Abstract
Temporomandibular joint (TMJ) disorder is clinically important because of its prevalence, chronicity, and therapy-refractoriness of the pain. In this study, we investigated the effect of infliximab in a mouse model of TMJ pain using a specially-engineered transducer for evaluating the changes in bite force (BF). The mice were randomly divided into three groups (7 mice per group): the control group, the complete Freund's adjuvant (CFA) group, and the infliximab group. BF was measured at day 0 (baseline BF). After measuring the baseline BF, CFA or incomplete Freund's adjuvant was injected into both TMJs and then the changes in BF were measured at days 1, 3, 5, 7, 9, and 13 after the TMJ injection. For measuring the BF, we used a custom-built BF transducer. Control, CFA, and infliximab groups showed similar baseline BF at day 0. From day 1, a significant reduction in BF was observed in the CFA group, and this reduction in BF was statistically significant compared to that in the control group (P < 0.05). This reduction in BF was maintained until day 7, and BF started to recover gradually from day 9. In the infliximab group also, the reduction in BF was observed on day 1, and this reduction was maintained until day 7. However, the degree of reduction in BF was less remarkable compared to that in the CFA group. The reduction in BF caused by injection of CFA into the TMJ could be partially alleviated by the injection of anti-tumor necrosis factor alpha, infliximab.
Graphical Abstract
Keywords: Bite Force, Infliximab, Temporomandibular Joint Pain
INTRODUCTION
Mastication is a very elaborate process, which includes food intake, intra-oral food transport, bolus formation and chewing in all of the mammals, and this activity is regulated by the motor and sensory components of the trigeminal system and their central projections (1, 2). Neural regulation of mastication, which can generate very high bite forces over milliseconds, comprises very rapid sensory feedback from innervated craniofacial structures that include the temporomandibular joint (TMJ), the masticatory muscles, and the teeth (1, 2, 3, 4). Under normal circumstances, mastication is an intrinsic activity that is not consciously perceived by humans and involves very rhythmic jaw movements which are produced by a Central Pattern Generator located in the pons and medulla (3). However, in cases of tissue injury to these structures, mastication can become painful and nonrhythmic, leading to reduced bite force (BF) (5, 6).
Temporomandibular joint disorder (TMJD) is known for its mastication-related pain, and it is clinically important because of its prevalence, chronicity, therapy-refractoriness of the pain, and the largely unknown pain mechanism (7, 8). TMJD is a continuum of various symptoms, which can give rise to progressive degenerative changes in the TMJ with progression of the disease. It encompasses a broad spectrum of conditions, including initial capsulitis or synovitis, more advanced forms of internal derangement, and eventually end-stage degenerative joint disease causing osteoarthritic changes (8).
There are several roadblocks to development of rationally targeted therapies, and one of them is shortcomings of available animal models for TMJD, especially the relative paucity of objective measurements that accurately represent patients' cardinal symptoms. Several studies of TMJ pain in a model frequently used the head withdrawal threshold to a von Frey filament for measuring the nociceptive response in the TMJ (7, 9, 10). However, this method has a limitation in reflecting the patients' cardinal symptom related to mastication, and it simply reflects the pain in the skin and subcutaneous tissue overlying the TMJ. Recently, Chen et al. (11) demonstrated a novel method measuring the BF changes in a mouse model of TMJ pain with a specially designed BF transducer. They also suggested that the direct measure of BF provides a novel quantitative approach to quantifying TMJ pain in the mouse model (11).
Tumor necrosis factor alpha (TNF-α) is known as a key proinflammatory molecule in human rheumatoid arthritis and other chronic inflammatory diseases (12). Recently, TNF-α has attracted remarkable attention and interest in pain research area as one of a putative pain mediators, because the application of TNF-α in healthy tissue could induce thermal hyperalgesia and synaptic long-term potentiation (13). Randomized, placebo-controlled, multi-center clinical trials of human TNF-α inhibitors such as infliximab or etanercept have demonstrated their consistent and remarkable efficacy in improving signs and symptoms, with a favorable safety profile (14, 15, 16).
Lee et al. (8) reported that synovial TNF-α and interleukin 6 levels were elevated in patients with TMJD compared to the normal healthy group, although there was no statistical significance. Therefore, we used Infliximab, a chimeric monoclonal antibody, for investigating whether this drug has any pain relieving effect in a mouse model of chronic TMJ pain. In this study, we evaluated the changes in BF in a mouse model of TMJ pain using a specially-engineered transducer and also investigated the effect of infliximab.
MATERIALS AND METHODS
Animals
Male ICR mice (20-25 g) were housed 5 per cage in a temperature controlled (22±2℃) vivarium under a 12-hr light/dark cycle. Mice were provided a standard rodent diet ad libitium and were allowed to acclimate for 5 days before the experimental procedure.
Induction of TMJ inflammation and intraperitoneal drug injections
The mice were randomly divided into three groups (7 mice per group): 1) the control group, which received only intraperitoneal injection of normal saline with TMJ injection of incomplete Freund's adjuvant (IFA; Chondrex, Redmond, WA, USA); 2) the CFA group, which received intraperitoneal injection of normal saline with TMJ injection of complete Freund's adjuvant (CFA, 5 mg/mL; Chondrex, Redmond, WA, USA); 3) the infliximab group, which received intraperitoneal injection of infliximab (10 mg/kg, dissolved in normal saline) with TMJ injection of CFA.
All mice were briefly anesthetized with 2% isoflurane and bilaterally injected using a 30-gauge needle fitted to a 10 µL Hamilton syringe. To easily identify and palpate the TMJ area, local hairs around the TMJ were trimmed with scissors. After carefully palpating the locally trimmed area considered to be the TMJ, a 30-gauge needle was inserted through the facial skin. The needle was carefully advanced superoanteriorly until the tip of the needle reached the zygomatic arch. Then the needle was slowly moved more inferiorly until it passed under the edge of the arch and ultimately entered into the joint space. Once the needle was located in the joint space, 10 µL of CFA or IFA was injected slowly over a period of 5 sec. Injections were given into both TMJs to minimize the fluctuation in the changes in BF. To evaluate the effect of TNF-alpha neutralizing drug, the infliximab group received a single intraperitoneal injection of infliximab. The control and CFA groups received intraperitoneal injection of the same volume of normal saline on the same day as the infliximab group. Intraperitoneal injection was administered immediately after local TMJ injection was given, and then it was administered daily for 13 days. BF was measured at day 0 (baseline bite force). After measuring the baseline BF, CFA or IFA was injected into both TMJs and then changes in BF were measured at days 1, 3, 5, 7, 9, and 13 after TMJ injection. All measurements were performed by one examiner who was blinded to the study groups.
Bite force analysis
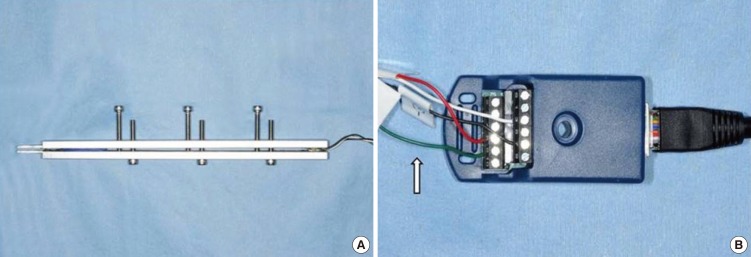
To measure the BF, we used a custom-built BF transducer which was manufactured by KTM Engineering Inc (Seongnam, Korea). This BF transducer was first developed by Williams et al. (17) and Chen et al. (11) also measured the BF reduction with this transducer. Briefly, the transducer consisted of two aluminum beams which were approximated to each other by 6 adjustable screws, and each beam was instrumented with two single-element strain gauges (Fig. 1A). The distance between the beams of the BF transducer was adjusted to 5.0 mm (-40% of maximal jaw opening), at which the mice can produce the maximum BF.
Fig. 1. Photograph showing the transducer (A) and bridge module (B). Arrow indicates the strain gauges.
The four strain gauges were connected in a bridge module (Fig. 1B), and they transmitted the BF signal to the data acquisition hardware. One end of each beam served as the bite plate and the tip of the bite plate was rounded to protect the animals' teeth and oral soft tissue.
Prior to use, the calibration of the BF transducer was performed and checked for linearity by suspending a series of calibration weights ranging from 100 to 500 g from the bite plates. The voltage output from each weight was regressed against the force exerted by calibration weights. Output for each set of calibrations was both linear and reproducible, with correlation coefficients (R2) ranging from 0.9902-0.9998 for each calibration. Mice were placed in a cylindrical plastic tube with an opening at one end for accommodating the mouse head. Placing the mice in a cylindrical tube which permits only head movement is a very stressful condition, and therefore, we spent several minutes to acclimate and calm the mice in a cylindrical tube. When the bite transducer was moved towards the mouse at 0.5-1 cm/sec, a bite was invariably induced. We could confirm the induced bite through the voltage output wave appearing in the monitor. When a proper and maximum bite was induced, the voltage output was recorded as a continuous wave at 500 Hz using Labview 2012 (National Instruments, Austin, TX, USA) (Fig. 2A, B). The peak voltage of each bite was determined and converted into force (Newton, N) based on the regression equation derived from calibration. Each animal was tested 3 times/testing day. The interval between two trials was >2 min. The maximum BF the mice produced per trial was recorded as the actual BF, and then averaged for all trials.
Fig. 2. Bite force voltage output wave through the computer monitor (A, B).
Histological evaluation of TMJ
To evaluate synovial inflammation, histological sections were prepared of the TMJs on day 13. The joint samples were fixed in 10% of neutralized formaldehyde and embedded in paraffin. Before tissue section, the paraffin blocks were decalcified for 1 hr in decalcification solution. Conventional 4 µm sections were obtained from the paraffin blocks and incubated in an oven at 60℃, overnight. Sections were then dewaxed in xylene for 10 min and rehydrated through graded alcohol to distilled water. The hydrated sections were stained with hematoxylin and eosin.
Statistical analysis
All data are expressed as mean±SD. We used one-way ANOVA followed by the Tukey post hoc test for group comparison. P<0.05 indicates a statistically significant difference.
Ethic statement
All animal protocols were approved by the Keimyung University Institutinal Animal Care and Use Committee (KM-2013-06R).
RESULTS
We could measure accurately the changes in BF in mice by using a custom-built aluminum transducer which was fitted to the opening of their mouth. Whenever the maximum bite was induced with this transducer, we could check its force by the voltage output wave through the computer monitor (Fig. 2A, B). However, after injecting CFA, several mice were very reluctant to bite the plate of the transducer due to the pain in the TMJ area. In such cases, we elicited the bite until the proper and maximum bite was induced.
Normal baseline BF without TMJ inflammation was around 20 to 21 N. The induced bite appeared aggressive in nature, in response to the slowly approaching transducer. We believe that the provoked aggression prompted the bite as a proven and valid method for measuring the actual BF because the recorded responses were immediate, robust, and reproducible.
TMJ inflammation was induced by microinjection of CFA, and control injections were composed of IFA. We could observe a prolonged and remarkable reduction in BF over 7 days with this method. Gradually, the BF recovered to more than 80% of the baseline value within 2 weeks. Body weight was reduced by less than 5% (data not shown). The reduction in BF without a reduction in body weight indicates the induction of masticatory sensitization rather than general inflammatory and anorexic effects of inflammation.
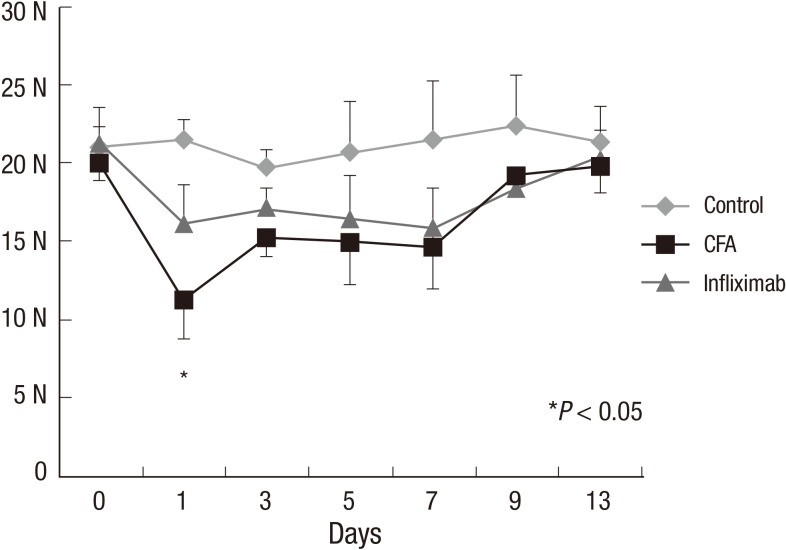
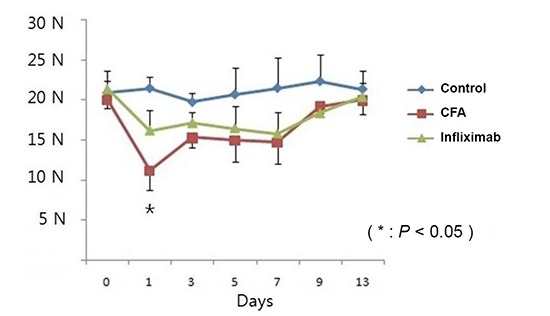
Control, CFA, and infliximab groups showed a similar baseline BF (20 to 21 N) at day 0. From day 1, a significant reduction in BF (nearly 50% of the baseline value) was observed in the CFA group and this reduction was statistically significant compared to that in the control group (Fig. 3) (P<0.05). The reduction in BF was maintained until day 7, and BF started to recover gradually from day 9.
Fig. 3. Changes in bite force from day 0 to day 13 in the control, complete Freund's adjuvant (CFA), and infliximab groups. *Statistically significant compared to that in the CFA group.
In the infliximab group also, the reduction in BF was observed on day 1, and this reduction was maintained until day 7. However, the degree of reduction was less remarkable compared to that in the CFA group (P<0.05) and it did not demonstrate a statistically significant change compared to that in the control group (Fig. 3).
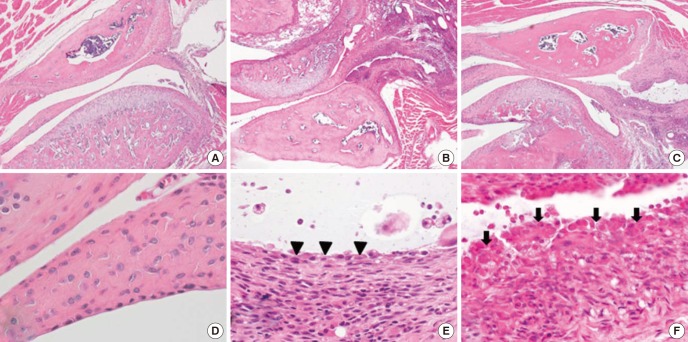
We analyzed the TMJ histology in the control, infliximab and CFA group. Normal, well preserved synovial lining was observed in the control group. CFA group demonstrated severe inflammation with marked synovial hyperplasia while infliximab group with moderate degree of chronic inflammation (Fig. 4A-F).
Fig. 4. Temeporomandibular joint of control (A), infliximab group (B), and CFA group (C) (H&E, ×40). The synovial membrane of control group (D) is smooth and lined by 1-2 synovial cells (H&E, ×400). In infliximab group (E), synovial membrane is lined by 2-3 cells (arrowhead) and shows moderate degree of chronic inflammation (H&E, ×400). The CFA group (F) shows severe inflammation and marked synovial hyperplasia which shows irregularly piled synovial cells (arrows) (H&E, ×400).
DISCUSSION
More than 20% of adults are affected by orofacial pain and some of the most difficult to treat forms of pain result from the TMJD. Patients with TMJD or masticatory muscle inflammation report that chewing induces the highest levels of pain. Patients with TMJD experience significant and prolonged pain compared to normal individuals during opening and closing of the jaw while chewing for an extended period of time (8, 18, 19). Duration of chewing is also related to the development of pain, and Gavish et al. (20) reported that patients with TMJD can experience a significant increase in pain after chewing for 9 min. Therefore, the cardinal symptom of TMJD occurs while chewing, and we tried to demonstrate this phenomenon by using a high throughput, objective assay for BF measurement.
With respect to using the BF in mice to quantify the nociceptive behavior in response to TMJ inflammation, another innovative method has been reported recently which also shows a remarkable similiarity to the clinical features of TMJD. Dolan et al. (21) used the dolognawmeter to quantify the gnawing function in three models of orofacial pain including TMJ inflammation, masticatory myositis, and head and neck cancer. Their method with the use of the dolognawmeter measures functional mechanical allodynia in the rodent of orofacial complex. However, facial mechanical allodynia is not associated with chewing or mastication related pain, which is a dominant clinical feature of TMJD. Taken together, we believe that the direct measurement of BF provides a novel quantitive approach for quantifying TMJ pain in the mouse model.
Our data of BF measurement from 3 groups demonstrated a robust, reproducible, and valid method for quantifying TMJ pain in the mouse model. Baseline BF in the 3 groups measured at day 0 was recorded as 20-21 N. The control group also showed a consistent value ranging from 19-21 N from day 0 to day 13. However, CFA injection into both TMJs dramatically attenuated the BF, which was observed in the CFA group. On day 1 in the CFA group, the BF was as low as 11 N which means a nearly 50% reduction from the baseline value or compared to that in the control group. The maximum attenuation of BF occurred on day 1, and from day 3 to day 7, BF was maintained at 14-15 N. From day 9, BF recovered to as much as 19 N. In contrast with the CFA group, the infliximab group maintained a fairly good BF after CFA injection into the TMJ. From day 1 to day 7, BF was maintained at 15-17 N, and this value was not statistically significant compared to that in the control group.
Wang et al. (7) assessed the nociceptive behavior of animals based on the head withdrawal test after injection of monosodium iodoacetate into the TMJ. The head withdrawal response was significantly decreased from day 1 and continued to decrease until the first 3 weeks after monosodium iodoacetate injection. There is some discrepancy between the period of reduction in BF in our results and the period of decrease in head withdrawal response. However, a direct comparison between these two results has some limitations because we measured the BF and Wang et al. (7) assessed the hyperalgesia of skin around the TMJ using the von Frey filament. Chen et al. (11) also measured the attenuation in BF after injection of CFA into the TMJ, and they reported a prolonged and significant attenuation of BF over 9 days, which was similar to our result.
TNF-α is one of various cytokines that are mainly produced by mono-macrophages, NK cells, T cells, B cells, endothelial cells, fibroblasts, and osteoblasts. Local stress or inflammatory condition of the overlying synovial cells in the TMJ can produce proinflammatory cytokines such as IL-6 and TNF-α. The clinical features of TMJD include dysfunction and pain during mastication, which are basically the symptoms of inflammation (8). In a study on TMJ synovial fluid analysis using ELISA, many cytokines such as TNF-α, IL-6, and IL-1 were found in the synovial fluid (8, 22, 23).
It is known that the effects of TNF-α neutralization are mediated by 2 receptors, TNF-RI and TNF-RII. Several studies demonstrated the expression of both TNF-RI and TNF-RII in rat dorsal root ganglion (DRG) neurons (24, 25, 26), however, other studies identified only TNF-RI in neurons, while localizing TNF-RII in non-neuronal cells in the DRG (27). Boettger et al. (12) demonstrated that systemic administration of the TNF-α neutralizers etanercept and infliximab significantly attenuated inflammation-induced changes in locomotor and pain related behavior in rats with antigen induced arthritis. Chen et al. (28) also reported that neutralization of TNF-α with infliximab partially alleviated ovariectomy induced mechanical and thermal hyperalgesia in rats, and they concluded that TNF-α plays an important role in estrogen deficiency induced mechanical and thermal hyperalgesia.
It is postulated that the main neuronal target of TNF-α is a trigeminal ganglion, and Chen et al. (11) demonstrated that TMJ inflammation following CFA injection caused a significant increase in TRPV4 in the trigeminal ganglion according to Western blotting, and it reached a peak at day 3 after induction of inflammation. They also reported that the increase in TRPV4 in the trigeminal ganglion showed a remarkable coincidence with the time course of BF attenuation.
The present study demonstrated that the reduction in BF caused by injection of CFA into the TMJ could be partially alleviated by the administration of TNF-α neutralizing drug, infliximab. Our experimental result supports the hypothesis that treatment with infliximab not only influences the inflammatory process, but also exerts direct antinociceptive effects. However, infliximab only partially improved BF reduction when compared with control group. Therefore, increased expression of TNF-α may be just one mechanism of BF reduction, other factors may also be contributed.
Infliximab is a chimeric anti-TNF monoclonal antibody that is composed of human IgG1 kappa (constant region) and a murine Fv (variable) region. Infliximab has been shown to bind with high affinity to both soluble and membrane bound TNF and is capable of neutralizing TNF in vitro and in vivo (16). We administered infliximab systemically via the intraperitoneal route; however, Ohtani et al. (29) demonstrated that the local intra-articular injection of anti-rabbit TNF-α monoclonal antibody in a monoarthritis model was also effective in controlling local inflammation and degenerative joint changes.
Our study has some limitations. We did not check the proinflammatory cytokines or chemokines in the blood or tissue. The study was not carried out regarding pain pathway such as nociceptors or neurotransmitters.
In conclusion, infliximab, a TNF-α neutralizing drug, could partially alleviate the reduction in BF caused by the injection of CFA into the TMJ.
Footnotes
This work was supported by the Bisa Research Funds, Daegu, Republic of Korea (grant number 2012-0029).
DISCLOSURE: The authors have no conflicts of interest to disclose.
AUTHOR CONTRIBUTION: Conception and coordination of the study: Kim SH, Son CN, Hong JH. Design of ethical issues: Cho HC, Jung SW, Hur JA, Baek WK, Jung HR. Acquisition of data: Lee HJ. Data review: Kim SH, Son CN, Baek WK, Hong JH. Statistical analysis: Son CN, Lee HJ, Hong JH. Manuscript preparation: Kim SH, Son CN, Cho HC, Jung SW, Hur JA, Jung HR. Manuscript approval: all authors.
References
- 1.Turman JE., Jr The development of mastication in rodents: from neurons to behaviors. Arch Oral Biol. 2007;52:313–316. doi: 10.1016/j.archoralbio.2006.08.013. [DOI] [PubMed] [Google Scholar]
- 2.Yamada Y, Yamamura K, Inoue M. Coordination of cranial motoneurons during mastication. Respir Physiol Neurobiol. 2005;147:177–189. doi: 10.1016/j.resp.2005.02.017. [DOI] [PubMed] [Google Scholar]
- 3.Westberg KG, Kolta A. The trigeminal circuits responsible for chewing. Int Rev Neurobiol. 2011;97:77–98. doi: 10.1016/B978-0-12-385198-7.00004-7. [DOI] [PubMed] [Google Scholar]
- 4.Cadden SW, Orchardson R. Mastication and swallowing: 2. control. Dent Update. 2009;36:390–392. 394–396, 398. doi: 10.12968/denu.2009.36.7.390. [DOI] [PubMed] [Google Scholar]
- 5.Marquezin MC, Kobayashi FY, Montes AB, Gavião MB, Castelo PM. Assessment of masticatory performance, bite force, orthodontic treatment need and orofacial dysfunction in children and adolescents. Arch Oral Biol. 2013;58:286–292. doi: 10.1016/j.archoralbio.2012.06.018. [DOI] [PubMed] [Google Scholar]
- 6.Kogawa EM, Calderon PS, Lauris JR, Araujo CR, Conti PC. Evaluation of maximal bite force in temporomandibular disorders patients. J Oral Rehabil. 2006;33:559–565. doi: 10.1111/j.1365-2842.2006.01619.x. [DOI] [PubMed] [Google Scholar]
- 7.Wang XD, Kou XX, He DQ, Zeng MM, Meng Z, Bi RY, Liu Y, Zhang JN, Gan YH, Zhou YH. Progression of cartilage degradation, bone resorption and pain in rat temporomandibular joint osteoarthritis induced by injection of iodoacetate. PLoS One. 2012;7:e45036. doi: 10.1371/journal.pone.0045036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee JK, Cho YS, Song SI. Relationship of synovial tumor necrosis factor alpha and interleukin 6 to temporomandibular disorder. J Oral Maxillofac Surg. 2010;68:1064–1068. doi: 10.1016/j.joms.2009.08.007. [DOI] [PubMed] [Google Scholar]
- 9.Nicoll SB, Hee CK, Davis MB, Winkelstein BA. A rat model of temporomandibular joint pain with histopathologic modifications. J Orofac Pain. 2010;24:298–304. [PubMed] [Google Scholar]
- 10.Wu YW, Bi YP, Kou XX, Xu W, Ma LQ, Wang KW, Gan YH, Ma XC. 17-Beta-estradiol enhanced allodynia of inflammatory temporomandibular joint through upregulation of hippocampal TRPV1 in ovariectomized rats. J Neurosci. 2010;30:8710–8719. doi: 10.1523/JNEUROSCI.6323-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen Y, Williams SH, McNulty AL, Hong JH, Lee SH, Rothfusz NE, Parekh PK, Moore C, Gereau RW, 4th, Taylor AB, et al. Temporomandibular joint pain: a critical role for Trpv4 in the trigeminal ganglion. Pain. 2013;154:1295–1304. doi: 10.1016/j.pain.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boettger MK, Hensellek S, Richter F, Gajda M, Stöckigt R, von Banchet GS, Bräuer R, Schaible HG. Antinociceptive effects of tumor necrosis factor alpha neutralization in a rat model of antigen-induced arthritis: evidence of a neuronal target. Arthritis Rheum. 2008;58:2368–2378. doi: 10.1002/art.23608. [DOI] [PubMed] [Google Scholar]
- 13.Gruber-Schoffnegger D, Drdla-Schutting R, Hönigsperger C, Wunderbaldinger G, Gassner M, Sandkühler J. Induction of thermal hyperalgesia and synaptic long-term potentiation in the spinal cord lamina I by TNF-α and IL-1β is mediated by glial cells. J Neurosci. 2013;33:6540–6551. doi: 10.1523/JNEUROSCI.5087-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Feldmann M, Maini RN. Anti-TNF alpha therapy of rheumatoid arthritis: what have we learned? Annu Rev Immunol. 2001;19:163–196. doi: 10.1146/annurev.immunol.19.1.163. [DOI] [PubMed] [Google Scholar]
- 15.Sanmarti R, Ruiz-Esquide V, Hernández MV. Rheumatoid arthritis: a clinical overview of new diagnostic and treatment approaches. Curr Top Med Chem. 2013;13:698–704. doi: 10.2174/15680266113139990092. [DOI] [PubMed] [Google Scholar]
- 16.Chen YF, Jobanputra P, Barton P, Jowett S, Bryan S, Clark W, Fry-Smith A, Burls A. A systematic review of the effectiveness of adalimumab, etanercept and infliximab for the treatment of rheumatoid arthritis in adults and an economic evaluation of their cost-effectiveness. Health Technol Assess. 2006;10:iii–iiv. xi–xiii, 1–229. doi: 10.3310/hta10420. [DOI] [PubMed] [Google Scholar]
- 17.Williams SH, Peiffer E, Ford S. Gape and bite force in the rodents Onychomys leucogaster and Peromyscus maniculatus: does jaw-muscle anatomy predict performance? J Morphol. 2009;270:1338–1347. doi: 10.1002/jmor.10761. [DOI] [PubMed] [Google Scholar]
- 18.Reiter S, Goldsmith C, Emodi-Perlman A, Friedman-Rubin P, Winocur E. Masticatory muscle disorders diagnostic criteria: the American Academy of Orofacial Pain versus the research diagnostic criteria/temporomandibular disorders (RDC/TMD) J Oral Rehabil. 2012;39:941–947. doi: 10.1111/j.1365-2842.2012.02337.x. [DOI] [PubMed] [Google Scholar]
- 19.Winocur E, Gavish A, Finkelshtein T, Halachmi M, Gazit E. Oral habits among adolescent girls and their association with symptoms of temporomandibular disorders. J Oral Rehabil. 2001;28:624–629. doi: 10.1046/j.1365-2842.2001.00708.x. [DOI] [PubMed] [Google Scholar]
- 20.Gavish A, Winocur E, Menashe S, Halachmi M, Eli I, Gazit E. Experimental chewing in myofascial pain patients. J Orofac Pain. 2002;16:22–28. [PubMed] [Google Scholar]
- 21.Dolan JC, Lam DK, Achdjian SH, Schmidt BL. The dolognawmeter: a novel instrument and assay to quantify nociception in rodent models of orofacial pain. J Neurosci Methods. 2010;187:207–215. doi: 10.1016/j.jneumeth.2010.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kubota E, Imamura H, Kubota T, Shibata T, Murakami K. Interleukin 1 beta and stromelysin (MMP3) activity of synovial fluid as possible markers of osteoarthritis in the temporomandibular joint. J Oral Maxillofac Surg. 1997;55:20–27. doi: 10.1016/s0278-2391(97)90438-9. discussion 7-8. [DOI] [PubMed] [Google Scholar]
- 23.Fu K, Ma X, Zhang Z, Pang X, Chen W. Interleukin-6 in synovial fluid and HLA-DR expression in synovium from patients with temporomandibular disorders. J Orofac Pain. 1995;9:131–137. [PubMed] [Google Scholar]
- 24.Hensellek S, Brell P, Schaible HG, Bräuer R, Segond von Banchet G. The cytokine TNFalpha increases the proportion of DRG neurones expressing the TRPV1 receptor via the TNFR1 receptor and ERK activation. Mol Cell Neurosci. 2007;36:381–391. doi: 10.1016/j.mcn.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 25.Schäfers M, Geis C, Brors D, Yaksh TL, Sommer C. Anterograde transport of tumor necrosis factor-alpha in the intact and injured rat sciatic nerve. J Neurosci. 2002;22:536–545. doi: 10.1523/JNEUROSCI.22-02-00536.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schäfers M, Lee DH, Brors D, Yaksh TL, Sorkin LS. Increased sensitivity of injured and adjacent uninjured rat primary sensory neurons to exogenous tumor necrosis factor-alpha after spinal nerve ligation. J Neurosci. 2003;23:3028–3038. doi: 10.1523/JNEUROSCI.23-07-03028.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xu JT, Xin WJ, Zang Y, Wu CY, Liu XG. The role of tumor necrosis factor-alpha in the neuropathic pain induced by Lumbar 5 ventral root transection in rat. Pain. 2006;123:306–321. doi: 10.1016/j.pain.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 28.Chen BL, Li YQ, Xie DH, He QL, Yang XX. Blocking TNF-α with infliximab alleviates ovariectomy induced mechanical and thermal hyperalgesia in rats. Neurol Sci. 2012;33:527–533. doi: 10.1007/s10072-011-0743-9. [DOI] [PubMed] [Google Scholar]
- 29.Ohtani T, Habu M, Khanal A, Yoshioka I, Matsukawa A, Tominaga K. Local effects of intra-articular injection of anti-rabbit tumor necrosis factor alpha monoclonal antibody in antigen-induced arthritis of the rabbit temporomandibular joint. J Oral Pathol Med. 2012;41:96–105. doi: 10.1111/j.1600-0714.2011.01056.x. [DOI] [PubMed] [Google Scholar]