A. C., Tanaka R. D., Callaway K., Fogelman A. M., Edwards P. A. Localization of 3-hydroxy-3-methylglutaryl CoA reductase and 3-hydroxy-3-methylglutaryl CoA synthase in the rat liver and intestine is affected by cholestyramine and mevinolin. J Lipid Res. 1988 Jun;29(6):781–796. [PubMed] [Google Scholar]
- Li Y. C., Wang D. P., Chiang J. Y. Regulation of cholesterol 7 alpha-hydroxylase in the liver. Cloning, sequencing, and regulation of cholesterol 7 alpha-hydroxylase mRNA. J Biol Chem. 1990 Jul 15;265(20):12012–12019. [PubMed] [Google Scholar]
- Lindros K. O., Penttilä K. E. Digitonin-collagenase perfusion for efficient separation of periportal or perivenous hepatocytes. Biochem J. 1985 Jun 15;228(3):757–760. doi: 10.1042/bj2280757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindros K. O., Penttilä K. E., Gaasbeek Janzen J. W., Moorman A. F., Speisky H., Israel Y. The gamma-glutamyltransferase/glutamine synthetase activity ratio. A powerful marker for the acinar origin of hepatocytes. J Hepatol. 1989 May;8(3):338–343. doi: 10.1016/0168-8278(89)90032-9. [DOI] [PubMed] [Google Scholar]
- Liscum L., Luskey K. L., Chin D. J., Ho Y. K., Goldstein J. L., Brown M. S. Regulation of 3-hydroxy-3-methylglutaryl coenzyme A reductase and its mRNA in rat liver as studied with a monoclonal antibody and a cDNA probe. J Biol Chem. 1983 Jul 10;258(13):8450–8455. [PubMed] [Google Scholar]
- Long T. T., 3rd, Jakoi L., Stevens R., Quarfordt S. The sources of rat biliary cholesterol and bile acid. J Lipid Res. 1978 Sep;19(7):872–878. [PubMed] [Google Scholar]
- Moorman A. F., De Boer P. A., Vermeulen J. L., Lamers W. H. Practical aspects of radio-isotopic in situ hybridization on RNA. Histochem J. 1993 Apr;25(4):251–266. doi: 10.1007/BF00159117. [DOI] [PubMed] [Google Scholar]
- Moorman A. F., de Boer P. A., Geerts W. J., van den Zande L., Lamers W. H., Charles R. Complementary distribution of carbamoylphosphate synthetase (ammonia) and glutamine synthetase in rat liver acinus is regulated at a pretranslational level. J Histochem Cytochem. 1988 Jul;36(7):751–755. doi: 10.1177/36.7.2898495. [DOI] [PubMed] [Google Scholar]
- Noshiro M., Nishimoto M., Okuda K. Rat liver cholesterol 7 alpha-hydroxylase. Pretranslational regulation for circadian rhythm. J Biol Chem. 1990 Jun 15;265(17):10036–10041. [PubMed] [Google Scholar]
- Princen H. M., Huijsmans C. M., Kuipers F., Vonk R. J., Kempen H. J. Ketoconazole blocks bile acid synthesis in hepatocyte monolayer cultures and in vivo in rat by inhibiting cholesterol 7 alpha-hydroxylase. J Clin Invest. 1986 Oct;78(4):1064–1071. doi: 10.1172/JCI112662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Princen H. M., Meijer P., Kwekkeboom J., Kempen H. J. Assay of cholesterol 7 alpha-hydroxylase activity in rat hepatocytes in primary monolayer culture. Anal Biochem. 1988 May 15;171(1):158–165. doi: 10.1016/0003-2697(88)90137-6. [DOI] [PubMed] [Google Scholar]
- Princen H. M., Meijer P., Wolthers B. G., Vonk R. J., Kuipers F. Cyclosporin A blocks bile acid synthesis in cultured hepatocytes by specific inhibition of chenodeoxycholic acid synthesis. Biochem J. 1991 Apr 15;275(Pt 2):501–505. doi: 10.1042/bj2750501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quistorff B. Gluconeogenesis in periportal and perivenous hepatocytes of rat liver, isolated by a new high-yield digitonin/collagenase perfusion technique. Biochem J. 1985 Jul 1;229(1):221–226. doi: 10.1042/bj2290221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reihnér E., Rudling M., Ståhlberg D., Berglund L., Ewerth S., Björkhem I., Einarsson K., Angelin B. Influence of pravastatin, a specific inhibitor of HMG-CoA reductase, on hepatic metabolism of cholesterol. N Engl J Med. 1990 Jul 26;323(4):224–228. doi: 10.1056/NEJM199007263230403. [DOI] [PubMed] [Google Scholar]
- Robins S. J., Fasulo J. M., Collins M. A., Patton G. M. Evidence for separate pathways of transport of newly synthesized and preformed cholesterol into bile. J Biol Chem. 1985 Jun 10;260(11):6511–6513. [PubMed] [Google Scholar]
- Scheibner J., Fuchs M., Schiemann M., Tauber G., Hörmann E., Stange E. F. Bile acid synthesis from newly synthesized vs. preformed cholesterol precursor pools in the rat. Hepatology. 1993 Jun;17(6):1095–1102. [PubMed] [Google Scholar]
- Sherman I. A., Fisher M. M. Hepatic transport of fluorescent molecules: in vivo studies using intravital TV microscopy. Hepatology. 1986 May-Jun;6(3):444–449. doi: 10.1002/hep.1840060321. [DOI] [PubMed] [Google Scholar]
- Singer I. I., Kawka D. W., Kazazis D. M., Alberts A. W., Chen J. S., Huff J. W., Ness G. C. Hydroxymethylglutaryl-coenzyme A reductase-containing hepatocytes are distributed periportally in normal and mevinolin-treated rat livers. Proc Natl Acad Sci U S A. 1984 Sep;81(17):5556–5560. doi: 10.1073/pnas.81.17.5556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stravitz R. T., Hylemon P. B., Heuman D. M., Hagey L. R., Schteingart C. D., Ton-Nu H. T., Hofmann A. F., Vlahcevic Z. R. Transcriptional regulation of cholesterol 7 alpha-hydroxylase mRNA by conjugated bile acids in primary cultures of rat hepatocytes. J Biol Chem. 1993 Jul 5;268(19):13987–13993. [PubMed] [Google Scholar]
- Su P., Rennert H., Shayiq R. M., Yamamoto R., Zheng Y. M., Addya S., Strauss J. F., 3rd, Avadhani N. G. A cDNA encoding a rat mitochondrial cytochrome P450 catalyzing both the 26-hydroxylation of cholesterol and 25-hydroxylation of vitamin D3: gonadotropic regulation of the cognate mRNA in ovaries. DNA Cell Biol. 1990 Nov;9(9):657–667. doi: 10.1089/dna.1990.9.657. [DOI] [PubMed] [Google Scholar]
- Tanaka R. D., Edwards P. A., Lan S. F., Knöppel E. M., Fogelman A. M. The effect of cholestyramine and Mevinolin on the diurnal cycle of rat hepatic 3-hydroxy-3-methylglutaryl coenzyme A reductase. J Lipid Res. 1982 Sep;23(7):1026–1031. [PubMed] [Google Scholar]
- Teixeira J., Gil G. Cloning, expression, and regulation of lithocholic acid 6 beta-hydroxylase. J Biol Chem. 1991 Nov 5;266(31):21030–21036. [PubMed] [Google Scholar]
- Twisk J., Lehmann E. M., Princen H. M. Differential feedback regulation of cholesterol 7 alpha-hydroxylase mRNA and transcriptional activity by rat bile acids in primary monolayer cultures of rat hepatocytes. Biochem J. 1993 Mar 15;290(Pt 3):685–691. doi: 10.1042/bj2900685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twisk J., de Wit E. C., Princen H. M. Suppression of sterol 27-hydroxylase mRNA and transcriptional activity by bile acids in cultured rat hepatocytes. Biochem J. 1995 Jan 15;305(Pt 2):505–511. doi: 10.1042/bj3050505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ugele B., Kempen H. J., Kempen J. M., Gebhardt R., Meijer P., Burger H. J., Princen H. M. Heterogeneity of rat liver parenchyma in cholesterol 7 alpha-hydroxylase and bile acid synthesis. Biochem J. 1991 May 15;276(Pt 1):73–77. doi: 10.1042/bj2760073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlahcevic Z. R., Pandak W. M., Hylemon P. B., Heuman D. M. Role of newly synthesized cholesterol or its metabolites on the regulation of bile acid biosynthesis after short-term biliary diversion in the rat. Hepatology. 1993 Sep;18(3):660–668. [PubMed] [Google Scholar]
- de Groot C. J., ten Voorde G. H., van Andel R. E., te Kortschot A., Gaasbeek Janzen J. W., Wilson R. H., Moorman A. F., Charles R., Lamers W. H. Reciprocal regulation of glutamine synthetase and carbamoylphosphate synthetase levels in rat liver. Biochim Biophys Acta. 1987 Apr 29;908(3):231–240. doi: 10.1016/0167-4781(87)90103-5. [DOI] [PubMed] [Google Scholar]