Abstract
Bacterial infection is an important cause of death in patients with liver cirrhosis. The aim of this study was to investigate the clinical characteristics and prognostic impact of bacterial infection in hospitalized patients with alcoholic liver disease (ALD). We retrospectively analyzed data from 409 patients consecutively admitted to a tertiary referral center with ALD diagnosis. Of a total of 544 admissions, 133 (24.4%) cases presented with bacterial infection, of which 116 were community-acquired whereas 17 were hospital-acquired. The common types of infection were pneumonia (38%), biliary tract infection (17%), soft tissue infection (12%), and spontaneous bacterial peritonitis (9%). Diabetes, serum Na <135 mM/L, albumin <2.5 g/dL, C-reactive protein ≥20 mg/L, systemic inflammatory response syndrome (SIRS) positivity were independently associated with bacterial infection in patients with ALD. Overall 30-day and 90-day mortalities in patients with bacterial infection were significantly (P < 0.001) higher than those without infection (22.3% vs. 5.1% and 32.3% vs. 8.2%, respectively). Furthermore, bacterial infection (HR, 2.2; 95% CI, 1.049-4.579, P = 0.037), SIRS positivity (HR, 2.5; 95% CI, 1.240-4.861, P = 0.010), Maddrey's discriminant function score ≥32 (HR, 2.3; 95% CI, 1.036-5.222, P = 0.041), and hemoglobin <12 g/dL (HR, 2.4; 95% CI, 1.081-5.450, P = 0.032) were independent predictors of short-term mortality. In conclusion, bacterial infection and SIRS positivity predicted short-term prognosis in hospitalized patients with ALD. A thorough evaluation at admission or on clinical deterioration is required to detect possible infection with prompt management.
Graphical Abstract
Keywords: Bacterial Infections; Liver Disease, Alcoholic; Prognosis
INTRODUCTION
Alcohol abuse can lead to alcoholic liver disease (ALD) including fatty liver, hepatitis, cirrhosis, and liver cancer (1). Alcohol is the second common cause of chronic liver disease, accounting for approximately 25%-30% cases of liver cirrhosis in Korea (2). Although fatty liver can be reversed after abstinence of alcohol, alcoholic hepatitis and cirrhosis are usually progressive and sometimes fatal. In a recent nation-wide study, the main causes of death in ALD were variceal bleeding (31.1%), liver failure (24.5%), and hepatorenal syndrome or sepsis (11.3%) (3).
ALD is closely associated with significant development of endotoxemia (4). Alcohol abuse increases the permeability of intestinal mucosa which facilitates the absorption of endotoxin and decreases the bactericidal activity of phagocytic cells. These factors contribute to the development of endeotoxemia, resulting in hypotension, altered renal function, and coagulopathy. Furthermore, patients with cirrhosis have multifactorial immune dysfunction locally and systemically (5). Previous studies have reported that bacterial infections are frequent in patients with cirrhosis, therefore increasing mortality (6, 7). Mortality rate after infection in cirrhosis was approximately 30% in 1-month which was 4-fold of the mortality rate in the general population (6). These findings suggest that bacterial infections may be common which have significant impact on short-term mortality in patients with ALD. Therefore, bacterial infection in ALD should be considered as important prognostic factor. The objectives of this study were to determine the clinical characteristics of recent bacterial infection in hospitalized patients with ALD and to investigate the prognostic impact of bacterial infection on short-term mortality in ALD patients.
MATERIALS AND METHODS
Study population and data collection
We retrospectively analyzed data of a total of 544 admissions from 409 patients who were consecutively admitted to Chonbuk National University Hospital and diagnosed with ALD between January 2010 and December 2012. The diagnosis of ALD in patients was based on the following: daily alcohol consumption exceeding 40 g in men and 20 g in women, with clinical, laboratory, radiological, or histological evidence of liver disease (2). Currently there was no definite diagnostic criteria for alcoholic hepatitis. In this study, we diagnosed alcoholic hepatitis based on the elevation of serum aspartate aminotransferase (AST) or alanine aminotransferase (ALT) when their levels were >2 fold of the upper normal limit or with evidence of histological findings compatible with alcoholic hepatitis. Alcoholic cirrhosis was diagnosed with clinical evidence, which included findings of portal hypertension (one of the followings: splenomegaly, varices, ascites, platelets count <100,000/µL) and radiologic findings of cirrhosis, or histological findings compatible with cirrhosis patients with ALD. Co-infections with other viruses including hepatitis A virus, hepatitis B virus, hepatitis D virus, human immunodeficiency virus, concomitant liver disease such as Wilson's disease, autoimmune hepatitis, co-existing liver cancer or extrahepatic malignancy, and use of hepatotoxic drugs were excluded from this study.
The following data were collected from electronic data system and medical records: age, gender, body mass index, amount of alcohol consumption, presence of diabetes, previous history of bacterial infection, vital signs, symptoms and laboratory findings at admission, clinical stages of ALD, presence of bacterial infection at admission or during hospitalization, total duration of hospitalization, and mortality at 30-day and 60-day. Child-Pugh score, model for end-stage liver disease (MELD) score, and Maddrey's discriminant function (DF) score were calculated from collected data. For patients with bacterial infections, types of infection, organisms isolated and their anti-microbial resistance profiles were determined.
Definitions and study endpoints
In this study, we divided study patients into the following two groups: bacterial infection group and non-infection group. We considered bacterial infection if patients presented with fever (>37.5℃) lasting more than 24 hr or leukocytosis (leukocyte count >10,000/µL) combining with one of the following criteria of infection. Pneumonia was diagnosed in radiologic evidence of pulmonary infiltration associated with purulent sputum. Spontaneous bacterial peritonitis (SBP) was diagnosed when ascitic fluid examination showed polymorphonuclear count ≥250 cells/µL, irrespective of the culture result. Urinary tract infection was diagnosed through urinary leukocyte count >15 cells/high power field and isolation of >106 bacteria/µL. Skin and soft tissue infection was diagnosed when patient presented with fever (>37.5℃) and cellulitis associated with leukocytosis. Spontaneous bacteremia was diagnosed when blood culture was positive but without any recognized source of infection. Other infections were diagnosed based on clinical, radiological and bacteriological data. Patients considered for bacterial infections but without positive culture or evidence of organ involvements were considered as undetermined infection. Community-acquired infection was considered if it was already present upon hospitalization or diagnosed within the first 48 hr. Hospital-acquired infection was considered if it occurred during hospitalization or diagnosed after 48 hr. Systemic inflammatory response syndrome (SIRS) was considered if patients fulfilled at least two of the following criteria: body temperature <36℃ or >38℃, heart rate >90 beats/min, respiratory rate >20 beats/min, leukocyte count <4,000 or >12,000 cells/µL or the presence of greater than 10% immature neutrophils (band forms).
Primary endpoints of this study were comparison of clinical features of the bacterial infection group versus the non-infection group and evaluation of 30-day and 90-day mortalities according to the presence of bacterial infection in hospitalized patients with ALD. Secondary endpoints were investigation of factors associated with bacterial infection and short term (30-day) mortality as well as cumulative survival rates according to prognostic factors in ALD patients.
Statistical analysis
Continuous variables were compared using the two-tailed Student's t-test. Categorical data were analyzed using the chi-square test or Fisher's exact test. Factors associated with bacterial infections were analyzed by binary linear regression models. Univariate binary logistic regression analysis was done using the factors represented with nominal variables. Then, multivariate analysis was done with the significant factors in the univariate analysis. Factors associated with short-term mortalities were analyzed by univariate and multivariate Cox regression models. Cumulative survival rates according to prognostic factors were evaluated by Kaplan-Meier analysis. Results were reported as mean±standard deviation (SD) or number (%). Data was collected in Microsoft EXCEL (Microsoft Excel 2007; Microsoft Corporation, Seattle, WA, USA) and analyzed using SPSS for Windows version 15.0 (SPSS, Chicago, IL, USA). Statistical significance was considered when P value was less than 0.05.
Ethics statement
This study was conducted in compliance with the World Medical Association Declaration of Helsinki and was approved by the institutional review board of Chonbuk National University Hospital (IRB number: 2013-05-003). Informed consent was waived by the board.
RESULTS
Clinical features of bacterial infection in patients with ALD
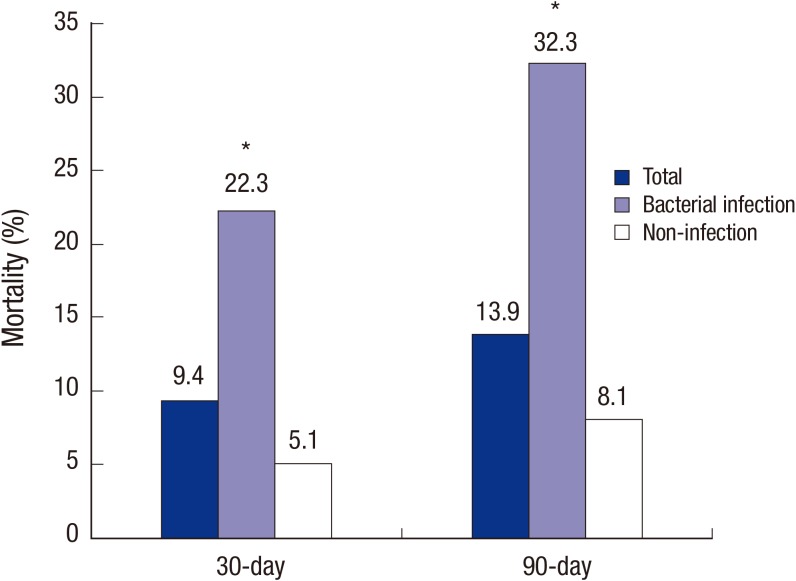
Of 544 consecutive admissions, 505 (92.8%) were males, 134 (24.6%) had diabetes, and 442 (81.3%) had pre-existing cirrhosis. The mean age of patients was 54.3±11.3 yr (Table 1). A total of 133 (24.4%) cases presented with bacterial infection at admission or during hospitalization. Clinical features were compared between the bacterial infection group and the non-infection group (Table 1). Bacterial infection group was significantly associated with older age (P<0.001), male sex (P=0.033), diabetes (P=0.032), previous bacterial infection (P=0.003) at admissions compared to the non-infection group. Meanwhile, non-infection group was more frequently combined with ascites (P=0.006) and gastrointestinal bleeding (P<0.001) compared to the infection group. In initial laboratory findings, compared to the non-infection group, the bacterial infection group had significantly higher levels of leukocytes counts (P<0.001), C-reactive protein (CRP) (P<0.001), and procalcitonin (P=0.013), but significantly lower levels of hemoglobin (P=0.003), serum Na (P<0.001), and albumin (P=0.012). In addition, the bacterial infection group exhibited significantly higher MELD score (15.5±9.8 vs. 13.2±9.0, P=0.015) and SIRS positivity (40.6% vs. 5.1%, P<0.001) compared to the non-infection group. The duration of hospitalization was significantly longer in the infection group compared to the non-infection group (16.7±14.1 vs. 12.0±10.7 days, P=0.001). Furthermore, the bacterial infection group had significantly higher 30- and 90-day mortality rates compared to the non-infection group (22.3% vs. 5.1%, P<0.001 and 32.3% vs. 8.2%, P<0.001, respectively) (Fig. 1). In multivariate analysis by binary linear regression models, diabetes (odd ratio [OR], 1.9; 95% confidence interval [CI], 1.013-3.415, P=0.045), serum Na<135 mM/L (OR, 2.6; 95% CI, 1.490-4.474, P=0.001), albumin <2.5 g/dL (OR, 2.6; 95% CI, 1.356-5.040, P=0.004), CRP ≥20 mg/L (OR, 10.1; 95% CI, 5.748-17.624, P<0.001), SIRS positivity (OR, 8.9; 95% CI, 4.294-18.542, P<0.001) were independently associated with bacterial infection in ALD patients (Table 2).
Table 1. Comparison of clinical features between the bacterial infection group and the non-infection group.
| Characteristics | Total (n = 544) | Infection group (n = 133) | Non-infection group (n = 411) | P value |
|---|---|---|---|---|
| Age (yr) | 54.4 ± 11.3 | 57.5 ± 11.6 | 53.3 ± 11.0 | <0.001 |
| Male | 505 (92.8%) | 129 (97.0%) | 376 (91.5%) | 0.033 |
| BMI (kg/m2) | 22.8 ± 3.5 | 22.3 ± 3.5 | 23.0 ± 3.5 | 0.132 |
| Diabetes mellitus | 134 (24.6%) | 42 (31.6%) | 92 (22.4%) | 0.032 |
| Pre-existing cirrhosis | 442 (81.3%) | 110 (82.7%) | 332 (80.8%) | 0.620 |
| Previous bacterial infection (%) | 41 (7.5) | 18 (13.5) | 23 (5.6) | 0.003 |
| Sign and symptoms at admission | ||||
| Ascites | 65 (11.9%) | 7 (5.3%) | 58 (14.1%) | 0.006 |
| Gastrointestinal bleeding | 184 (33.8%) | 10 (7.5%) | 174 (42.3%) | <0.001 |
| Hepatic encephalopathy | 33 (6.1%) | 8 (6.0%) | 25 (6.1%) | 0.977 |
| Laboratory findings at admission | ||||
| WBC ( × 103/µL) | 8,887.5 ± 5,483.6 | 10,711.0 ± 7,265.0 | 8,297.5 ± 4,626.5 | <0.001 |
| Hemoglobin (g/dL) | 10.9 ± 2.8 | 11.4 ± 2.3 | 10.7 ± 2.9 | 0.003 |
| Platelets ( × 103/µL) | 116.6 ± 70.1 | 115.0 ± 85.1 | 117.2 ± 64.7 | 0.783 |
| Prothrombin time (INR) | 1.6 ± 4.3 | 1.5 ± 0.5 | 1.7 ± 4.9 | 0.681 |
| Serum Na (mM/L) | 134.8 ± 6.0 | 132.9 ± 6.5 | 135.5 ± 5.7 | <0.001 |
| Serum AST (IU/L) | 375.8 ± 1,522.2 | 231.4 ± 716.4 | 422.6 ± 1,701.8 | 0.068 |
| Serum ALT (IU/L) | 150.0 ± 589.1 | 121.6 ± 484.2 | 159.2 ± 619.6 | 0.469 |
| GGT (IU/L) | 406.2 ± 556.6 | 343.4 ± 514.6 | 425.6 ± 568.2 | 0.165 |
| Total bilirubin (mg/dL) | 4.5 ± 6.2 | 4.3 ± 5.1 | 4.6 ± 6.6 | 0.628 |
| Albumin (g/dL) | 3.1 ± 0.7 | 3.0 ± 0.7 | 3.2 ± 0.7 | 0.012 |
| Creatinine (mg/dL) | 1.6 ± 7.0 | 1.6 ± 1.6 | 1.6 ± 8.1 | 0.995 |
| Glucose (mg/dL) | 165.6 ± 93.7 | 164.2 ± 125.5 | 166.0 ± 80.9 | 0.897 |
| Cholesterol (mg/dL) | 137.6 ± 50.6 | 141.4 ± 81.4 | 136.3 ± 39.7 | 0.850 |
| LDH (IU/L) | 863.6 ± 1,117.5 | 825.0 ± 654.2 | 875.4 ± 1,225.8 | 0.730 |
| CRP (mg/L) | 25.9 ± 44.6 | 67.5 ± 64.2 | 12.5 ± 23.9 | <0.001 |
| Procalcitonin (ng/mL) | 4.2 ± 11.1 | 7.5 ± 15.1 | 1.0 ± 1.4 | 0.013 |
| SIRS positivity | 75 (13.8%) | 54 (40.6%) | 21 (5.1%) | <0.001 |
| Child-pugh score | 8.2 ± 2.3 | 8.5 ± 2.4 | 8.1 ± 2.3 | 0.057 |
| MELD score | 13.7 ± 9.2 | 15.5 ± 9.8 | 13.2 ± 9.0 | 0.015 |
| Maddrey's DF score | 36.4 ± 48.2 | 37.0 ± 40.7 | 36.3 ± 50.5 | 0.884 |
| Hospitalization (days) | 13.1 ± 11.8 | 16.7 ± 14.1 | 12.0 ± 10.7 | 0.001 |
Values are mean±standard deviation or number (%). BMI, body mass index; GGT, gamma glutamyl peptidase; AST, aspartate aminotransferase; ALT, alanine aminotransferase; LDH, lactate dehydrogenase; CRP, C-reactive protein; MELD, model for end stage liver disease; DF, discriminant function; SIRS, systemic inflammatory response syndrome.
Fig. 1. Mortalities at 30- and 90-day according to bacterial infections in hospitalized patients with alcoholic liver disease (ALD). *P < 0.01 compared to non-infection group.
Table 2. Factors associated with bacterial infection by binary linear regression analysis.
| Characteristics | Multivariate adjusted OR | 95% CI | P value |
|---|---|---|---|
| Diabetes mellitus | 1.9 | 1.013-3.415 | 0.045 |
| Initial laboratory findings | |||
| Serum Na < 135 mM/L | 2.6 | 1.490-4.474 | 0.001 |
| Serum Albumin < 2.5 g/dL | 2.6 | 1.356-5.040 | 0.004 |
| CRP ≥ 20 mg/L | 10.1 | 5.748-17.624 | <0.001 |
| SIRS positivity | 8.9 | 4.294-18.542 | <0.001 |
OR, odds ratio; CI, confidence interval; CRP, C-reactive protein; SIRS, systemic inflammatory response syndrome.
Types of bacterial infection and profiles of isolated organisms
Of a total of 133 bacterial infections, 116 (87.2%) were community-acquired, 17 (12.8%) were hospital-acquired. Common types of bacterial infection were pneumonia or pleurisy (35.3%), biliary tract infection (17.3%), soft tissue infection (12.8%), SBP (9.8%), pulmonary tuberculosis (4.5%), urinary tract infection (3.8%), septic arthritis (3.8%), acute pancreatitis (3.8%), spontaneous bacteremia (3.8%), and others (9.8%) (Table 3).
Table 3. Incidence of different types of bacterial infection.
| Types | Total (n = 133) |
|---|---|
| Pneumonia or pleurisy | 47 (35.3%) |
| Biliary tract infection | 23 (17.3%) |
| Soft tissue infection | 17 (12.8%) |
| Spontaneous bacterial peritonitis | 13 (9.8%) |
| Pulmonary tuberculosis | 6 (4.5%) |
| Urinary tract infection | 5 (3.8%) |
| Septic arthritis | 5 (3.8%) |
| Acute pancreatitis | 5 (3.8%) |
| Spontaneous bacteremia | 4 (3%) |
| Other infections* | 13 (9.8%) |
*Other infections; scrub typhus (n=4), infective spondylitis (n=1), colitis (n=2), brain abscess (n=1), catheter related infection (n=1), undetermined (n=4).
There were 65 microorganisms isolated from bacterial cultures, including 45 (70.3%) Gram-negative and 18 (28.1%) Gram-positive bacteria plus one Candida (Table 4). The most common Gram-negative organisms were: Escherichia coli, Klebsiella pneumoniae, Klebsiella oxytoca, Pseudomonas species, and Acinetobacter species. Antimicrobial resistance profiles of Gram-negative bacteria showed high rate of resistance to ampicillin or oxacillin (90.2%), ciprofloxacin or levofloxacin (51.2%), and cefotaxime or ceftriaxone (53.7%). Extended-spectrum beta-lactamase (ESBL) producing bacteria mainly Escherichia coli and Klebsiella species were detected in 22.0% of organisms. The common Gram-positive organisms were Staphylococcus and Enterococcus species. Similarily, antimicorobial resistance profiles of Gram-positive organisms showed high rate of resistance to ampicillin or oxacillin (78.2%) and ciprofloxacin or levofloxacin (69.6%). We investigated the history of previous hospitalization for the 65 bacterial culture positive cases. Fifty seven cases showed a resistance to one or more antimicrobials and 36 cases (63.2%) of them had a history of previous hospitalization with antimicrobial use.
Table 4. Profiles of isolated organisms with antibiotic resistance in culture-positive infection.
| Organisms | Total (n = 65) | Ampicillin or oxacillin | Ciprofloxacin or levofloxacin | Ceftriaxone or cefotaxime | ESBL | Vancomycin |
|---|---|---|---|---|---|---|
| Gram negative organisms | 41 | 37 (90.2) | 21 (51.2) | 22 (53.7) | 9 (22.0) | |
| Escherichia coli | 11 | 8 (72.7) | 6 (54.5) | 5 (45.5) | 5 (45.5) | - |
| Klebsiella pneumoniae | 10 | 10 (100.0) | 3 (30.0) | 3 (30.0) | 3 (30.0) | - |
| Klebsiella oxytoca | 2 | 2 (100.0) | 1 (50.0) | 1 (50.0) | 1 (50.0) | - |
| Pseudomonas species | 7 | 7 (100.0) | 5 (71.4) | 6 (85.7) | - | - |
| Acinetobacter species | 7 | 7 (100.0) | 6 (85.7) | 7 (100.0) | - | - |
| Aeromonas species | 2 | 2 (100.0) | 0 (0.0) | 0 (0.0) | - | - |
| Enterobacter species | 1 | 1 (100.0) | 0 (0.0) | 0 (0.0) | - | - |
| Others | 1 | 0 (0.0) | 0 (0.0) | 0 (0.0) | - | |
| Gram positive organisms | 23 | 18 (78.2) | 16 (69.6) | - | - | 0 (0.0) |
| Staphylococcus species | 15 | 13 (86.7) | 11 (73.3) | - | - | 0 (0.0) |
| Enterococcus species | 7 | 5 (71.4) | 5 (71.4) | - | - | 0 (0.0) |
| Others | 1 | - | - | - | - | - |
| Candida species | 1 | |||||
| Candida albicans | 1 | - | - | - | - | - |
ESBL, Extended spectrum β-lactamase; Data are expresed as number (%).
Risk factors associated with short-term (30-day) mortality
We analyzed factors associated with short-term (30-day) mortality in ALD patients. In univariate analysis, Child-Pugh class B or C (P=0.016) , MELD score ≥21 (P<0.001), Maddrey's DF score ≥32 (P<0.001), SIRS positivity (P<0.001), bacterial infection (P<0.001), leukocyte count ≥10,000/µL (P<0.001), hemoglobin level <12 g/dL (P=0.016), serum albumin level <2.5 g/dL (P=0.009), serum creatinine level ≥1.5 mg/dL (P<0.001), and serum CRP level ≥20 mg/L (P<0.001) were significant factors (Table 5). In multivariate analysis, bacterial infection (HR, 2.2; 95% CI, 1.049, 4.579, P=0.037), SIRS positivity (HR, 2.5; 95% CI,1.240-4.861, P=0.010), Maddrey's DF score ≥32 (HR, 2.3; 95% CI, 1.036-5.222, P=0.041), and hemoglobin level <12 g/dL (HR, 2.4; 95% CI, 1.081-5.450, P=0.032) were independent significant factors associated with short-term (30-day) mortality (Table 6).
Table 5. Factors associated with short-term (30-day) mortality in univariate analysis.
| Characteristics | Survival group (n = 498) | Non-survival group (n = 46) | Univariate adjusted HR (95% CI) | P value |
|---|---|---|---|---|
| Age ≥ 50 yr | 321 (64.5%) | 32 (69.6%) | 1.3 (0.655-2.425) | 0.487 |
| Male sex | 459 (92.2%) | 46 (100.0%) | 1.1 (1.070-1.131) | 0.065 |
| Alcohol consumption ( ≥ 60 g/day) | 376 (75.7%) | 39 (84.8%) | 1.8 (0.782-4.113) | 0.163 |
| Diabetes mellitus | 122 (24.5%) | 12 (26.1%) | 1.1 (0.546-2.167) | 0.811 |
| Previous bacterial infection | 35 (7.0%) | 6 (13.0%) | 2.0 (0.787-5.001) | 0.139 |
| Sign and symptoms at admission | ||||
| Ascites | 60 (12.0%) | 5 (10.9%) | 0.9 (0.339-2.341) | 0.814 |
| Gastrointestinal bleeding | 173 (34.7%) | 11 (23.9%) | 0.6 (0.293-1.192) | 0.138 |
| Hepatic encephalopathy | 29 (5.8%) | 4 (8.7%) | 1.5 (0.517-4.590) | 0.435 |
| Child-Pugh class, B or C | 352 (70.7%) | 40 (87.0%) | 2.8 (1.148-6.663) | 0.016 |
| MELD score ≥ 21 | 97 (19.5%) | 22 (47.8%) | 3.8 (2.039-7.041) | <0.001 |
| Maddrey's DF score ≥ 32 | 167 (33.5%) | 28 (60.9%) | 3.1 (1.657-5.735) | <0.001 |
| SIRS positivity | 55 (11.0%) | 20 (43.5%) | 6.2 (3.245-11.830) | <0.001 |
| Bacterial infection | 106 (21.3%) | 27 (58.7%) | 5.3 (2.813-9.817) | <0.001 |
| Initial laboratory results | ||||
| WBC ≥ 10,000/µL | 139 (27.9%) | 26 (56.5%) | 3.4 (1.815-6.210) | <0.001 |
| Hemoglobin < 12 g/dL | 324 (65.1%) | 38 (82.6%) | 2.6 (1.164-5.589) | 0.016 |
| Serum Na < 135 mM/L | 196 (39.4%) | 24 (52.2%) | 1.7 (0.863-3.172) | 0.090 |
| Serum AST ≥ 80 IU/L | 282 (56.6%) | 31 (67.4%) | 1.6 (0.834-3.006) | 0.158 |
| Serum ALT ≥ 80 IU/L | 122 (24.5%) | 10 (21.7%) | 0.9 (0.413-1.776) | 0.676 |
| Total bilirubin ≥ 8 mg/dL | 67 (13.5%) | 9 (19.6%) | 1.6 (0.723-3.388) | 0.253 |
| Albumin < 2.5 g/dL | 93 (18.7%) | 16 (34.8%) | 2.3 (1.216-4.437) | 0.009 |
| Creatinine ≥ 1.5 mg/dL | 94 (18.9%) | 19 (41.3%) | 3.0 (1.613-5.669) | <0.001 |
| CRP ≥ 20 mg/L | 129 (25.9%) | 29 (63.0%) | 4.9 (2.595-9.175) | <0.001 |
| Procalcitonin ≥ 0.5 ng/mL | 30 (6.0%) | 13 (28.3%) | 6.1 (2.931-12.885) | <0.001 |
Values are represented by mean±standard deviations or numbers (%). HR, hazard ratio; CI, confidence interval; MELD, model for end stage liver disease; DF, discriminant function; SIRS, systemic inflammatory response syndrome; WBC, white blood cells; Na, sodium; AST, aspartate aminotransferase; ALT, alanine aminotransferase; CRP, C-reactive protein.
Table 6. Factors associated with short-term (30-day) mortality in multivariate analysis.
| Risk factors | Multivariate adjusted HR | 95% CI | P value |
|---|---|---|---|
| Bacterial infection | 2.2 | 1.049-4.579 | 0.037 |
| SIRS positivity | 2.5 | 1.240-4.861 | 0.010 |
| Maddrey's DF score ≥ 32 | 2.3 | 1.036-5.222 | 0.041 |
| Hemoglobin < 12 g/dL | 2.4 | 1.081-5.450 | 0.032 |
HR, hazard ratio; CI, confidence interval; DF, discriminant function; SIRS, systemic inflammatory response syndrome.
Cumulative survival rates according to each prognostic factor
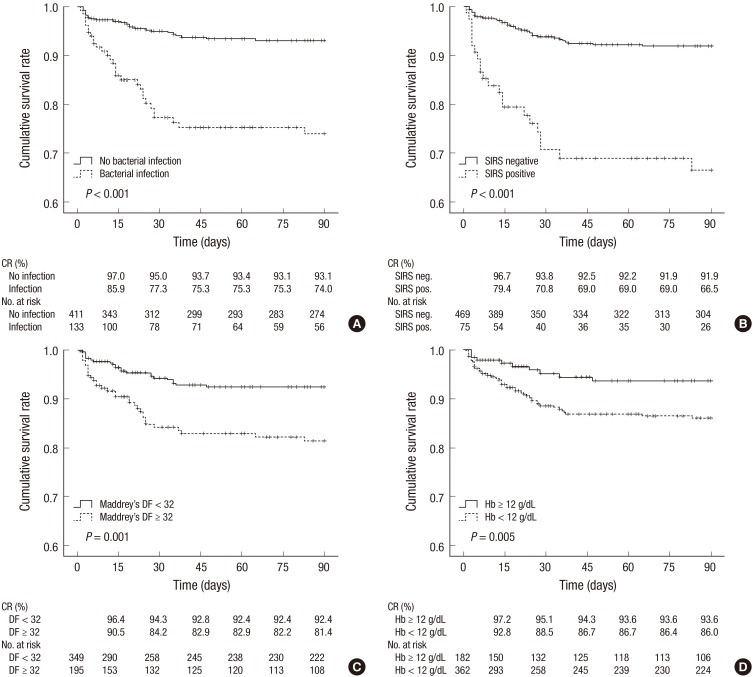
We analyzed cumulative survival rates up to 90-day follow-up according to the presence of prognostic factors which were found to be significant in the multivariate analysis (Fig. 2). Patients with bacterial infections had significantly lower survival rates compared to those without bacterial infection (77.3% vs. 95.0%, 75.3% vs. 93.4%, and 74.0% vs. 93.1% at 30-, 60-, and 90-day, respectively, P<0.001) (Fig. 2A). Patients with SIRS positive showed significantly lower survival rates compared to those with SIRS negative (70.8% vs. 93.8%, 69.0% vs. 92.2%, and 66.5% vs. 91.9% at 30-, 60-, and 90-day, respectively, P<0.001) (Fig. 2B). Patients with Maddrey's DF score ≥32 had significantly lower survival rates compared to those with Maddrey's DF score <32 (84.2% vs. 94.3%, 82.9% vs. 92.4%, and 81.4% vs. 92.4% at 30-, 60-, and 90-day, respectively, P=0.001) (Fig. 2C). In addition, patients with hemoglobin level <12 g/dL also showed significantly lower survival rates compared to those with hemoglobin level ≥12 g/dL (88.5% vs. 95.1%, 86.7% vs. 93.6%, and 86.0% vs. 93.6% at 30-, 60-, and 90-day, respectively, P=0.005) (Fig. 2D).
Fig. 2. Cumulative mortalities up to 90-day according to the prognostic factors including bacterial infection (A), SIRS positive (B), Maddrey's DF score ≥ 32 (C), and hemoglobin level < 12 mg/dL (D). CR, cumulative rate.
DISCUSSION
In this study, 24.4% of hospitalized patients with ALD presented with bacterial infections (87.2% and 12.8% were community- and hospital-acquired, respectively). Diabetes, serum Na<135 mM/L, albumin <2.5 g/dL, CRP ≥20 mg/L, and SIRS positivity were significantly associated with bacterial infections. Patients with bacterial infections showed significantly higher 30- and 90-day mortalities compared to those without infection (22.3% vs. 5.1% and 32.3% vs. 8.2%, respectively, P<0.001). In the multivariate analysis, bacterial infection (HR, 2.2, P=0.037), SIRS positivity (HR, 2.5, P=0.010), Maddrey's discriminant score ≥32 (HR, 2.3, P=0.041), and hemoglobin <12 g/dL (HR, 2.4, P=0.032) were independently associated with short-term (30-day) mortality.
Patients with ALD are well known to be susceptible to bacterial infections. Alcohol consumption has been reported to delay endotoxin clearance from the circulation, causing intestinal bacterial overgrowth and dysfunctional gut barrier or increased intestinal permeability, resulting in endotoxemia (4). Many patients with ALD encompass underlying liver cirrhosis at multifactorial immunodeficiency state (8). Liver dysfunction, portosystemic shunting, gut dysbiosis, increased bacterial translocation, cirrhosis-associated immune dysfunction and genetic factors are associated with the risk of bacterial infection in cirrhosis (9). Bacterial infections have been identified in approximately 15%-47% of hospitalized patients with cirrhosis (10, 11, 12, 13). Rosa et al. (14) reported that the prevalence of bacterial infection was higher in alcoholic cirrhotics compared to nonalcoholic cirrhotics. In the present study, of 544 patients with ALD including 442 cirrhotics, 133 (24.4%) had bacterial infection upon or during hospitalization. The initial presentation of a bacterial infection in some patients with ALD may be obscure or not specific, which make it difficult for an early diagnosis. Poor liver function, variceal bleeding, low ascitic fluid protein levels, prior SBP and hospitalization are known factors associated with increased risk of infection in cirrhosis (9). However, in this study, non-infection group presented ascites or gastrointestinal bleeding more frequently compared to the infection group. This may be explained by that those patients are commonly treated with prophylactic antibiotics at admission. Our study demonstrated that diabetes (OR, 1.9, P=0.045), serum Na <135 mM/L (OR, 2.6, P=0.001), albumin <2.5 g/dL (OR, 2.6, P=0.004), CRP ≥20 mg/L (OR, 10.1, P<0.001), SIRS positivity (OR, 8.9, P<0.001) were independently associated with bacterial infections in ALD patients.
It is well known that bacterial infections can induce SIRS. In this study, the bacterial infection group was more likely to be SIRS positive compared to the non-infection group (40.6% vs. 5.1%). However, SIRS may be presented in cirrhotic patients without bacterial infection because hyperdynamic circulation, hepatic encephalopathy, tense ascites and hypersplenism may affect vital signs and white-cell count (9). Indeed, patients with decompensated cirrhosis without bacterial infection might be SIRS positive in 10%-30% patients (15, 16). In addition, it is difficult to diagnose sepsis with conventional parameters of SIRS in these patients (15).
Recently, two acute phase serum proteins, serum CRP and procalcitonin have been suggested for the diagnosis and prediction of bacterial infection in cirrhosis (17, 18, 19, 20). A CRP value >10 ng/mL was independently associated with clinically significant bacterial infections in patients with cirrhosis without overt infections (18). For cut off values of 24.7 ng/mL of CRP and 0.49 ng/mL of procalcitonin, the area under the ROC curve for predicting sepsis was 0.811 and 0.89, respectively, in patients with cirrhosis (20). Our study also revealed higher levels of CRP and procalcitonin in the bacterial infection group compared to the non-infection group. However, patients with cirrhosis may present reduced CRP in response to infection and factors such as inflammation and bacterial translocation which might induce production of acute phase proteins without infection.
In this study, the common types of infection in ALD patients were pneumonia (38%), biliary tract infection (17%), soft tissue infection (12%), and spontaneous bacterial peritonitis (9%). Main causative organisms of infections were Gram-negative bacteria (70.3%) from intestinal origin. However, Gram-positive bacteria (28.1%) were also frequent cause of infection in hospitalized patients with ALD. In recent years, infections caused by multidrug-resistant bacteria are becoming important clinical problems in many countries (13). Common treatment options for bacterial infections occurring in cirrhosis are quinolones and third-generation cephalosporins. However, our study showed high rates of antimicrobial resistance to quinolones (51.2%) and third-generation cephalosporins (53.7%). Even ESBL producing organisms known as the most frequent multidrug-resistant bacteria were also positive in 22.0%. These antimicrobial resistance profiles may differ in various hospital settings. However, the empirical antibiotic strategy should consider high resistance rates for commonly used initial antimicrobials. This study also showed high rate (63.2%) of previous history of hospitalization with prior antimicrobial use in the patients with antimicrobial resistance. Indeed, a recent prospective study suggested that multiresistant infections in cirrhosis are frequently isolated in nosocomial origin of infection, long-term norfloxacin prophylaxis, recent infection by multiresistant bacteria, and recent use of β-lactams (13). Therefore, the antibiotic strategy should be properly adapted according to local epidemiological data, specific conditions of patients, and treatment response.
In patients with severe alcoholic hepatitis, Maddrey's DF and MELD scores are useful to estimate short term prognosis and guide treatment decisions (2). Glasgow alcoholic hepatitis score and ABIC (Age, Bilirubin, INR, Creatinine) score also show promise as prognosis predictive models for alcoholic hepatitis (2). A Korean study has demonstrated that the Maddrey's DF score has superior prognosis prediction ability for alcoholic hepatitis, with comparable results to that from the MELD score (3). Meanwhile, despite recent improvements in prevention and management, bacterial infections are major cause of morbidity and mortality in patients with cirrhosis. In a recent prospective study, infection is the most common cause of mortality in patients with 'acute-on-chronic liver failure' characterized in a more advanced stage by liver failure associated with multiple other end-organ failure (21). Positive SIRS criteria and ductular bilirubinostasis are early markers of acute-on-chronic liver failure which might allow more rapid identification of high-risk patients. Consistently, in this study, the 30- and 90-day mortality rates were significantly higher in the bacterial infection group compared to the non-infection group. Furthermore, bacterial infection and SIRS positivity as well as Maddrey's DF score ≥32 were independently associated with short-term (30-day) mortality. Several recent studies also reported that high levels of serum CRP and procalcitonin were associated with poor short-term mortality in cirrhotic patients (17, 18, 19, 20). However, in our study, high levels of serum CRP and procalcitonin were not significant in the multivariate analysis. On the other hand, low hemoglobin level was significantly associated with poor short-term mortality in our study. Alcohol is toxic to erythropoiesis in the bone marrow. Alcoholics often develop secondary malnutrition, a manifestation of which may be anemia caused by folic acid deficiency. In addition, as ALD progresses, acute or chronic hemorrhage into gastrointestinal tract and impaired blood coagulation due to deficiency of coagulation factors and/or thrombocytopenia could exacerbate anemia, therefore affecting the prognosis of patients (22).
This study has several limitations. Firstly, this study was retrospectively designed. Some data collection about treatment strategy were limited. Secondly, because this study was carried out in a single center, data about antimicrobial resistance and treatment of bacterial infection might be affected by specific circumstance of our institution. Therefore, future prospectively designed, multi-center studies including a larger number of patients should be performed to overcome these limitations.
In conclusion, bacterial infection and SIRS positivity should be considered as important prognostic factor for short-term mortality in hospitalized patients with ALD. Because untreated bacterial infection might lead to detrimental effect on survival, a thorough evaluation at admission or on clinical deterioration is required to detect possible infection with prompt management.
Footnotes
This study was supported by the Fund of Chonbuk National University Hospital Research Institute of Clinical Medicine 2014.
DISCLOSURE: The authors have no conflicts of interest to disclose.
AUTHOR CONTRIBUTION: Study design: Kim IH. Data generation and collection: Park JK, Lee CH, Kim SM, Jang JW, Kim SH. Data analysis: Park JK, Lee CH. Writing and revision: Kim IH. Supervision of study and manuscript writing: Kim SW, Lee SO, Lee ST, Kim DG. Manuscript approval: Kim IH.
References
- 1.Mandayam S, Jamal MM, Morgan TR. Epidemiology of alcoholic liver disease. Semin Liver Dis. 2004;24:217–232. doi: 10.1055/s-2004-832936. [DOI] [PubMed] [Google Scholar]
- 2.KASL clinical practice guidelines: management of alcoholic liver disease. Clin Mol Hepatol. 2013;19:216–254. doi: 10.3350/cmh.2013.19.3.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yim HJ, Kim DJ, Kim JH, Heo J, Woo HY, Bae SH, Lee BS, Kim MW, Yang JM, Han SY, et al. Prognosis of patients with alcoholic liver disease in Korea: Comparisons of prognostic models by a national-wide survey. Hepatol Int. 2013;7:S44. [Google Scholar]
- 4.Rao R. Endotoxemia and gut barrier dysfunction in alcoholic liver disease. Hepatology. 2009;50:638–644. doi: 10.1002/hep.23009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bonnel AR, Bunchorntavakul C, Reddy KR. Immune dysfunction and infections in patients with cirrhosis. Clin Gastroenterol Hepatol. 2011;9:727–738. doi: 10.1016/j.cgh.2011.02.031. [DOI] [PubMed] [Google Scholar]
- 6.Arvaniti V, D'Amico G, Fede G, Manousou P, Tsochatzis E, Pleguezuelo M, Burroughs AK. Infections in patients with cirrhosis increase mortality four-fold and should be used in determining prognosis. Gastroenterology. 2010;139:1246–1256. 1256.e1–1256.e5. doi: 10.1053/j.gastro.2010.06.019. [DOI] [PubMed] [Google Scholar]
- 7.Christou L, Pappas G, Falagas ME. Bacterial infection-related morbidity and mortality in cirrhosis. Am J Gastroenterol. 2007;102:1510–1517. doi: 10.1111/j.1572-0241.2007.01286.x. [DOI] [PubMed] [Google Scholar]
- 8.Runyon BA. Bacterial infections in patients with cirrhosis. J Hepatol. 1993;18:271–272. doi: 10.1016/s0168-8278(05)80267-3. [DOI] [PubMed] [Google Scholar]
- 9.Jalan R, Fernandez J, Wiest R, Schnabl B, Moreau R, Angeli P, Stadlbauer V, Gustot T, Bernardi M, Canton R, et al. Bacterial infections in cirrhosis: a position statement based on the EASL Special Conference 2013. J Hepatol. 2014;60:1310–1324. doi: 10.1016/j.jhep.2014.01.024. [DOI] [PubMed] [Google Scholar]
- 10.Caly WR, Strauss E. A prospective study of bacterial infections in patients with cirrhosis. J Hepatol. 1993;18:353–358. doi: 10.1016/s0168-8278(05)80280-6. [DOI] [PubMed] [Google Scholar]
- 11.Yoshida H, Hamada T, Inuzuka S, Ueno T, Sata M, Tanikawa K. Bacterial infection in cirrhosis, with and without hepatocellular carcinoma. Am J Gastroenterol. 1993;88:2067–2071. [PubMed] [Google Scholar]
- 12.Fernández J, Navasa M, Gómez J, Colmenero J, Vila J, Arroyo V, Rodés J. Bacterial infections in cirrhosis: epidemiological changes with invasive procedures and norfloxacin prophylaxis. Hepatology. 2002;35:140–148. doi: 10.1053/jhep.2002.30082. [DOI] [PubMed] [Google Scholar]
- 13.Fernández J, Acevedo J, Castro M, Garcia O, de Lope CR, Roca D, Pavesi M, Sola E, Moreira L, Silva A, et al. Prevalence and risk factors of infections by multiresistant bacteria in cirrhosis: a prospective study. Hepatology. 2012;55:1551–1561. doi: 10.1002/hep.25532. [DOI] [PubMed] [Google Scholar]
- 14.Rosa H, Silvério AO, Perini RF, Arruda CB. Bacterial infection in cirrhotic patients and its relationship with alcohol. Am J Gastroenterol. 2000;95:1290–1293. doi: 10.1111/j.1572-0241.2000.02026.x. [DOI] [PubMed] [Google Scholar]
- 15.Cazzaniga M, Dionigi E, Gobbo G, Fioretti A, Monti V, Salerno F. The systemic inflammatory response syndrome in cirrhotic patients: relationship with their in-hospital outcome. J Hepatol. 2009;51:475–482. doi: 10.1016/j.jhep.2009.04.017. [DOI] [PubMed] [Google Scholar]
- 16.Thabut D, Massard J, Gangloff A, Carbonell N, Francoz C, Nguyen-Khac E, Duhamel C, Lebrec D, Poynard T, Moreau R. Model for end-stage liver disease score and systemic inflammatory response are major prognostic factors in patients with cirrhosis and acute functional renal failure. Hepatology. 2007;46:1872–1882. doi: 10.1002/hep.21920. [DOI] [PubMed] [Google Scholar]
- 17.Tsiakalos A, Karatzaferis A, Ziakas P, Hatzis G. Acute-phase proteins as indicators of bacterial infection in patients with cirrhosis. Liver Int. 2009;29:1538–1542. doi: 10.1111/j.1478-3231.2009.02088.x. [DOI] [PubMed] [Google Scholar]
- 18.Papp M, Vitalis Z, Altorjay I, Tornai I, Udvardy M, Harsfalvi J, Vida A, Kappelmayer J, Lakatos PL, Antal-Szalmas P. Acute phase proteins in the diagnosis and prediction of cirrhosis associated bacterial infections. Liver Int. 2012;32:603–611. doi: 10.1111/j.1478-3231.2011.02689.x. [DOI] [PubMed] [Google Scholar]
- 19.Lazzarotto C, Ronsoni MF, Fayad L, Nogueira CL, Bazzo ML, Narciso-Schiavon JL, de Lucca Schiavon L, Dantas-Corrêa EB. Acute phase proteins for the diagnosis of bacterial infection and prediction of mortality in acute complications of cirrhosis. Ann Hepatol. 2013;12:599–607. [PubMed] [Google Scholar]
- 20.Li CH, Yang RB, Pang JH, Chang SS, Lin CC, Chen CH, Chen HY, Chiu TF. Procalcitonin as a biomarker for bacterial infections in patients with liver cirrhosis in the emergency department. Acad Emerg Med. 2011;18:121–126. doi: 10.1111/j.1553-2712.2010.00991.x. [DOI] [PubMed] [Google Scholar]
- 21.Katoonizadeh A, Laleman W, Verslype C, Wilmer A, Maleux G, Roskams T, Nevens F. Early features of acute-on-chronic alcoholic liver failure: a prospective cohort study. Gut. 2010;59:1561–1569. doi: 10.1136/gut.2009.189639. [DOI] [PubMed] [Google Scholar]
- 22.Gonzalez-Casas R, Jones EA, Moreno-Otero R. Spectrum of anemia associated with chronic liver disease. World J Gastroenterol. 2009;15:4653–4658. doi: 10.3748/wjg.15.4653. [DOI] [PMC free article] [PubMed] [Google Scholar]