Abstract
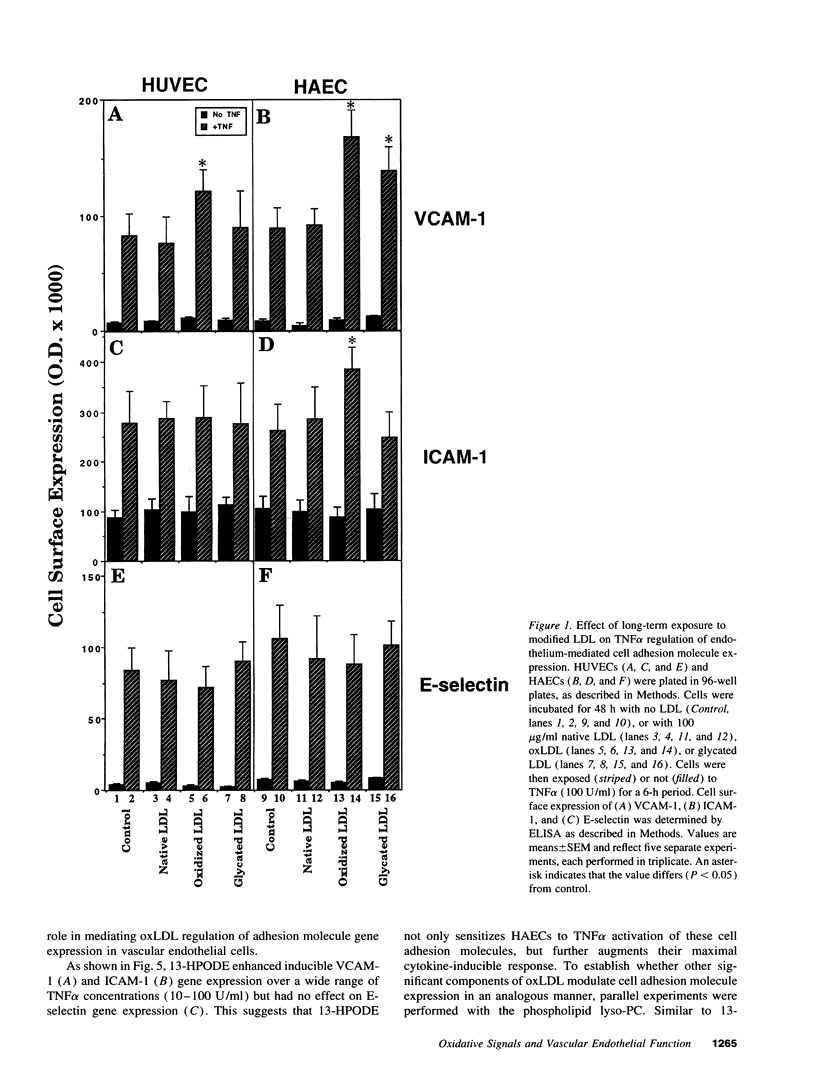
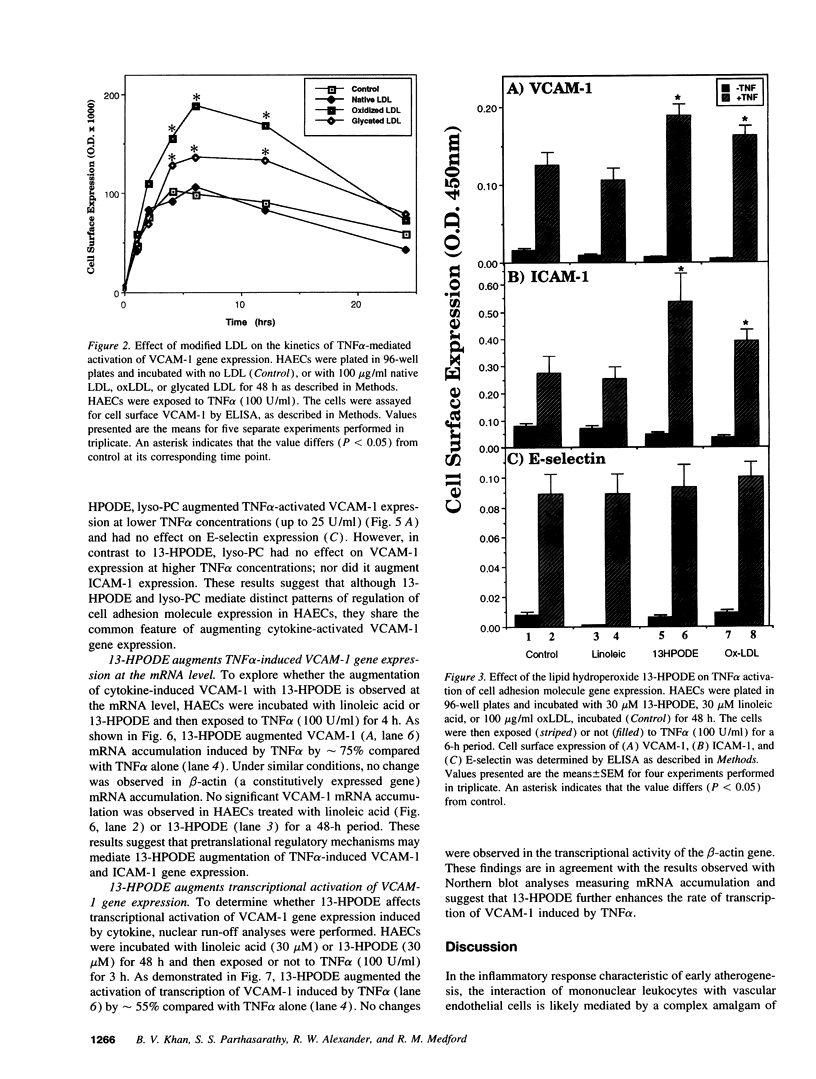
Early features in the pathogenesis of atherosclerosis include accumulation of oxidized LDL (oxLDL) and endothelial expression of the vascular adhesion molecule VCAM-1. Because antioxidants inhibit endothelial VCAM-1 expression, we tested the hypothesis that oxLDL functions as a prooxidant signal in atherogenesis to augment VCAM-1 activation by inflammatory signals. Cultured human aortic endothelial cells (HAECs) or human umbilical vein endothelial cells (HUVECs) were incubated with unmodified LDL, oxLDL, or glycated LDL for 48 h. No change in VCAM-1, intercellular cell adhesion molecule-1 (ICAM-1), or E-selectin expression from control was observed by ELISA. However, dose-response and time course studies demonstrated that oxLDL enhanced VCAM-1 expression induced by the cytokin tumor necrosis factor alpha (TNF alpha) 63% in HAECs and 45% in HUVECs over unmodified LDL or control. Using flow cytometry analysis, oxLDL augmented TNF alpha-induced VCAM-1 expression in a uniform HAEC population. oxLDL had no effect on E-selection induction. oxLDL augmented TNF alpha-induced ICAM-1 expression 44% in HAECs but not in HUVECs. Glycated LDL augmented TNF alpha-induced VCAM-1 expression 35% in HAECs but not HUVECs. Similar results were obtained with 13-HPODE or lysophosphatidylcholine, significant components of oxLDL. 13-HPODE augmented TNF alpha-induced mRNA accumulation and transcriptional activation of VCAM-1 in HAECs. These results suggest that as long-term regulatory signals, specific oxidized fatty acid and phospholipid components of oxLDL augment the ability of vascular endothelial cells to express cytokine-mediated VCAM-1. These studies link oxidant signals conferred by oxLDL to oxidation-sensitive regulatory mechanisms controlling the expression of endothelial cell adhesion molecules involved in early atherosclerosis.
Full text
PDF


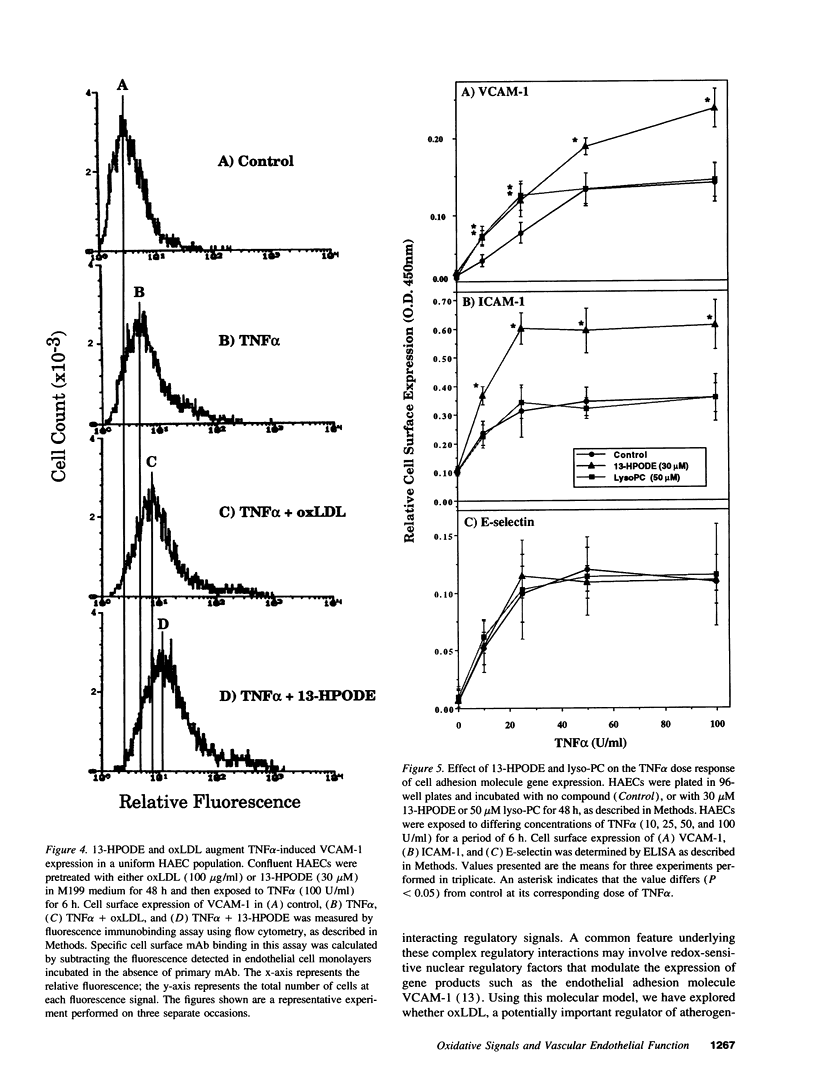
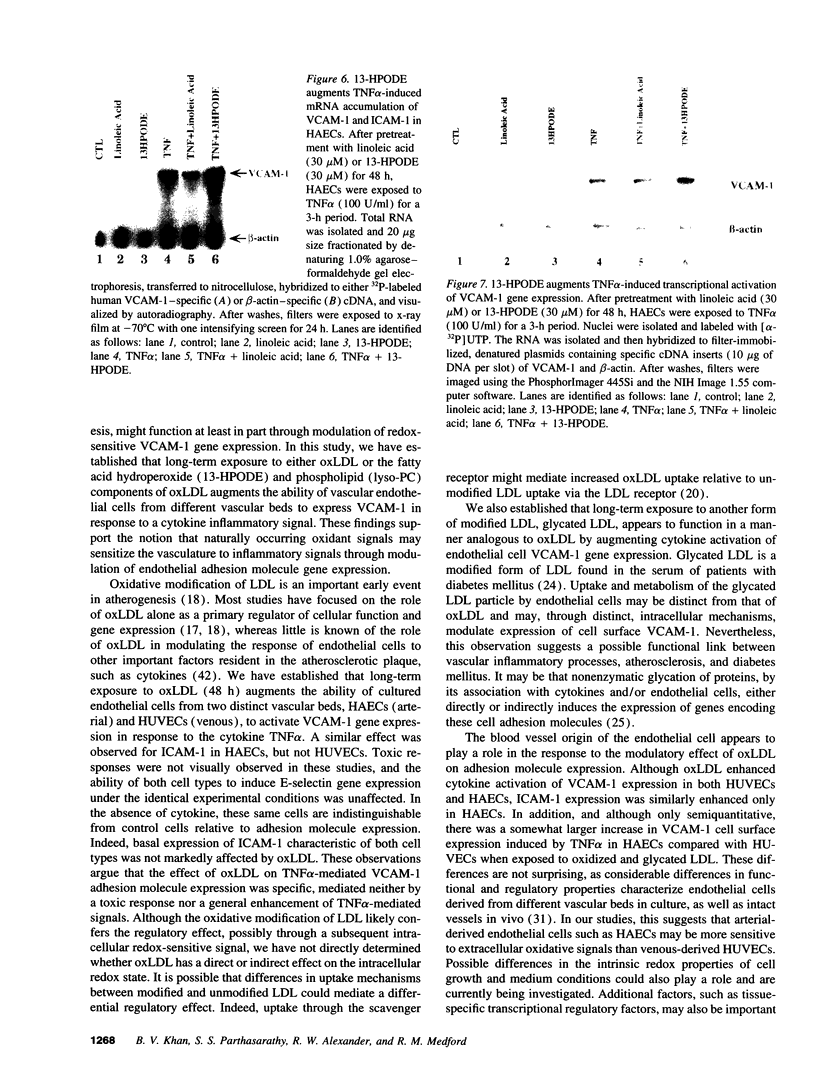


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barath P., Fishbein M. C., Cao J., Berenson J., Helfant R. H., Forrester J. S. Detection and localization of tumor necrosis factor in human atheroma. Am J Cardiol. 1990 Feb 1;65(5):297–302. doi: 10.1016/0002-9149(90)90291-8. [DOI] [PubMed] [Google Scholar]
- Berliner J. A., Territo M. C., Sevanian A., Ramin S., Kim J. A., Bamshad B., Esterson M., Fogelman A. M. Minimally modified low density lipoprotein stimulates monocyte endothelial interactions. J Clin Invest. 1990 Apr;85(4):1260–1266. doi: 10.1172/JCI114562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevilacqua M. P., Pober J. S., Wheeler M. E., Cotran R. S., Gimbrone M. A., Jr Interleukin 1 acts on cultured human vascular endothelium to increase the adhesion of polymorphonuclear leukocytes, monocytes, and related leukocyte cell lines. J Clin Invest. 1985 Nov;76(5):2003–2011. doi: 10.1172/JCI112200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butcher E. C. Leukocyte-endothelial cell recognition: three (or more) steps to specificity and diversity. Cell. 1991 Dec 20;67(6):1033–1036. doi: 10.1016/0092-8674(91)90279-8. [DOI] [PubMed] [Google Scholar]
- Carlos T. M., Schwartz B. R., Kovach N. L., Yee E., Rosa M., Osborn L., Chi-Rosso G., Newman B., Lobb R., Rosso M. Vascular cell adhesion molecule-1 mediates lymphocyte adherence to cytokine-activated cultured human endothelial cells. Blood. 1990 Sep 1;76(5):965–970. [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Clinton S. K., Underwood R., Hayes L., Sherman M. L., Kufe D. W., Libby P. Macrophage colony-stimulating factor gene expression in vascular cells and in experimental and human atherosclerosis. Am J Pathol. 1992 Feb;140(2):301–316. [PMC free article] [PubMed] [Google Scholar]
- Cushing S. D., Berliner J. A., Valente A. J., Territo M. C., Navab M., Parhami F., Gerrity R., Schwartz C. J., Fogelman A. M. Minimally modified low density lipoprotein induces monocyte chemotactic protein 1 in human endothelial cells and smooth muscle cells. Proc Natl Acad Sci U S A. 1990 Jul;87(13):5134–5138. doi: 10.1073/pnas.87.13.5134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonen B., Baenziger J., Schonfeld G., Jacobson D., Farrar P. Nonenzymatic glycosylation of low density lipoproteins in vitro. Effects on cell-interactive properties. Diabetes. 1981 Oct;30(10):875–878. doi: 10.2337/diab.30.10.875. [DOI] [PubMed] [Google Scholar]
- Hahne M., Jäger U., Isenmann S., Hallmann R., Vestweber D. Five tumor necrosis factor-inducible cell adhesion mechanisms on the surface of mouse endothelioma cells mediate the binding of leukocytes. J Cell Biol. 1993 May;121(3):655–664. doi: 10.1083/jcb.121.3.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajjar D. P., Pomerantz K. B. Signal transduction in atherosclerosis: integration of cytokines and the eicosanoid network. FASEB J. 1992 Aug;6(11):2933–2941. doi: 10.1096/fasebj.6.11.1644257. [DOI] [PubMed] [Google Scholar]
- Hamilton T. A., Ma G. P., Chisolm G. M. Oxidized low density lipoprotein suppresses the expression of tumor necrosis factor-alpha mRNA in stimulated murine peritoneal macrophages. J Immunol. 1990 Mar 15;144(6):2343–2350. [PubMed] [Google Scholar]
- Henriksen T., Mahoney E. M., Steinberg D. Enhanced macrophage degradation of low density lipoprotein previously incubated with cultured endothelial cells: recognition by receptors for acetylated low density lipoproteins. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6499–6503. doi: 10.1073/pnas.78.10.6499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henriksson P., Hamberg M., Diczfalusy U. Formation of 15-HETE as a major hydroxyeicosatetraenoic acid in the atherosclerotic vessel wall. Biochim Biophys Acta. 1985 Apr 25;834(2):272–274. doi: 10.1016/0005-2760(85)90166-3. [DOI] [PubMed] [Google Scholar]
- Jeng J. R., Chang C. H., Shieh S. M., Chiu H. C. Oxidized low-density lipoprotein enhances monocyte-endothelial cell binding against shear-stress-induced detachment. Biochim Biophys Acta. 1993 Aug 18;1178(2):221–227. doi: 10.1016/0167-4889(93)90013-f. [DOI] [PubMed] [Google Scholar]
- Jougasaki M., Kugiyama K., Saito Y., Nakao K., Imura H., Yasue H. Suppression of endothelin-1 secretion by lysophosphatidylcholine in oxidized low density lipoprotein in cultured vascular endothelial cells. Circ Res. 1992 Sep;71(3):614–619. doi: 10.1161/01.res.71.3.614. [DOI] [PubMed] [Google Scholar]
- Krolewski A. S., Warram J. H., Valsania P., Martin B. C., Laffel L. M., Christlieb A. R. Evolving natural history of coronary artery disease in diabetes mellitus. Am J Med. 1991 Feb 21;90(2A):56S–61S. doi: 10.1016/0002-9343(91)90040-5. [DOI] [PubMed] [Google Scholar]
- Kugiyama K., Kerns S. A., Morrisett J. D., Roberts R., Henry P. D. Impairment of endothelium-dependent arterial relaxation by lysolecithin in modified low-density lipoproteins. Nature. 1990 Mar 8;344(6262):160–162. doi: 10.1038/344160a0. [DOI] [PubMed] [Google Scholar]
- Kume N., Arai H., Kawai C., Kita T. Receptors for modified low-density lipoproteins on human endothelial cells: different recognition for acetylated low-density lipoprotein and oxidized low-density lipoprotein. Biochim Biophys Acta. 1991 Jan 10;1091(1):63–67. doi: 10.1016/0167-4889(91)90223-k. [DOI] [PubMed] [Google Scholar]
- Kume N., Cybulsky M. I., Gimbrone M. A., Jr Lysophosphatidylcholine, a component of atherogenic lipoproteins, induces mononuclear leukocyte adhesion molecules in cultured human and rabbit arterial endothelial cells. J Clin Invest. 1992 Sep;90(3):1138–1144. doi: 10.1172/JCI115932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kume N., Gimbrone M. A., Jr Lysophosphatidylcholine transcriptionally induces growth factor gene expression in cultured human endothelial cells. J Clin Invest. 1994 Feb;93(2):907–911. doi: 10.1172/JCI117047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Liao F., Andalibi A., deBeer F. C., Fogelman A. M., Lusis A. J. Genetic control of inflammatory gene induction and NF-kappa B-like transcription factor activation in response to an atherogenic diet in mice. J Clin Invest. 1993 Jun;91(6):2572–2579. doi: 10.1172/JCI116495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libby P., Hansson G. K. Involvement of the immune system in human atherogenesis: current knowledge and unanswered questions. Lab Invest. 1991 Jan;64(1):5–15. [PubMed] [Google Scholar]
- Marui N., Offermann M. K., Swerlick R., Kunsch C., Rosen C. A., Ahmad M., Alexander R. W., Medford R. M. Vascular cell adhesion molecule-1 (VCAM-1) gene transcription and expression are regulated through an antioxidant-sensitive mechanism in human vascular endothelial cells. J Clin Invest. 1993 Oct;92(4):1866–1874. doi: 10.1172/JCI116778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navab M., Imes S. S., Hama S. Y., Hough G. P., Ross L. A., Bork R. W., Valente A. J., Berliner J. A., Drinkwater D. C., Laks H. Monocyte transmigration induced by modification of low density lipoprotein in cocultures of human aortic wall cells is due to induction of monocyte chemotactic protein 1 synthesis and is abolished by high density lipoprotein. J Clin Invest. 1991 Dec;88(6):2039–2046. doi: 10.1172/JCI115532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien K. D., Allen M. D., McDonald T. O., Chait A., Harlan J. M., Fishbein D., McCarty J., Ferguson M., Hudkins K., Benjamin C. D. Vascular cell adhesion molecule-1 is expressed in human coronary atherosclerotic plaques. Implications for the mode of progression of advanced coronary atherosclerosis. J Clin Invest. 1993 Aug;92(2):945–951. doi: 10.1172/JCI116670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborn L. Leukocyte adhesion to endothelium in inflammation. Cell. 1990 Jul 13;62(1):3–6. doi: 10.1016/0092-8674(90)90230-c. [DOI] [PubMed] [Google Scholar]
- Parhami F., Fang Z. T., Fogelman A. M., Andalibi A., Territo M. C., Berliner J. A. Minimally modified low density lipoprotein-induced inflammatory responses in endothelial cells are mediated by cyclic adenosine monophosphate. J Clin Invest. 1993 Jul;92(1):471–478. doi: 10.1172/JCI116590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parhami F., Fang Z. T., Fogelman A. M., Andalibi A., Territo M. C., Berliner J. A. Minimally modified low density lipoprotein-induced inflammatory responses in endothelial cells are mediated by cyclic adenosine monophosphate. J Clin Invest. 1993 Jul;92(1):471–478. doi: 10.1172/JCI116590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perchellet E. M., Maatta E. A., Abney N. L., Perchellet J. P. Effects of diverse intracellular thiol delivery agents on glutathione peroxidase activity, the ratio of reduced/oxidized glutathione, and ornithine decarboxylase induction in isolated mouse epidermal cells treated with 12-O-tetradecanoylphorbol-13-acetate. J Cell Physiol. 1987 Apr;131(1):64–73. doi: 10.1002/jcp.1041310111. [DOI] [PubMed] [Google Scholar]
- Pober J., Cotran R. S. What can be learned from the expression of endothelial adhesion molecules in tissues? Lab Invest. 1991 Mar;64(3):301–305. [PubMed] [Google Scholar]
- Richardson M., Hadcock S. J., DeReske M., Cybulsky M. I. Increased expression in vivo of VCAM-1 and E-selectin by the aortic endothelium of normolipemic and hyperlipemic diabetic rabbits. Arterioscler Thromb. 1994 May;14(5):760–769. doi: 10.1161/01.atv.14.5.760. [DOI] [PubMed] [Google Scholar]
- Rollins B. J., Yoshimura T., Leonard E. J., Pober J. S. Cytokine-activated human endothelial cells synthesize and secrete a monocyte chemoattractant, MCP-1/JE. Am J Pathol. 1990 Jun;136(6):1229–1233. [PMC free article] [PubMed] [Google Scholar]
- Schuh J., Fairclough G. F., Jr, Haschemeyer R. H. Oxygen-mediated heterogeneity of apo-low-density lipoprotein. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3173–3177. doi: 10.1073/pnas.75.7.3173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu Y., Newman W., Tanaka Y., Shaw S. Lymphocyte interactions with endothelial cells. Immunol Today. 1992 Mar;13(3):106–112. doi: 10.1016/0167-5699(92)90151-V. [DOI] [PubMed] [Google Scholar]
- Simon B. C., Cunningham L. D., Cohen R. A. Oxidized low density lipoproteins cause contraction and inhibit endothelium-dependent relaxation in the pig coronary artery. J Clin Invest. 1990 Jul;86(1):75–79. doi: 10.1172/JCI114718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparrow C. P., Parthasarathy S., Steinberg D. Enzymatic modification of low density lipoprotein by purified lipoxygenase plus phospholipase A2 mimics cell-mediated oxidative modification. J Lipid Res. 1988 Jun;29(6):745–753. [PubMed] [Google Scholar]
- Witztum J. L., Steinberg D. Role of oxidized low density lipoprotein in atherogenesis. J Clin Invest. 1991 Dec;88(6):1785–1792. doi: 10.1172/JCI115499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ylä-Herttuala S., Lipton B. A., Rosenfeld M. E., Särkioja T., Yoshimura T., Leonard E. J., Witztum J. L., Steinberg D. Expression of monocyte chemoattractant protein 1 in macrophage-rich areas of human and rabbit atherosclerotic lesions. Proc Natl Acad Sci U S A. 1991 Jun 15;88(12):5252–5256. doi: 10.1073/pnas.88.12.5252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ylä-Herttuala S. Macrophages and oxidized low density lipoproteins in the pathogenesis of atherosclerosis. Ann Med. 1991;23(5):561–567. doi: 10.3109/07853899109150518. [DOI] [PubMed] [Google Scholar]
- Ylä-Herttuala S., Palinski W., Rosenfeld M. E., Parthasarathy S., Carew T. E., Butler S., Witztum J. L., Steinberg D. Evidence for the presence of oxidatively modified low density lipoprotein in atherosclerotic lesions of rabbit and man. J Clin Invest. 1989 Oct;84(4):1086–1095. doi: 10.1172/JCI114271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ylä-Herttuala S., Rosenfeld M. E., Parthasarathy S., Glass C. K., Sigal E., Witztum J. L., Steinberg D. Colocalization of 15-lipoxygenase mRNA and protein with epitopes of oxidized low density lipoprotein in macrophage-rich areas of atherosclerotic lesions. Proc Natl Acad Sci U S A. 1990 Sep;87(18):6959–6963. doi: 10.1073/pnas.87.18.6959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ylä-Herttuala S., Rosenfeld M. E., Parthasarathy S., Sigal E., Särkioja T., Witztum J. L., Steinberg D. Gene expression in macrophage-rich human atherosclerotic lesions. 15-lipoxygenase and acetyl low density lipoprotein receptor messenger RNA colocalize with oxidation specific lipid-protein adducts. J Clin Invest. 1991 Apr;87(4):1146–1152. doi: 10.1172/JCI115111. [DOI] [PMC free article] [PubMed] [Google Scholar]