Abstract
The introduction of perforator flaps represented a significant advance in microsurgical reconstruction. However, confusion has developed due to the erroneous belief that perforator flaps are different from conventional flaps. The concept of the perforator is not new, but is an idea that evolved from the conventional flap. In fact, some of the flaps used by microsurgeons were perforator flaps. The only difference is the anatomical level of the blood vessels involved; the perforator concept is focused on the distal circulation, so-called 'perforator'. Therefore, thinner sections of tissue can be taken from the conventional donor sites of myocutaneous flaps. With the use of perforators, there are no longer "flap of choice" for specific reconstructions, because conventional donor sites have become universal donor sites, enabling the harvesting of a variety of flaps. Moreover, depending on the surgeon's ability, any flap can be utilized as a perforator-based island flap whose source vessel has been completely preserved. Therefore, tissues can be efficiently customized and tailored into any configuration required for reconstruction. The application of perforator flap technique enables more precise dissection, and allows more selective harvesting of thinner flaps, which will expand options in reconstructive surgery. No doubt the technique will continue to evolve.
Graphical Abstract
Keywords: Surgical Flaps, Perforator Flap, Microsurgery, Reconstruction
INTRODUCTION
The use of perforator flaps in reconstructive surgery is a superior surgical technique when compared to the use of conventional flaps. Initially, the concept of the flap based on a perforating vessel, or 'perforator' was viewed as being new and distinct from the older concept of the conventional flap (1, 2). However, the perforator technique has in fact evolved directly from, and is not very different from, the conventional approach. The lack of experience of surgeons with the techniques involved in perforator flap surgery, and confusion about the nature of the technique, led to the misconception that perforator flap surgery was difficult to perform. This misconception has extended to confusion about the distinction between perforator flaps and earlier classical flaps. In fact, many of the flaps that surgeons have used for years were actually perforator flaps according to the current definition, although they were described by many different names at the time (1, 2, 3).
The development of the perforator flap technique has resulted in an improved understanding of how flaps receive their blood supply (3, 4). Major source vessels supply nutritional blood flow to all tissues they traverse. These major vessels and their main branches send other smaller branches to supply the surrounding muscles, connective tissue, and skin (5). Axial pattern flaps were designed to include axial vessels at the flap base, to provide blood supply (2). It was therefore important to know the precise vascular anatomy to design the flap, which was a branch-based concept. However, as the flap concept developed, flap design evolved from being based on a source vessel or branch concept, to being based on a specific perforating vessel. The successful harvest of a perforator flap requires the identification of a perforating vessel, and dissection in a retrograde fashion (from distal to proximal) (6). Therefore, the route of access to the pedicle is changed: a direct approach to the source vessel is essential in the harvest of a conventional flap, but when harvesting a perforator flap, the approach starts from the distal part of circulation at the subcutaneous tissue layer, and proceeds to the source vessel. As a matter of fact, the flap harvest is frequently free-style depending on the anatomical variations of the pedicle (3, 6, 7). The source and path of a perforating vessel are not the essential considerations of the operation although it is obvious that a perforator always comes from a source vessel (6). Sometimes, a perforator alone can be selectively used as a pedicle (4), so eliminating the proximal dissection (3).
Here, we review historical aspects of the development of the flap technique and provide an overview of perforator flaps. The application of the perforator flap technique enables more precise dissection, and allows more selective harvesting of thinner flaps, which has expanded options in reconstructive surgery. There is no doubt that the technique will continue to develop.
CONCEPT AND CLASSIFICATION OF PERFORATOR FLAPS
Overall, the vasculature of the skin and subcutaneous tissue is arranged in five vascular plexuses: the subepidermal plexus, dermal plexus, subdermal plexus, subcutaneous plexus, and fascial plexus (subfascial and suprafascial). Conventional musculocutaneous or fasciocutaneous flaps are connected to a source vessel under the muscle, or to vessels at the fascial level (5). On the other hand, a perforator flap is connected to vessels of the subdermal or subcutaneous plexus, which therefore involve a connection to more distal vascularity than a conventional flap (8, 9).
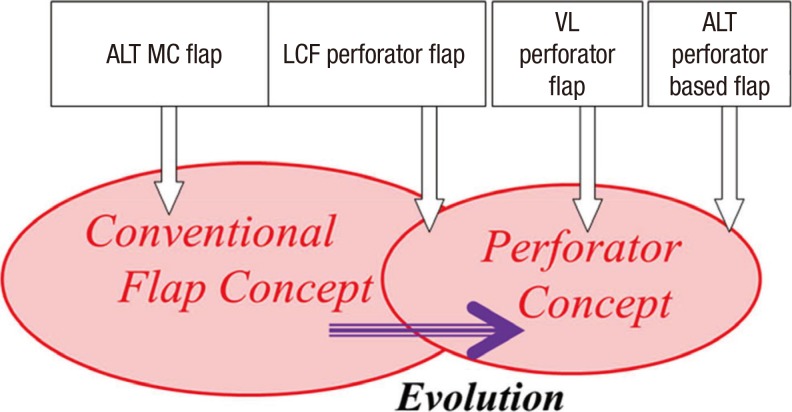
Based on the path taken by the perforating vessel, perforators can be categorized as direct cutaneous, septocutaneous, and musculocutaneous perforators (10). The exact definition of perforator is still controversial. When the concept of the perforator was first introduced, Wei el al. (11) defined perforating vessels as those of which the source artery is deep and the branch that carries blood directly to the fasciocutaneous tissues, in its course to reach the skin, passes through the overhanging muscular tissue without exclusively following the intermuscular septum. By this definition, only musculocutaneous perforators are considered true perforators, and flaps based on other types of perforator are not regarded as perforator flaps. However, this rigid definition has caused confusion about the concept of the perforator. The anterolateral thigh (ALT) flap, one of the most popular perforator flaps, is a representative example that demonstrates the differences between perforator flaps and conventional flaps. The anterolateral thigh flap is based on a perforator connected to the descending branch of the lateral circumflex femoral system (6, 12, 13). Most of the time, the perforator comes through the vastus lateralis muscle (82%) and is therefore musculocutaneous, or it is a septocutaneous perforator, which is easier to harvest and use than the musculocutaneous perforator (14). According to strict definitions, the flap based on the musculocutaneous perforator is a perforator flap and the flap based on the septocutaneous perforator is not a perforator flap. These flaps differ from each other in terms of the ease of pedicle dissection - tedious transmuscular dissection in musculocutaneous flaps, as opposed to easy and rapid dissection between muscles in septocutaneous flaps (6, 14). Most microsurgeons tried to select septocutaneous perforators to save operating time before the development of the perforator concept, but now transmuscular dissection is more popular due to the reliability of the perforator. Moreover, when the perforator is reliable and the length is sufficient, pedicle dissection stops at the level of the perforator without requiring transmuscular dissection (6). Therefore, the ALT flap has evolved from a myocutaneous flap, to a range of flaps based on either septocutaneous or musculocutaneous perforators. As it is important to distinguish between these flaps, workers have named the different flaps 'ALT myocutaneous flaps,' 'lateral circumflex femoral perforator flaps,' 'vastus lateralis perforator flaps,' and 'ALT perforator based flaps,' depending on the perforators involved (10). The former two patterns are conventional flaps while the latter three are perforator flaps. The lateral circumflex femoral flap can be included in both groups: it was initially designated an ALT (conventional) flap but is now considered a perforator flap, which illustrates the confusion surrounding the perforator concept. That is the reason why we insist that the concept of the perforator flap is not completely distinct from that of the conventional flap concept (Fig. 1). We therefore conclude that flaps previously described as conventional flaps were in fact perforator flaps, and that flaps based on septocutaneous perforators should be considered perforator flaps (3, 10).
Fig. 1. Diagram of a conventional flap and its derived perforator flap. The ALP MC flap and LCF perforator flap (SCp-based) were commonly used flaps before the perforator concept was introduced. After the perforator concept was introduced, the LCF perforator flap was regarded as a perforator flap, and the VL perforator flap (MCp-based) and ALT perforator based flaps were challenged. The LCF perforator flap is now included in both concepts and it is a perforator flap. Therefore, perforators can not be completely distinguished from the conventional flaps and some classical flaps were in fact perforator flaps, even though they were described by different names. ALT MC flap, anterolateral thigh myocutaneous flap; LCF perforator flap, lateral femoral circumflex perforator flap; SCp, septocutaneous perforator; VL perforator flap, vastus lateralis perforator flap; MCp, musculocutaneous perforator.
Most skin flaps are now elevated based on their perforator instead of on their source vessel or branch, and direct cutaneous perforators (DCp) should also be included in the perforator group (3, 10, 15). However, in terms of the level of surgical dissection required septocutaneous perforators and DCps, which are not very different from each other, should be differentiated from musculocutaneous perforators, which require transmuscular dissection during elevation (14). Therefore, perforator flaps based on musculocutaneous perforators are named after the muscle involved. On the other hand, flaps based on a septocutaneous perforator or direct cutaneous perforator are named after their source vessel (Fig. 2, 3) (10). A flap based on a perforator itself, without further proximal dissection, is referred to as a perforator-"based" flap and is named after the associated muscle (MCp) or vessel (SCp) (Fig. 4) (10, 16).
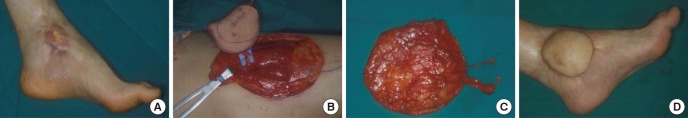
Fig. 2. Vastus lateralis perforator flap. (A) A 57-yr-old male patient has cellulitis after a crush injury to the right foot that required amputation. (B, C) A 12×15 cm Vastus lateralis perforator flap is elevated based on musculocutaneous perforators. (D) Postoperative view at 12-month follow-up.
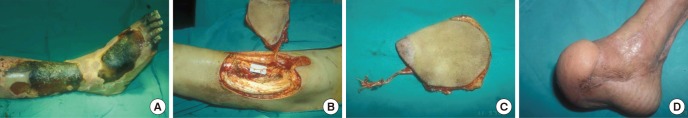
Fig. 3. Lateral circumflex femoral perforator flap. (A) A 46-yr-old patient has an open tibia fracture fixed with an external fixator. However, the overlying skin becomes necrotic with exposure of the tibia. (B, C) A 10×8 cm lateral circumflex femoral perforator flap is elevated in flow-through pattern. (D) Two months after flap transfer good contour is maintained.
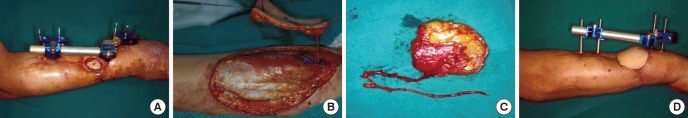
Fig. 4. Vastus lateralis perforator-based flap. (A) Recurrent ulceration is present on an unstable burn scar in the medial malleolus area. (B, C) After debridement, an 8×8 cm Vastus lateralis perforator-based flap was elevated as a free flap, and perforator-to-perforator anastomosis was performed. (D) Postoperative 3-month view.
HISTORICAL PERESPECTIVE
The concept of axial flaps developed in the 1970s. A major discovery was the pedicle groin flap described by McGregor and Morgan (2) and McGregor and Jackson (17), based on an incorporated axial blood supply. The importance of the blood supply to the skin flap limited the design of the flap in specific regions of the body to the axial blood supply (2, 5, 18). Subsequently, more detailed knowledge of vascular anatomy allowed better flap selection and refinement (Fig. 5). Owing to the presence of multiple perforators in the inguinal region, flaps became more versatile, and free-style design became possible. Therefore, the term, "groin flap" was not commonly used. Instead, names such as the 'superficial circumflex iliac perforator flap', 'superficial inferior epigastric perforator flap', or 'external pudendal perforator flap' were used (Fig. 6, 7, 8). Before the development of the perforator concept, the method of dissection of an inguinal flap harvest was described in textbooks, and guidelines or landmarks were provided for a safe and successful flap harvest. However, with development of the perforator concept, a greater variety of flap designs became possible and the size and composition of the harvested perforator flaps could be more freely modified. For example, thin perforator flaps from the inguinal region can be used for thin resurfacing of the extremities; a pedicle flap can be used for genital resurfacing; vascularized lymph nodes can be transferred; and bulky flaps for volume replacement can be harvested, such as bulky superficial inferior epigastric perforator flaps for breast reconstruction (8, 19). The more advanced surgical techniques made possible by the perforator concept allowed the conventional donor site to become a "universal donor site", which enabled greater variation in the design, composition, and use of flaps according to the specific needs of the patient. The perforator flap is not a new, difficult flap, but is rather the product of the application of evolving and improved surgical techniques to more common conventional flaps. The inguinal pedicled flap used to be the "flap of choice" for resurfacing of the hand, but now the inguinal area can be source of a wide variety of perforator flaps, depending on what is required.
Fig. 5. Groin flap and perforator flaps in the right groin area. Inguinal flaps were conventional flaps harvested in textbook style, and frequently used for emergent hand surfacing. After the introduction of the perforator concept, several different perforator flaps became available for various reconstructive purposes; for example, SIEp, SCIp, EPp flaps for thin resurfacing, vascularized lymph node transfer or breast reconstruction. The design and incision is free-style, without regard to any landmarks and the flap harvest is simpler and faster than for the conventional inguinal flap. SIEp , superficial inferior epigastric perforator flap; SCIp, superficial circumflex iliac perforator flap; EPp, external pudendal perforator flap.
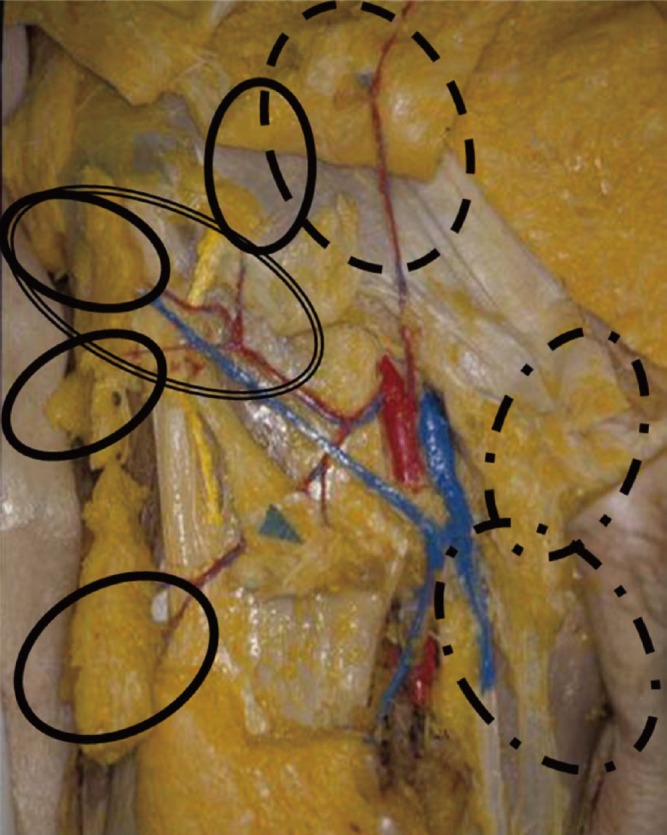
Fig. 6. Thin resurfacing with a superficial inferior epigastric perforator flap. (A) 32-yr-old patient who underwent an amputation of a crushed left hand. (B) After replantation, the hand dorsum required thin resurfacing, and a superficial inferior epigastric perforator flap is harvested. (C) Postoperative 6-month view after resurfacing.
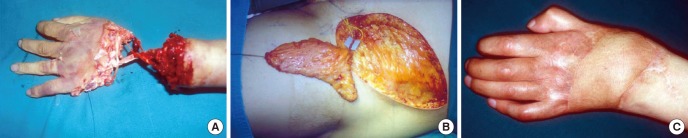
Fig. 7. Thin resurfacing with a superficial circumflex iliac perforator flap. (A) A 47-yr-old patient who had a degloving injury of all the fingers. (B) A 15×22 cm superficial circumflex iliac perforator flap was harvested for resurfacing followed by division of the flap after 3 months. (C) The atient was able to pinch and grasp, with natural contours of the fingers.
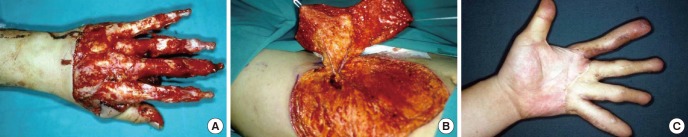
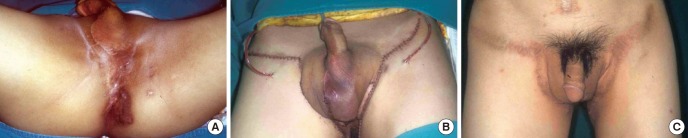
Fig. 8. Scrotal reconstruction using an external pudendal perforator-based island flap. (A) A 29-yr-old patient has scrotal loss with buried testis. (B) Scrotal reconstruction is performed with bilateral external pudendal perforator-based island flaps. (C) 6-month after the reconstruction, the natural shape of the scrotum is obtained by thin flap reconstruction, and the donor site scar along the inguinal crease is concealed.
It was shown that the skin obtains its blood supply through the underlying muscle in many regions of the body (5). This finding led to the development of musculocutaneous flaps. The successful use of these flaps was attributable to the robust blood supply of muscle (14, 20, 21, 22, 23). However, harvesting muscle often resulted in a subsequent loss of donor site function, and the bulkiness of the resulting flaps often led to inaccurate reconstruction and poor aesthetic results (1). In the same period, the inclusion of the deep fascia, and reliance on vessels at the fascial level (subfascial and suprafascial plexus), resulted in the development of the very reliable fasciocutaneous flap (14, 24, 25, 26). In 1989, Koshima and Soeda (27) introduced a new type of the flap based on perforators, which was composed exclusively of skin and subcutaneous tissue. This flap is a different from fasciocutaneous flaps. The fascial plexus is not necessary for vascularization (3, 27, 28). Exclusion of the muscle is possible if a small artery perforating the muscle and vascularizing the skin (a muscle perforator) is preserved (3, 14, 27). The use of the perforator flap evolved as an improvement over the use of the musculocutaneous flap, and made it possible for the muscle itself to be left behind (1, 14, 19, 27, 29).
EVOLUTION OF FLAPS
Resurfacing with thin flaps
The ability to conduct thin resurfacing is one of the advantages of perforator flaps (3, 9, 13). The thickness of a flap for contouring or resurfacing can be reduced by elevating a thin flap containing only the superficial adipose layers (10, 16). The subcutaneous layer is composed of superficial and deep adipose layer (30). The superficial adipose layer has a constant thickness, more vascularity, and is composed of small pinkish fat granules. On the other hand, the deep adipose layer has a variable thickness, less vascularity, and is composed of large yellow fat granules. A skin flap of constant thickness based on a perforator can be elevated over a large distance by excluding the deep adipose layer (16). Using monopolar electrocautery and with the flap held under tension with skin hooks, the flap can be thinned between the superficial and deep adipose layers (16, 31).
Conventional donor sites turn universal
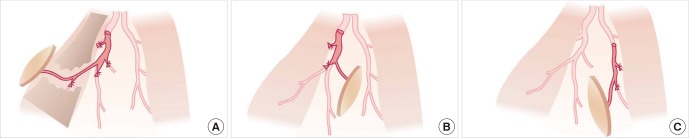
The lateral thoracic area, from which the latissimus dorsi myocutaneous flaps can be harvested, is now used as donor site for perforator flaps, such as the latissimus dorsi perforator (LDp) flap, thoracodorsal perforator (TDp) flap, and lateral thoracic perforator (LTp) flap (Fig. 9) (16, 31). The skin overlying the lateral thoracic area has a rich blood supply from three rows of perforators that originate from the thoracodorsal and lateral thoracic vessels (31, 32). From anterior to posterior, the three groups of perforators in the lateral thoracic region are: 1) direct cutaneous perforators from the lateral thoracic artery, 2) septocutaneous perforators form the thoracodorsal artery, and 3) musculocutaneous perforators also from the thoracodorsal artery (16, 31, 32). These perforators have the same reliability as a pedicle of the perforator flap. Since they can all be considered to originate from the same donor site (31, 32), they have to be distinguished by a different nomenclatures. According to the our nomenclature, flaps based on a septocutaneous perforator or direct cutaneous perforator are named after the proximal vessel, while flaps based on the musculocutaneous perforator are named after the muscle (10, 31). Accordingly, the 'latissimus dorsi perforator flap' signifies the perforator flap based on the musculocutaneous perforator, the 'thoracodorsal perforator flap' is the perforator flap based on the septocutaneous perforator, and the 'lateral thoracic perforator flap' is based on the direct cutaneous perforator from the lateral thoracic artery (10, 33). Therefore, the conventional donor site of muscle or myocutaneous flaps turns into a "universal donor site" in the context of the perforator concept.
Fig. 9. Three perforator flaps from the lateral thoracic area. (A) Latissimus dorsi perforator (LDp) flap. (B) Thoracodorsal perforator (TDp) flap. (C) Lateral thoracic perforator (LTp) flap.
No more "flap of choice"
When planning primary closure of the donor site in the lateral thoracic area, thin flaps up to 20 cm in length and 10 cm in width can be safely designed (31). These flaps can be used effectively for resurfacing of the hand, pretibia, or foot (16). Moreover, they can be easily shaped and designed to suit the defect. In the case of tongue reconstruction, flaps can be shaped into a pyramidal form in a thin wrapping pattern, or with moderate thickness to create the tongue base. Also, a larger flap can be tailored into a tubed flap for esophageal reconstruction, which is much easier than the use of abdominal surgery for jejunal flap harvest. In another case, a perforator flap can be used for hemilaryngeal reconstruction (Fig. 10). The forearm and jejunal flaps were "flap of choice" for head, neck and esophageal reconstruction. Now, these flaps are being replaced by perforator flaps, since the latter are more diverse and versatile in terms of their design and composition (12, 15). Improved knowledge and understanding of vascular anatomy has enabled better tailoring of the flaps, and refinement of the reconstructive surgery involved (15, 19).
Fig. 10. Hemilaryngeal reconstruction. (A) Lateral thoracic perforator flaps are elevated for hemilaryngeal reconstruction in a laryngeal cancer patient. (B, C) A superthin lateral thoracic perforator flap is folded and shaped to create the curvature of a true vocal cord and a false vocal cord.
Customized/tailored reconstruction
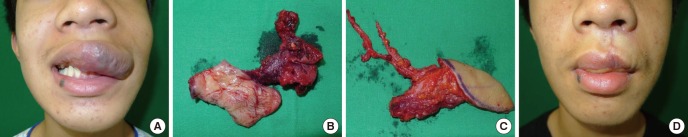
Improved knowledge of the blood supply, together with development of the perforator concept, have increased the variety of soft tissue compositions of flaps available to surgeons (15, 16). Dermoadiposal flaps, adiposal flaps, adipofascial flaps, and myoadipofascial flaps can all be harvested from the same donor sites, allowing for improved tailoring of the flaps and more refinement in their reconstruction (13, 33, 34). For a patient with maxillary cancer, each perforator component of the flap had to be tailored to the size and composition of the defect, which required the reconstruction of the oral lining, nasal lining, and outer resurfacing of the cheek (35). This degree of customized or tailored reconstruction was possible because the perforator flaps can have an appropriate composition that allows them to be inserted into the defect like an assembly of missing blocks. For another patient with a venous malformation of the upper lip, a free flap could be utilized to obliterate the dead space after tumor ablation and to prevent tumor recurrence. After radical resection of the vascular tumor, a perforator flap was tailored to resemble the resected tissue from the tumor (Fig. 11).
Fig. 11. Tailored reconstruction of the upper lip. (A) A 14-yr-old male presents with a venous malformation on his upper lip and left cheek. (B) The venous malformation is radically resected. (C) A 5×3 cm, thin lateral thoracic perforator flap is designed for upper lip reconstruction. (D) Six months after the surgery, the venous malformation has subsided without recurrence.
Perforator-based island flaps
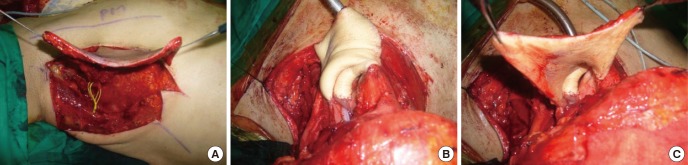
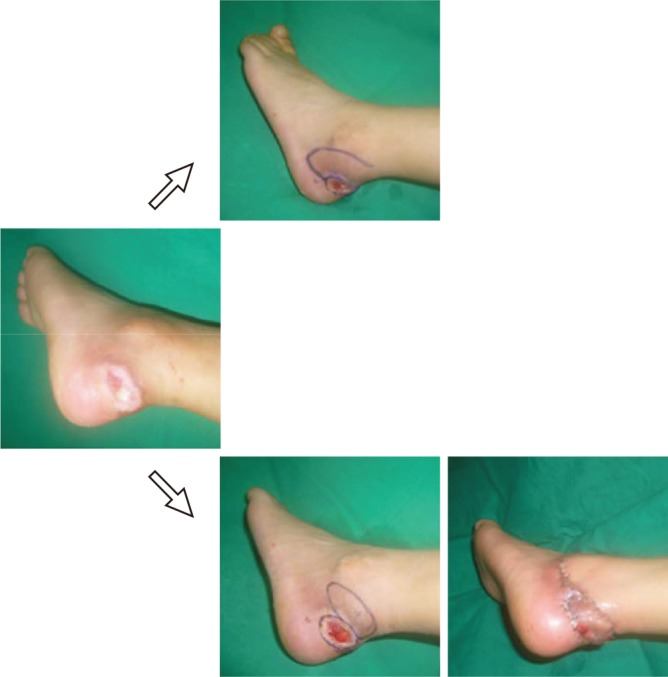
One potential application of the perforator flaps is the use of tissue adjacent to the defect as a perforator-based island flap (PBIF). The term 'perforator-based' is used for the flap when it is truly based on the perforator itself, with the proximal vessel saved (10, 16); the dissection is suspended at the suprafascial or intramuscular level without requiring the sacrifice of the proximal vessel (31). A PBIF can be elevated from any region where perforators exist, offering greater freedom in the design of the flap (16, 31, 34, 36, 37). Reconstruction becomes simple, providing more options in the selection of an appropriate design, and resolves the problems that previously required a free flap (25, 36, 37). For example, the forearm flap is a fasciocutaneous flap with the septum and deep fascia included, requiring sacrifice of the radial artery and vein. With the use of a single perforator, the associated flap is comparable to a similarly sized island pedicle flap (15). In the case of a small defect that results in Achilles tendon exposure, the conventional treatment involves a lateral calcaneal flap, which requires the sacrifice of source vessels as well as a skin graft at the donor site. However, by using a PBIF, the source vessel can be preserved and the donor site can be closed without the need of a skin graft (Fig. 12).
Fig. 12. Conventional design vs. perforator-based island flaps. Example of a conventional design (above) and a design based on a perforator-based island flap (PBIF) (below) for reconstructing a defect of the Achilles tendon. The PBIF is more acceptable than the conventional lateral calcaneal flap for several reasons: there is no sacrifice of the lateral calcaneal vessel, no need for a skin graft, and primary closure of donor site.
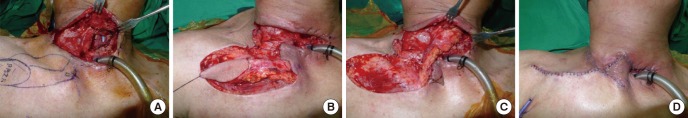
The perforator-based island flap is one of the most successful clinical applications of the perforator concept: the same size of flap can be elevated based on a perforator, without requiring the sacrifice of the source vessel (16, 31). The elevation of more challenging island flaps is made possible by their simple design and easy dissection, and the straightforward closure of the donor site (31). Moreover, a reliable flap can be harvested that can be rotated sufficiently to cover the defect (24, 25, 37). Based on multiple perforators available adjacent to a defect, a PBIF can be harvested quickly and reliably, and more efficient transfer of harveted tissues to the defect is possible (15, 24).
The use of flaps in reconstructive surgery has progressed from the use of musculocutaneous flaps to fasciocutaneous flaps, then to perforator flaps, and finally to perforator-based island flaps. Random pattern flaps were a misnomer, since their blood supply was preserved by the presence of tiny perforators that surgeons were unaware of, and such flaps are in fact now considered to be perforator-based island flaps (1, 15).
Moreover, a wide variety of types and designs of flaps are available, depending on the surgeon's creativity and ability (1, 16). For example, a patient suffered a fistula at the neoesophagus after head and neck surgery. To reconstruct the fistula, multiple surgical options were available: local flaps such as conventional pectoralis major island flaps, or free flaps, such as forearm flaps. Perforators from the internal mammary vessels were detected near the defect, and a perforator-based island flap was designed, based on those perforators. The flap was elevated and turned over to the defect with primary closure of the donor site (Fig. 13). This simple and efficient design of a PBIF was made possible by understanding the structure, reliability, and function (the provision of a vascular supply to the skin flap) of the perforator (16). The perforator concept allow the efficient use of tissues in the design of perforator flaps (1, 35). Therefore, surgeons can reduce times and efforts required by surgical procedures to elevate a flap. A skilled surgeon who is familiar with the concept and techniques of perforators can achieve excellent results.
Fig. 13. A perforator-based island flap. (A) A fistula developed in the neoesophagus. (B, C) A perforator-based island flap (PBIF) based on internal mammary vessels is elevated and turned over to the defect to repair the esophageal fistula. (D) The donor site was closed primarily.
CONCLUSION
The introduction of perforator flaps represented a significant advance in the field of microsurgical reconstruction. Undoubtedly, perforator dissection is a tedious procedure for beginners, involving a long and steep learning curve, and requiring both a great deal of experiences in tiny pedicle dissection, and confidence in anatomical knowledge (1, 16). The perforator concept is not new, but is an idea that evolved from the concept of conventional flaps. The perforator concept allows flaps to be harvested and manipulated with more reliability and confidence, even in challenging cases. Perforator flaps have multiple advantages over conventional flaps (16). Thin flap resurfacing is possible and the thickness of the flap can be well-controlled while preserving the deep adipose layer. There are no longer any "flap of choice" for specific surgical reconstructions because conventional donor sites for 'flap of choice' of a specific surgical composition have become universal donor sites, allowing increased diversity and versatility in the design and composition of flaps. Moreover, depending on the skill and creativity of the surgeon, any flap can be used as a perforator-based island flap in which the source vessel is completely preserved. In that way, tissues can be used very efficiently in customized and tailored reconstruction (15).
The use of perforator flaps is a new paradigm in reconstructive surgery. Perforator flaps are not very different from conventional flaps, but they do replace some conventional flaps. Therefore the long held idea of a "flap of choice" in a given reconstruction is no longer valid. However, misconceptions concerning perforators have caused surgeons to avoid using the technique in favor of conventional flaps. Because many of the challenging reconstructions carried out before the introduction of the perforator concept involved flaps similar to perforator flaps, the latter are best viewed as evolved products of the conventional flap.
Footnotes
DISCLOSURE: The authors have no conflicts of interest to disclose.
References
- 1.Geddes CR, Morris SF, Neligan PC. Perforator flaps: evolution, classification, and applications. Ann Plast Surg. 2003;50:90–99. doi: 10.1097/00000637-200301000-00016. [DOI] [PubMed] [Google Scholar]
- 2.McGregor IA, Morgan G. Axial and random pattern flaps. Br J Plast Surg. 1973;26:202–213. doi: 10.1016/0007-1226(73)90003-9. [DOI] [PubMed] [Google Scholar]
- 3.Sinna R, Boloorchi A, Mahajan AL, Qassemyar Q, Robbe M. What should define a "perforator flap"? Plast Reconstr Surg. 2010;126:2258–2263. doi: 10.1097/PRS.0b013e3181f61824. [DOI] [PubMed] [Google Scholar]
- 4.Blondeel PN, Morris SF, Hallock GG, Heligan PC. Perforator flaps: anatomy, technique and clinical application. St. Louis: Quality Medical; 2006. [Google Scholar]
- 5.Taylor GI, Palmer JH. The vascular territories (angiosomes) of the body: experimental study and clinical applications. Br J Plast Surg. 1987;40:113–141. doi: 10.1016/0007-1226(87)90185-8. [DOI] [PubMed] [Google Scholar]
- 6.Wei FC, Celik N. Perforator flap entity. Clin Plast Surg. 2003;30:325–329. doi: 10.1016/s0094-1298(03)00033-6. [DOI] [PubMed] [Google Scholar]
- 7.Wei FC, Mardini S. Free-style free flaps. Plast Reconstr Surg. 2004;114:910–916. doi: 10.1097/01.prs.0000133171.65075.81. [DOI] [PubMed] [Google Scholar]
- 8.Grover R, Nelson JA, Fischer JP, Kovach SJ, Serletti JM, Wu LC. The impact of perforator number on deep inferior epigastric perforator flap breast reconstruction. Arch Plast Surg. 2014;41:63–70. doi: 10.5999/aps.2014.41.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saint-Cyr M, Schaverien MV, Rohrich RJ. Perforator flaps: history, controversies, physiology, anatomy, and use in reconstruction. Plast Reconstr Surg. 2009;123:132e–145e. doi: 10.1097/PRS.0b013e31819f2c6a. [DOI] [PubMed] [Google Scholar]
- 10.Kim JT. New nomenclature concept of perforator flap. Br J Plast Surg. 2005;58:431–440. doi: 10.1016/j.bjps.2004.12.009. [DOI] [PubMed] [Google Scholar]
- 11.Wei FC, Jain V, Suominen S, Chen HC. Confusion among perforator flaps: what is a true perforator flap? Plast Reconstru Surg. 2001;107:874–876. doi: 10.1097/00006534-200103000-00037. [DOI] [PubMed] [Google Scholar]
- 12.Kang MJ, Chung CH, Chang YJ, Kim KH. Reconstruction of the lower extremity using free flaps. Arch Plast Surg. 2013;40:575–583. doi: 10.5999/aps.2013.40.5.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Agostini T, Russo GL, Zhang YX, Spinelli G, Lazzeri D. Adipofascial anterolateral thigh flap safety: applications and complications. Arch Plast Surg. 2013;40:91–96. doi: 10.5999/aps.2013.40.2.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee MJ, Yun IS, Rah DK, Lee WJ. Lower extremity reconstruction using vastus lateralis myocutaneous flap versus anterolateral thigh fasciocutaneous flap. Arch Plast Surg. 2012;39:367–375. doi: 10.5999/aps.2012.39.4.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pribaz JJ, Chan RK. Where do perforator flaps fit in our armamentarium? Clin Plast Surg. 2010;37:571–579. doi: 10.1016/j.cps.2010.06.007. [DOI] [PubMed] [Google Scholar]
- 16.Kim JT. Latissimus dorsi perforator flap. Clin Plast Surg. 2003;30:403–431. doi: 10.1016/s0094-1298(03)00041-5. [DOI] [PubMed] [Google Scholar]
- 17.McGregor IA, Jackson IT. The groin flap. Br J Plast Surg. 1972;25:3–16. doi: 10.1016/s0007-1226(72)80003-1. [DOI] [PubMed] [Google Scholar]
- 18.Zayakova Y, Stanev A, Mihailov H, Pashaliev N. Application of local axial flaps to scalp reconstruction. Arch Plast Surg. 2013;40:564–569. doi: 10.5999/aps.2013.40.5.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yoo KW, Shin HW, Lee HK. A case of urethral reconstruction using a superficial circumflex iliac artery. Arch Plast Surg. 2012;39:253–256. doi: 10.5999/aps.2012.39.3.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Han DH, Park MC, Park DH, Song H, Lee IJ. Role of muscle free flap in the salvage of complicated scalp wounds and infected prosthetic dura. Arch Plast Surg. 2013;40:735–741. doi: 10.5999/aps.2013.40.6.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yoon SK, Song SH, Kang N, Yoon YH, Koo BS, Oh SH. Reconstruction of the head and neck region using lower trapezius musculocutaneous flaps. Arch Plast Surg. 2012;39:626–630. doi: 10.5999/aps.2012.39.6.626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McCraw JB, Vasconez LO. Musculocutaneous flaps: principles. Clin Plast Surg. 1980;7:9–13. [PubMed] [Google Scholar]
- 23.Mathes SJ, Nahai F. Clinical atlas of muscle and musculocutaneous flaps. St. Louis: CV Mosby; 1979. [Google Scholar]
- 24.Yang J, Ko SH, Oh SJ, Jung SW. Reconstruction of a perineoscrotal defect using bilateral medial thigh fasciocutaneous flaps. Arch Plast Surg. 2013;40:72–74. doi: 10.5999/aps.2013.40.1.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shin IS, Lee DW, Rah DK, Lee WJ. Reconstruction of pretibial defect using pedicled perforator flaps. Arch Plast Surg. 2012;39:360–366. doi: 10.5999/aps.2012.39.4.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pontén B. The fasciocutaneous flap: its use in soft tissue defects of the lower leg. Br J Plast Surg. 1981;34:215–220. doi: 10.1016/s0007-1226(81)80097-5. [DOI] [PubMed] [Google Scholar]
- 27.Koshima I, Soeda S. Inferior epigastric artery skin flaps without rectus abdominis muscle. Br J Plast Surg. 1989;42:645–648. doi: 10.1016/0007-1226(89)90075-1. [DOI] [PubMed] [Google Scholar]
- 28.Park JS, Roh SG, Lee NH, Yang KM. Versatility of the distally-based sural artery fasciocutaneous flap on the lower leg and foot in patients with chronic disease. Arch Plast Surg. 2013;40:220–225. doi: 10.5999/aps.2013.40.3.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tan S, Lim J, Yek J, Ong WC, Hing CH, Lim TC. The deep inferior epigastric perforator and pedicled transverse rectus abdominis myocutaneous flap in breast reconstruction: a comparative study. Arch Plast Surg. 2013;40:187–191. doi: 10.5999/aps.2013.40.3.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kimura N, Satoh K. Consideration of a thin flap as an entity and clinical applications of the thin anterolateral thigh flap. Plast Reconstr Surg. 1996;97:985–992. doi: 10.1097/00006534-199604001-00016. [DOI] [PubMed] [Google Scholar]
- 31.Kim JT. Two options for perforator flaps in the flank donor site: latissimus dorsi and thoracodorsal perforator flaps. Plast Reconstr Surg. 2005;115:755–763. doi: 10.1097/01.prs.0000152427.09893.80. [DOI] [PubMed] [Google Scholar]
- 32.Kim JT, Ng SW, Naidu S, Kim JD, Kim YH. Lateral thoracic perforator flap: additional perforator flap option from the lateral thoracic region. J Plast Reconstr Aesthet Surg. 2011;64:1596–1602. doi: 10.1016/j.bjps.2011.06.048. [DOI] [PubMed] [Google Scholar]
- 33.Yang JD, Ryu DW, Lee JW, Choi KY, Chung HY, Cho BC, Park HY, Byun JS. Usefulness of a lateral thoracodorsal flap after breast conserving surgery in laterally located breast cancer. Arch Plast Surg. 2013;40:367–373. doi: 10.5999/aps.2013.40.4.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jeong JH, Hong JM, Imanishi N, Lee Y, Chang H. Face reconstruction using lateral intercostal artery perforator-based adipofascial free flap. Arch Plast Surg. 2014;41:50–56. doi: 10.5999/aps.2014.41.1.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.De la Parra M, Sanchez G, Lopez J, Perez A, Naal N. Total maxillary reconstruction using a double-barreled and double skin paddle fibular flap after total maxillectomy. Arch Plast Surg. 2013;40:779–782. doi: 10.5999/aps.2013.40.6.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yoon TH, Yun IS, Rha DK, Lee WJ. Reconstruction of various perinasal defects using facial artery perforator-based nasolabial island flaps. Arch Plast Surg. 2013;40:754–760. doi: 10.5999/aps.2013.40.6.754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lazzeri D, Huemer GM, Nicoli F, Larcher L, Dashti T, Grassetti L, Li Q, Zhang Y, Spinelli G, Agostini T. Indications, outcomes, and complications of pedicled propeller perforator flaps for upper body defects: a systematic review. Arch Plast Surg. 2013;40:44–50. doi: 10.5999/aps.2013.40.1.44. [DOI] [PMC free article] [PubMed] [Google Scholar]