Abstract
Topiramate reduces drinking, but little is known about the mechanisms that precipitate this effect. This double-blind randomized placebo-controlled study assessed the putative mechanisms by which topiramate reduces alcohol use among 96 adult nontreatment-seeking heavy drinkers in a laboratory-based alcohol cue reactivity assessment and in the natural environment using ecological momentary assessment methods. Topiramate reduced the quantity of alcohol heavy drinkers consumed on drinking days and reduced craving while participants were drinking but did not affect craving outside of drinking episodes in either the laboratory or in the natural environment. Topiramate did not alter the stimulant or sedative effects of alcohol ingestion during the ascending limb of the blood alcohol curve. A direct test of putative mechanisms of action using multilevel structural equation mediation models showed that topiramate reduced drinking indirectly by blunting alcohol-induced craving. These findings provide the first real-time prospective evidence that topiramate reduces drinking by reducing alcohol’s priming effects on craving and highlight the importance of craving as an important treatment target of pharmacotherapy for alcoholism.
Keywords: Craving, Medication Action, Topiramate
Introduction
An estimated 38 million adults in the United States engage in heavy drinking on a nearly weekly basis, yet most of these individuals do not meet criteria for an alcohol use disorder (Kanny et al. 2013). Beyond the harmful effects of acute intoxication, prolonged excessive alcohol consumption is associated with numerous health problems (Corrao et al. 2004; Smith et al. 1999). Despite the high prevalence of alcohol misuse, the development of new treatments is hampered by the fact that researchers have struggled to elucidate the precise mechanisms by which medications reduce drinking (Heilig & Egli 2006). A better understanding of the processes by which treatments exert their beneficial effect could advance personalized treatment planning and inform the development of novel interventions.
One promising medication whose mechanisms remain largely unstudied is topiramate, which in several clinical trials reduced drinking in general and heavy drinking in particular both among alcohol-dependent individuals and among nondependent heavy drinkers (Baltieri et al. 2008; Johnson et al. 2003, 2007; Kranzler et al. 2014a; Rubio et al. 2009). Topiramate is an AMPA/kainite glutamate antagonist that also facilitates gamma aminobutyric acid (GABA) function. It is purported to affect drinking via corticomesolimbic dopamine neurotransmission, which is tonically under GABAergic inhibitory control and glutamatergic excitatory control (Johnson et al. 2003). Based on this mechanism, topiramate is theorized to inhibit dopamine release in the midbrain following alcohol consumption, thereby attenuating motivation (i.e., craving) to continue drinking (Johnson et al. 2003).
Several studies have examined topiramate’s effects on craving. In the Johnson et al. (2003) trial, topiramate reduced alcohol craving reported at weekly assessments compared to placebo, with reductions in craving and drinking becoming more pronounced as the trial progressed. Rubio and colleagues (2009) reported similar effects among alcohol dependent men. Although none of the remaining placebo-controlled clinical trials reported data on craving (Baltieri et al. 2008; Johnson et al. 2007; Kranzler et al. 2014a), several open-label studies observed similar effects (Paparrigopoulos et al. 2011; Rubio et al. 2004). In a preliminary human laboratory study, we tested the effects of 200 mg and 300 mg of topiramate, compared to placebo, on measures of general craving, alcohol cue-elicited craving, and the subjective effects of alcohol in a nontreatment-seeking sample (Miranda et al. 2008). Topiramate did not affect either index of craving but attenuated the stimulant effects of alcohol in the 200 mg condition, although not in the 300 mg condition.
On the whole, findings from clinical trials suggest that topiramate reduces alcohol use, at least in part, by blunting craving. But it remains unclear whether topiramate first reduced craving and thus reduced drinking, or whether topiramate affected another aspect of alcohol use that reduced drinking, which in turn reduced craving. In a recent secondary analysis, Kranzler et al (2014b) examined whether topiramate’s effects on desire to drink or positive alcohol expectancies mediated its effect on drinking. Although topiramate reduced drinking, desire to drink, and positive expectancies among individuals with a specific genotype at a glutamate receptor gene, these effects did not explain how topiramate affected drinking. Despite null findings, this study reflects an important step toward trying to elucidate how medications exert beneficial effects. Improvements in ecological momentary assessment (EMA) methods with respect to the timing and scope of sampling schedules and consideration to the temporal order of potential mediators and drinking within a given day or monitoring period may yield more sensitive ways of detecting biobehavioral mechanisms of medication effects (Miranda et al. 2013; Tidey et al. 2008).
In the present investigation, a distinct subsequent study to our initial dose-response trial (Miranda et al. 2008), we used EMA methods to further clarify the biobehavioral mechanisms by which topiramate affects drinking. Although human laboratory studies are the standard for understanding medication mechanisms, EMA methods can provide important information not obtainable from laboratory paradigms by assessing how individuals behave in their daily lives. In addition, laboratory paradigms typically cannot inform the temporal sequence of putative mechanisms on drinking. Therefore, even when laboratory studies show medication effects on a putative mechanism of action (e.g., craving), such findings only test the first link of the proposed causal chain from medication to putative mechanism of action and leave the second link from the mechanism to the outcome to be assumed from other research. EMA can provide a more complete understanding of the processes by which medications reduce drinking and thereby could identify the most important treatment targets for disrupting patterns of pathological drinking.
The present study examined topiramate’s effects on drinking, craving, and subjective responses to alcohol among nontreatment-seeking adult heavy drinkers in their natural environment. It was hypothesized that topiramate (200 mg/day), as compared to placebo, would reduce daily quantities of alcohol consumption. In terms of putative mechanisms, it was hypothesized that topiramate would decrease the intensity of craving outside drinking episodes, as observed in clinical trials. For maximum resolution for examining craving, we paired EMA with laboratory-based alcohol cue reactivity assessment (CRA) methods. We also examined the effects of topiramate on craving, stimulation, and sedation while drinking. It was hypothesized that topiramate would blunt alcohol-induced craving and stimulation and enhance sedation. Finally, we examined the temporal relationship between craving and subjective responses to alcohol in natural drinking settings and daily drinking levels to investigate whether topiramate reduces daily drinking levels, at least in part, by blunting craving and altering alcohol’s subjective effects.
Materials and Methods
Participants
We recruited adult (≥ 18 years) heavy drinkers (≥ 14 standard drinks per week in the past 90 days for women and ≥ 18 drinks for men) from the community for a pharmacotherapy study on alcohol misuse. Exclusion criteria included treatment seeking or a history of treatment for alcohol problems in the past 30 days; significant alcohol withdrawal (> 10 on the Clinical Institute Withdrawal Assessment for Alcohol-revised, Sullivan et al. 1989); positive urine screen for narcotics, amphetamines, or sedative hypnotics or self-reported drug use other than alcohol, cannabis, or nicotine in the past 30 days; medical and psychiatric conditions (e.g., actively suicidal or psychotic) or prescribed medications that contraindicated taking topiramate; weight < 110 or > 250 lbs; or living with someone enrolled in the study. Females were ineligible if they were pregnant, nursing, or unwilling to use a barrier method of birth control.
Materials and Procedure
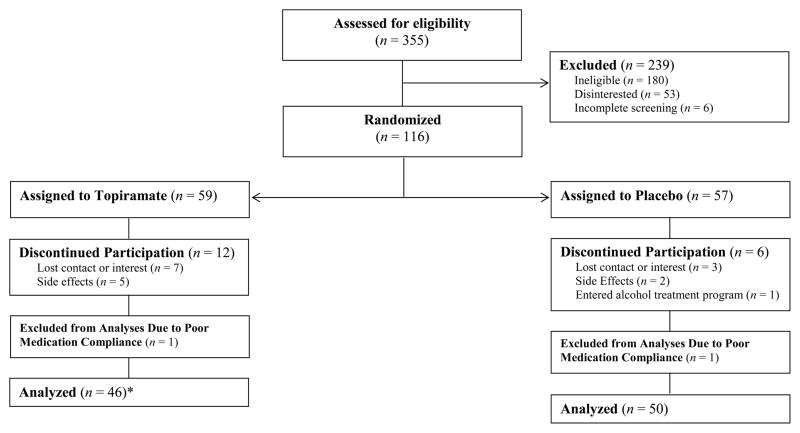
Figure 1 illustrates the flow of participants through the study. Volunteers provided consent and underwent a screening that included a medical/psychiatric history, urine and blood tests, and a physical exam. Participants were randomized to topiramate or placebo for 5 weeks, completed baseline measures and were taught the EMA protocol. Those randomized to topiramate began with the same 3-week titration period used in the Miranda et al. (2008) study followed by two weeks at the target dose (200 mg/day). During study week 5, participants completed a laboratory-based alcohol CRA. The Brown University Institutional Review Board approved this study.
Figure 1.
Participant flow through the randomized double-blind study; *One participant randomized to the topiramate condition did not consume alcohol while at the target dose and therefore was not included in analyses on subjective responses to alcohol. This participate was included in analyses of drinking outcomes.
Medication administration and compliance
Topiramate was compounded from bulk into dose-specific capsules. Placebo capsules contained pharmacologically inert microcrystalline cellulose filler and were identical to topiramate capsules except for content. Participants were assigned two capsules daily, one morning and one evening dose, administered in a double-blind fashion. All capsules contained riboflavin to assess compliance in both conditions. Two blinded raters independently evaluated urine samples for riboflavin (Del Boca et al. 1996). A third rater resolved discrepancies. Participants were considered compliant if their urine was positive for riboflavin in study week 4 or 5.
EMA
Our EMA software was implemented on handheld electronic devices (iPAQ hx2490b, Hewlett Packard, Palo Alto, CA). Instructions were in simple English and participants recorded data by tapping directly on the screen. Response options included: visual analog bars; multiple checkboxes when more than one option was appropriate; and categorical checkboxes when only one response was warranted. An alarm-clock feature prevented assessments from occurring while sleeping.
Participants were trained to discern standard drink volumes (14 g of alcohol; National Institute on Alcohol Abuse and Alcoholism, 2005). Participants completed EMA reports upon waking, immediately before the first alcoholic drink of an episode, directly after each of the first three standard drinks of an episode, and in response to auditory prompts delivered at randomly selected times once within each 3-hour block (e.g., 3–6pm) throughout the day except when sleeping or otherwise unable to respond (e.g., driving). We set the number of drinks assessed at three based on evidence that subjective effects are detectable at BACs of 0.04g/dl (Davidson et al. 1997). By consuming three drinks participants were expected to reach this level.
Upon waking, participants recorded the number of standard alcoholic drinks consumed yesterday. At the start of the first drink of an episode, participants initiated a begin-drink report. Begin-drink reports first queried participants about whether they had started their drink and, if so, how many minutes had elapsed since they began drinking. Participants then rated their subjective craving, stimulation, and sedation. After finishing each of the first three alcoholic drinks of an episode, participants completed end-drink reports where they indicated how many minutes elapsed since they finished the drink, selected the beverage type, recorded the ounces consumed, and rated their subjective states.
At random assessments, participants rated their subjective states and selected one of three response options to indicate whether alcohol was present (not visible, visible directly [bottle, glass, etc.], or visible indirectly [television, advertisement, etc.]). As done previously (Ramirez & Miranda 2014), we dichotomized alcohol cues as not present (0) versus directly or indirectly present (1) for all analyses.
Alcohol cue reactivity
We modeled the CRA on published protocols (Miranda et al. 2008). Participants tested negative for breath alcohol before the session (Intoximeters Inc., Saint Louis, MO) and were fitted with a blood pressure cuff (Criticare Systems Inc., Waukesha, WI) to assess mean arterial pressure (MAP) and heart rate. Participants then underwent a 3-min relaxation period to habituate to the inflation cycle and setting. Following relaxation, participants were presented with a glass half full of water accompanied by its commercially labeled bottle. Audio recordings instructed participants to sniff the glass when high tones signaled and stop sniffing when low tones signaled; thirteen 5-s exposures occurred in variable intervals during each trial. Participants then underwent another 3-min relaxation period followed by two alcohol cue exposure trials that were identical to the water trial except the glass contained their most commonly consumed alcoholic beverage and was accompanied by its commercially labeled bottle. Two alcohol trials, which were averaged, ensured a stable estimate of participants’ reactions to alcohol cues. Participants rated their craving after each trial (see Measures). Trials were always presented in the same order due to carryover effects (Monti et al. 1987).
Measures
Participant characteristics
Participants completed a demographics questionnaire. Alcohol diagnoses were derived from the Structured Clinical Interview for DSM-IV Axis I Disorders – Patient Version (First et al. 1997).
Alcohol use
Drinking prior to the study was assessed using the 90-day Timeline Follow-back interview (TLFB; Sobell & Sobell 1992). EMA data provided our primary outcome measure during the trial, with missing data culled from the TLFB (Carney et al. 1998). Dependent measures were the likelihood of drinking on any given day and the number of standard drinks consumed per drinking day.
Momentary subjective states
Momentary ratings of craving, stimulation, and sedation were assessed at three time points: 1) random assessments, 2) drinking onset, and 3) directly after each of the first three drinks of an episode. Craving was measured in the laboratory and via EMA using the same single item, rated from 0 (no urge) to 10 (strongest ever), which has been widely used (Miranda et al. 2008, 2013, 2014; Monti et al. 1987). Two items from the stimulation (energized, excited) and sedation (sedated, sluggish) subscales of the Biphasic Alcohol Effects Scale (Martin et al. 1993) were administered to reduce burden and facilitate compliance. Items were rated on visual analog scales from 0 (not at all) to 10 (extremely) and combined into a mean score for each dimension. Reliability coefficients supported the internal consistency of these subscales (Cronbach’s α for begin- and end-drink reports, respectively: Stimulation = 0.85, 0.86; Sedation = 0.72, 0.79). For all items, participants rated their feelings ‘right now.’ Items used to assess stimulation and sedation were general descriptors of affect independent from alcohol-specific effects to avoid psychometric issues involved with asking participants to deconstruct the degree to which changes in affect are attributable to alcohol (Rueger et al. 2009).
Estimated blood alcohol concentrations (eBAC)
In order to capture gradations of alcohol consumption we calculated eBAC at each momentary assessment in the natural environment using a standard equation shown to produce high intraclass correlations with actual BACs (Hustad & Carey 2005; Matthews & Miller 1979). This approach was used successfully in previous EMA research (Miranda et al. 2013, 2014; Piasecki et al. 2012; Ray et al. 2010).
Adverse effects
The Systematic Assessment for Treatment Emergent Effects Interview (Johnson et al. 2005; Levine & Schooler 1986) was used to assess side effects at weekly visits. To assess expected and unexpected events, side effects were collected in an open-ended way first and then categorized. Staff then queried about known topiramate effects.
Data Analytic Strategy
Analyses were conducted in SAS 9.3 (SAS Institute Inc. 2012) and Mplus 7.0 (Muthén & Muthén 1998–2013). Pretreatment differences between conditions were evaluated using independent sample t-tests and chi-squared analyses. Primary analyses focused on repeated assessments of drinking-related variables from each participant across time. The nested data structure, variable number of diary records for each participant, and unique timing of reports made these data suitable for multilevel modeling (MLM; Gibbons et al. 2010; Raudenbush & Bryk 2002; Singer & Willet 2003). Multilevel models were fit with an unstructured variance/covariance matrix and between/within degrees of freedom. Time-invariant covariates (baseline drinking variables but excluding sex) were grand-mean centered; eBAC (time-varying covariate) was person-mean centered.
Our first set of hypotheses predicted that at the target dose topiramate reduces the likelihood of drinking on any given day and number of standard drinks consumed each drinking day. Days (level 1) were nested within persons (level 2). Likelihood of drinking was modeled with a binary distribution and logit link function, whereas number of drinks was modeled as a continuous variable. Final models tested whether effects remained significant when sex and baseline drinking variables were covaried.
Next, we tested whether topiramate dampened craving in the laboratory and in the natural environment outside of drinking episodes. For the multilevel analysis of laboratory data, cue type (alcohol vs water; level 1) was nested within persons (level 2). EMA data were culled from random assessments recorded before drinking each day to curtail confounding effects with alcohol intoxication. Diary responses (level 1) were nested within persons (level 2). Final models tested whether effects remained significant when sex and baseline percent drinking days were entered as covariates.
We then examined effects of topiramate on craving, stimulation, and sedation in drinking and nondrinking moments across drinking days. Diary responses (level 1) were nested within persons (level 2). A Medication Condition × Drinking Moment interaction was included to contrast effects of topiramate on subjective states prior to (nondrinking moment = 0) and while drinking (drinking moment = 1). This approach disentangles the subjective effects of alcohol from how participants were feeling prior to drinking on a given day. Consistent with other EMA studies (Miranda et al. 2013, 2014; Ray et al. 2010; Tidey et al. 2008), drinking episodes where the participant initiated the begin-drink report > 5 min after drinking began (n = 216 of 2,342; 9.22%) were excluded from analyses to curtail the pharmacological effects of alcohol consumption on these measures.1 Begin drink reports recorded within 5 minutes of drinking onset were included in analyses as nondrinking moments. We also evaluated whether drink reports were recorded during the ascending or descending limb of the blood alcohol curve by computing successive differences in eBAC across reports within each drinking episode (Miranda et al. 2013, 2014; Piasecki et al. 2012). Few reports were recorded during the descending limb (n = 2 of 2,346 drink reports). We restricted analyses to data collected during the ascending limb to facilitate interpretation of results. Final models tested whether effects remained significant when covariates (eBAC, sex, baseline percent drinking days) were included.
Finally, we used multilevel structural equation modeling (MSEM) with fixed slopes and a Bayesian estimation method with diffuse (non-informative) priors to test the hypothesis that topiramate reduces drinks consumed across drinking episodes, at least in part, by altering subjective effects (craving, stimulation, sedation) on the ascending limb of intoxication. Diffuse-prior Bayesian estimation can be preferable to a likelihood approach when the distribution of the indirect effect parameter is skewed, as was the case in the present analyses (Muthén 2010). MSEM offers a key advantage over MLM-based approaches to mediation analyses. Using MLM, between-subjects (level 2) and within-subject (level 1) effects are combined in estimating the indirect effect, which conflates the estimate (Preacher et al. 2010). MSEM mitigates this bias by treating the between-subject level component of the within-subject variable as a latent variable (Preacher et al. 2010).
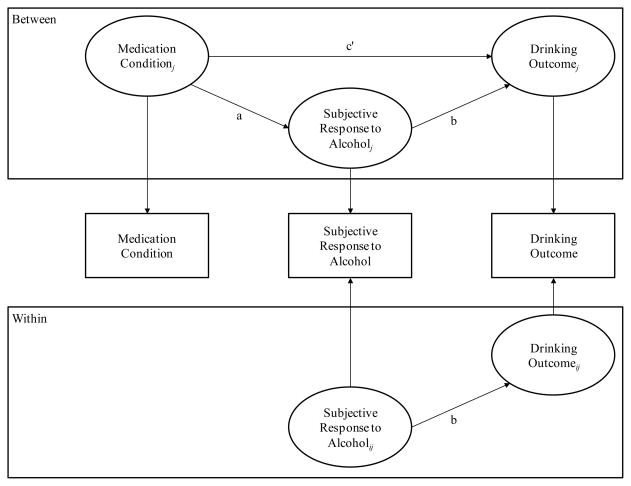
A separate model was conducted to test each putative mediator. As illustrated in Figure 2, the independent variable (IV) was medication condition, the dependent variable (DV) was the number of standard drinks consumed each drinking day during the second week at target dose (study week 5), and the mediating variable (MV) was the putative mechanism (craving, stimulation, sedation) measured via EMA during the second week at target dose (study week 5). In each model, we controlled for the corresponding subjective response variable and drinks per drinking day measured during the first week at target dose (study week 4). This sequenced approach afforded a rigorous test of our hypothesis that altered subjective responses to alcohol early in a drinking episode (i.e., after each of the first 3 standard drinks) predict how much total alcohol an individual will consume that day. Moreover, based on our dose-response study (Miranda et al. 2008) we expected the effective dose to be 200mg and this study was specifically designed to test the effects of topiramate at that level. Medication dose during the titration period (study weeks 1–3) was variable, with participants changing dosages every few days. Focusing on data from study week 5 allowed participants on topiramate to stabilize at the target dose, providing the best test of the medication’s effects. Controlling for subjective responses and drinking levels in study week 4 allowed us to address the notion that changes in subjective responses at target dose predict changes in the number of drinks consumed across drinking episodes at target dose.
Figure 2.
Example illustration of the 2-1-1 mediation models. Medication Condition = randomly-assigned medication condition; Subjective Response to Alcohol = participant-reported subjective responses to alcohol (i.e., craving, stimulation, sedation) across drinking episodes; Drinking Outcome = participant-reported drinks per drinking day. Carryover effects of subjective response and drinking variables from study week 4 were controlled for in all models. For simplicity, these covariates are not shown.
As shown in Figure 2, medication condition is measured at the between-subjects level (level 2), meaning that it does not have within-subject variability (i.e., participants were randomized to one condition). As such, medication condition can only predict between-level variability in subjective responses and drinking, even though these variables are measured at level 1, and thus mediation can only exist at the between-subjects level (for more details, see Preacher et al. 2010).
Results
Of the 116 randomized participants, 98 (85%) completed the study (see Figure 1). Urine assays indicated all but two of the participants who completed the study were medication compliant; noncompliant participants (placebo = 1, topiramate = 1) were excluded from analyses.2 Characteristics of the final sample (N = 96) are presented in Table 1. Participants were 18 to 60 years old and approximately half the sample met diagnostic criteria for current alcohol abuse (9.4%) or dependence (43.8%). Medication groups were equivalent on baseline study variables (see Table 1).
Table 1.
Participant Characteristics and Comparisons by Medication Condition
| Characteristic | Topiramate (n =
46) |
Placebo (n = 50)
|
t or χ2 | p |
|---|---|---|---|---|
| N (%) or M ± SD | N (%) or M ± SD | |||
| Age | 35.91 ± 12.04 | 35.74 ± 12.99 | −0.68 | .946 |
| Sex (female) | 18 (39.1) | 20 (40.0) | 0.00 | .931 |
| Race | 3.39 | .640 | ||
| White | 33 (71.7) | 36 (72.0) | ||
| African-American | 9 (19.6) | 10 (20.0) | ||
| American Indian | 1 (2.2) | 1 (2.0) | ||
| Asian/Pacific Islander | 2 (4.3) | 1 (2.0) | ||
| Mixed Race | 1 (2.2) | 2 (4.0) | ||
| Ethnicity (Hispanic)a | 5 (10.9) | 7 (14.3) | 0.25 | .616 |
| Cigarette smoker | 24 (52.2) | 23 (46.0) | 0.37 | .545 |
| Alcohol abuseb | 4 (8.7) | 5 (10.0) | 0.05 | .827 |
| Alcohol dependenceb | 19 (41.3) | 23 (46.0) | 0.22 | .643 |
| Drinks per drinking dayc | 6.60 ± 3.26 | 7.18 ± 5.62 | 0.61 | .543 |
| Drinking daysc | 73.23 ± 19.26 | 70.60 ± 21.70 | −0.63 | .533 |
| Heavy drinking daysc | 47.00 ± 27.76 | 45.16 ± 24.31 | −0.34 | .732 |
Note.
Ethnicity and race were not mutually exclusive;
Diagnostic status was identified in accordance with the Diagnostic and Statistical Manual of Mental Disorders (Fourth Edition, Text Revision) using the Structured Clinical Interview for DSM-IV Axis I Disorders;
Derived from the 90-day Timeline Follow-Back interview conducted at baseline.
Table 2 presents side effects reported by ≥ 10% of participants in either condition. Participants on topiramate reported paresthesias, fatigue, change in taste, and memory problems more frequently than placebo. Otherwise, there were no differences between conditions.
Table 2.
Adverse Events Reported by ≥ 10% of Participants in Either Medication Condition
| Adverse Event | Topiramate
(n = 46) |
Placebo (n
= 50) |
χ2 (1, N = 96) | p | ||
|---|---|---|---|---|---|---|
| n | % | n | % | |||
| Paresthesias | 22 | 47.8 | 6 | 12.0 | 14.884 | < .001 |
| Fatigue | 17 | 37.0 | 8 | 16.0 | 5.463 | .019 |
| Decreased appetite | 14 | 30.4 | 7 | 14.0 | 3.787 | .052 |
| Change in taste | 14 | 30.4 | 4 | 8.0 | 7.915 | .005 |
| Difficulty sleeping | 8 | 17.4 | 6 | 12.0 | 0.559 | .455 |
| Irritability | 6 | 13.0 | 7 | 14.0 | 0.031 | .860 |
| Nervousness/anxiety | 4 | 8.7 | 8 | 16.0 | 1.169 | .280 |
| Difficulty concentrating | 8 | 17.4 | 5 | 10.0 | 1.118 | .290 |
| Gastrointestinal problems | 6 | 13.0 | 6 | 12.0 | 0.024 | .877 |
| Anomic aphasia | 7 | 15.2 | 4 | 8.0 | 1.230 | .267 |
| Memory problems | 8 | 17.4 | 2 | 4.0 | 4.604 | .032 |
| Weight loss | 6 | 13.0 | 3 | 6.0 | 1.399 | .237 |
| Restlessness | 3 | 6.5 | 6 | 12.0 | 0.846 | .358 |
| Dizziness | 5 | 10.9 | 3 | 6.0 | 0.744 | .388 |
Effects of Topiramate on Alcohol Use
Table 3 presents drinking data across the trial. At target dose (study weeks 4–5), participants in the placebo condition drank on 61.43% (SD = 28.46) of study days and consumed 5.02 (SD = 3.69) drinks per drinking day on average, whereas participants in the topiramate condition drank on 53.49% (SD = 30.67) of study days and consumed 3.63 (SD = 1.80) drinks per drinking day on average. There was a main effect of medication condition on drinking levels at target dose, such that individuals randomized to topiramate consumed fewer drinks per drinking day, b = −1.35, 95%CI = [−2.62, −0.08], SE = 0.64, p = .03. Effects remained significant following the addition of sex and baseline number of drinks per drinking day, b = −1.25, 95%CI = [−2.45, −0.06], SE = 0.60, p = .03. The univariate effect of medication condition on the likelihood of drinking was not significant, p = .17; however, the multivariate model including sex and baseline percent drinking days as covariates suggested a marginal trend, OR = .63, 95%CI = [0.37, 1.05], p = .07.
Table 3.
Drinking Behavior Across the Trial by Medication Condition: Percentage or Mean (With Standard Deviation in Parentheses)
| Condition and Outcomes | Baseline | Study Week |
||||
|---|---|---|---|---|---|---|
| Titration Period |
Target Dose |
|||||
| 1 | 2 | 3 | 4 | 5 | ||
| Placebo (n = 50) | ||||||
| Drinks per drinking daya | 7.2 (5.6) | 5.6 (3.5) | 5.2 (3.3) | 5.1 (4.9) | 5.2 (4.3) | 5.1 (3.5) |
| Percent drinking days | 70.6 (21.7) | 75.7 (20.0) | 73.1 (24.3) | 68.0 (27.6) | 63.4 (30.4) | 59.4 (29.9) |
| Topiramate (n = 46) | ||||||
| Drinks per drinking daya | 6.6 (3.3) | 4.7 (3.1) | 3.8 (2.1) | 3.8 (2.5) | 3.7 (2.3) | 3.6 (2.3) |
| Percent drinking days | 72.7 (19.1) | 71.4 (23.1) | 64.1 (27.7) | 59.7 (25.5) | 54.6 (32.6) | 52.4 (31.2) |
Note.
Measured in standard drink volumes
Effects of Topiramate on Alcohol Cue-Elicited Craving Outside Drinking Episodes
EMA findings
Models testing unconditional main effects showed that across both conditions participants reported greater craving outside drinking episodes when exposed to alcohol cues, as compared to settings without alcohol cues, b = 0.76, 95% CI [0.59, 0.93], p < .001. The main effect of medication condition was not significant, b = 0.12, 95% CI [−0.88, 1.12], p = .81. As shown in Table 4, the Medication Condition × Cue interaction was not significant.
Table 4.
Multilevel Models Predicting Momentary Subjective Craving Outside of Drinking Episodes in the Natural Environment and Laboratory from Alcohol Cues and Medication Condition
| Model and Predictors | B | SE | 95% CI |
p | |
|---|---|---|---|---|---|
| LL | UL | ||||
| Natural Environment | |||||
| Intercept | 2.71 | 0.35 | 2.02 | 3.40 | < .001 |
| Alcohol visibility | 0.77 | 0.12 | 0.53 | 1.00 | < .001 |
| Medication condition | 0.10 | 0.50 | −0.90 | 1.11 | .837 |
| Medication condition × Alcohol visibility | −0.01 | 0.17 | −0.35 | 0.33 | .953 |
| Laboratory | |||||
| Intercept | 2.80 | 0.45 | 1.91 | 3.69 | <.001 |
| Cue type | 2.14 | 0.33 | 1.48 | 2.80 | <.001 |
| Medication condition | −0.37 | 0.65 | −1.66 | 0.93 | .576 |
| Cue type × Medication condition | −0.52 | 0.48 | −1.48 | 0.44 | .284 |
Note. CI = confidence interval; LL = lower limit; UL = upper limit. Values are from multivariate/simultaneous models. Therefore, the Alcohol visibility and Medication condition terms are conditional effects (i.e., effect of each term when all other terms in the model = 0). Effects for Alcohol visibility and Cue type remained significant after the addition of sex and baseline percent drinking days.
Laboratory findings
Unconditional models showed that alcohol cues increased craving relative to water cues, b = 1.89, 95% CI [1.41, 2.37], p < .001; the main effect of medication condition was not significant, b = −0.63, 95% CI [−1.82, 0.57], p = .30. Results of the multivariate model showed the Medication Condition × Cue interaction was not significant (see Table 4).
In terms of physiological reactivity, an unconditional model testing the main effect of medication condition showed that topiramate reduced MAP, b = −4.90, 95% CI [−9.54, −0.25], p = .04. The unconditional main effect of cue type was not significant, b = 0.67, 95% CI [−0.45, 1.79], p = .24. The multivariate model showed the Medication Condition × Cue interaction also was not significant, b = 0.46, 95% CI [−1.79, 2.71], p = .69. The unconditional main effects of medication condition and cue type on heart rate were not significant (ps = .23 and .17, respectively). The multivariate model showed the Cue Type × Medication Condition interaction on heart rate also was nonsignificant (p = .53).
Effects of Topiramate on Alcohol-elicited Craving and Subjective Responses to Alcohol
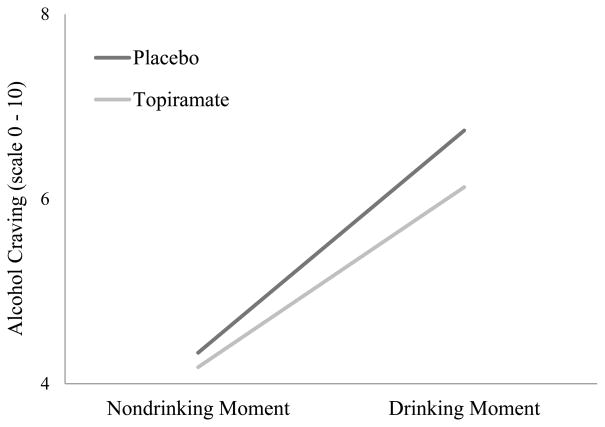
The Medication Condition × Drinking Moment interaction was significant, indicating that participants randomized to topiramate experienced reduced craving while drinking compared to participants randomized to placebo (see Table 5). This interaction was probed by calculating simple slopes with equations appropriate for multilevel data (Preacher et al. 2006). As shown in Figure 3, craving increased while drinking, but this effect was attenuated by topiramate. Unconditional models showed that participants reported greater stimulation while drinking, b = 0.23, 95% CI [0.15, 0.32], p < .001; drinking did not affect sedation, b = 0.03, 95% CI [−0.04, 0.10], p = .36. Unconditional main effects of medication condition on stimulation and sedation were not significant (ps = .82 and .19, respectively). As shown in Table 5, the Medication Condition × Drinking Moment interaction effects of topiramate on stimulation and sedation were not significant.
Table 5.
Multilevel Models Predicting Momentary Subjective Responses to Alcohol from Medication Condition across Drinking Days
| B | SE | 95% CI |
p | ||
|---|---|---|---|---|---|
| LL | UL | ||||
| Craving | |||||
| Intercept | 4.18 | 0.31 | 3.57 | 4.78 | < .001 |
| Drinking moment | 2.47 | 0.10 | 2.27 | 2.67 | < .001 |
| Medication condition | −0.11 | 0.45 | −1.00 | 0.77 | .801 |
| Medication condition × Drinking moment | −0.47 | 0.17 | −0.79 | −0.14 | .005 |
| Stimulation | |||||
| Intercept | 6.00 | 0.30 | 5.40 | 6.60 | <.001 |
| Drinking moment | 0.23 | 0.05 | 0.12 | 0.33 | <.001 |
| Medication condition | −0.09 | 0.44 | −0.97 | 0.78 | .831 |
| Medication condition × Drinking moment | 0.02 | 0.09 | −0.16 | 0.19 | .852 |
| Sedation | |||||
| Intercept | 1.50 | 0.23 | 1.05 | 1.96 | <.001 |
| Drinking moment | 0.03 | 0.04 | −0.06 | 0.11 | .550 |
| Medication condition | 0.44 | 0.33 | −0.22 | 1.11 | .191 |
| Medication condition × Drinking moment | 0.01 | 0.07 | −0.13 | 0.16 | .846 |
Note. CI = confidence interval; LL = lower limit; UL = upper limit. Values are from multivariate/simultaneous models. Therefore, the Drinking-moment and Medication-condition terms are conditional effects (i.e., effect of each term when all other terms in the model = 0). The pattern of results did not change when estimated blood alcohol concentration, sex, and baseline percent drinking days were added to the models.
Figure 3.
Interaction plot between medication condition and drinking moment on alcohol craving in the natural environment; Nondrinking moment = ecological momentary assessments of craving recorded in the natural environment during all momentary reports prior to alcohol use on a given day; Drinking moment = ecological momentary assessments recorded in the natural environment while drinking alcohol.
Prediction of Topiramate’s Effects on Drinking by Alcohol-Induced Craving
Table 6 displays within and between effect estimates and Bayesian posterior standard deviations from MSEM models predicting the number of standard drinks consumed per drinking day from medication condition and subjective responses to alcohol in the natural environment. One-tailed, posterior p-values represent the proportion of the posterior distribution that is below (positive-effect estimates) or above (negative-effect estimates) zero, with significance indicated at p < .025. The direct effect of medication condition on the number of drinks consumed across drinking days was significant, Est = −1.225, SE = .607, p < .001 (see Table 6, column c).
Table 6.
Effect Estimates and Bayesian Posterior Standard Deviations from Multilevel Structural Equation Models Predicting Drinks Consumed Per Drinking Day from Medication Condition and Subjective Responses to Alcohol in the Natural Environment
| Subjective Response (SR) | Effect of Medication on SR (a) | Effect of SR on Drinking (b) | Effect of Medication on Drinking (c) | Effect of Medication on Drinking after SR (c′) | Indirect effect (a × b) | 95% CI for indirect effect |
|---|---|---|---|---|---|---|
| Between | ||||||
| Craving | −0.601† (0.338) | 0.613 (0.280) | −0.823 (0.569) | −0.323† (0.290) | −1.010, 0.029 | |
| Stimulation | −0.125 (0.277) | 0.354 (0.325) | −1.225*** (0.607) | −1.156* (0.579) | −0.022 (0.132) | −0.351, 0.183 |
| Sedation | −0.236 (0.162) | 0.183 (0.799) | −1.119† (0.637) | −0.022 (0.224) | −0.528, 0.418 | |
| Within | ||||||
| Craving | –––– | 0.168*** (0.048) | –––– | –––– | –––– | |
| Stimulation | –––– | 0.117 (0.061) | –––– | –––– | –––– | |
| Sedation | –––– | −0.108 (0.076) | –––– | –––– | –––– | |
Note. SR = subjective response; CI = credible interval. Significance of effects is based on Bayesian one-tailed posterior p-values, at the .025 level. For positive effects, the Bayesian p-value is the proportion of the posterior distribution below zero; for negative effects, the Bayesian p-value is the proportion of the posterior distribution above zero. All models controlled for the carryover effects of subjective responses and drinking from study week 4. The pattern of results did not change when sex and baseline percent drinking days were included.
p< .05.
p< .025.
p< .01.
p< .001.
Next, we fit separate mediation models for craving, stimulation, and sedation to determine which, if any, of these subjective effects accounted for topiramate’s effect on reduced drinking. Within-subjects results suggested that participant-reported craving on the ascending limb of intoxication was significantly associated with greater alcohol consumption across drinking episodes, Est = 0.168, SE = .048, p < .001, whereas effects of stimulation and sedation were not significant (see Table 6, column b). Between-subjects results from mediation models suggested that, on average, the direct effect of topiramate on drinking was not significant after accounting for the effect of craving, Est = −0.823, SE = .569, p = .072 (see Table 6, column c′), suggesting that the effect of topiramate on drinking can be explained, at least in part, by topiramate’s negative association with participant-reported craving while drinking. In contrast, the direct effect of topiramate on drinking remained significant after accounting for the effect of stimulation, Est = −1.156, SE = .579, p = .020, and marginally significant after accounting for the effect of sedation, Est = −1.119, SE = .637, p = .047. A formal test of the indirect effect suggested partial support for the hypothesis that blunted alcohol-induced craving on the ascending limb of intoxication mediates, at least in part, topiramate’s ability to reduce the number of drinks consumed across drinking episodes, Est = −0.323, SE = .290, p = .043 (see Table 6, column a × b).
Discussion
This study focused on elucidating the mechanisms by which topiramate reduces drinking. Topiramate blunted craving to drink more alcohol when participants were drinking but did not affect alcohol-induced stimulation or sedation. The fact that alcohol potentiated stimulation but had no effect on sedation supports the internal validity of our EMA approach for capturing the ascending limb of the blood alcohol curve. Conversely, topiramate did not blunt cue-induced craving outside drinking episodes in the laboratory (consistent with Miranda et al., 2008) or natural environment. Topiramate suppressed MAP during cue reactivity in the laboratory; however, neither the main effect of cue type nor the medication by cue type interaction was significant. This reduction in blood pressure is consistent with findings from clinical trials that examined the effects of topiramate on hypertension (Engeli & Jordan 2013). Thus, topiramate’s beneficial effects on drinking reductions may be due to its ability to reduce craving to drink more alcohol once drinking has begun rather than craving to start drinking. A treatment implication of these findings is that topiramate may be especially beneficial for heavy drinkers whose primary treatment objective is to reduce their drinking to less harmful levels. These findings also suggest that another pharmacologic or behavioral strategy may be needed to supplement topiramate to address craving while abstinent.
Consistent with the above point, mediation models showed that topiramate reduced drinking indirectly by blunting alcohol-induced craving. Specifically, alcohol produced acute increases in craving among heavy drinkers that potentiated subsequent daily drinking levels. Participants randomized to topiramate experienced less craving while drinking, however, and this effect in turn led to reduced volumes of alcohol consumed on drinking days. Our findings provide the first prospective, momentary test of whether craving while drinking mediates the effects of topiramate on drinking under real-world conditions. This suggests that medications that target craving while drinking have potential to reduce heavy drinking.
Our finding that topiramate reduced how much alcohol heavy drinkers consume when drinking is consistent with previous research. Six placebo-controlled clinical trials of topiramate treatment on drinking outcomes have been published to date (Baltieri et al. 2008; Johnson et al. 2003, 2007; Kranzler et al. 2014a; Likhitsathian et al. 2013; Rubio et al. 2009). All but one trial found that topiramate reduced heavy drinking and four trials found that topiramate increased abstinence rates; one trial found no effect of topiramate on heavy drinking or abstinence rates among individuals recently treated for alcohol-dependence in a residential program (Likhitsathian et al. 2013). While the amount of drinking was decreased in the present study, the effect of topiramate on the likelihood of drinking each day was only marginally significant. This marginal finding may be due to the short two-week period that participants remained at the target dose and may also be due to the non-treatment-seeking status of the participants. Nonetheless, it is notable that, extending the findings of Miranda et al. (2008), this is the second study to show that topiramate reduces drinking among heavy drinkers not actively seeking to change their drinking. Finally, participants in our study tolerated topiramate quite well, with similar completion rates in the two medication conditions. This finding, which parallels those reported by Kranzler et al. (2014a), suggests that topiramate may be particularly well tolerated by heavy drinkers. Studies with heavy drinkers used a lower dosage, however, than trials that recruited individuals with alcohol dependence (e.g., Johnson et al. 2007), which may have contributed to the high rate of tolerability.
This study has limitations that should be considered. First, the short duration of treatment at the target dose may have reduced our ability to detect effects of topiramate on abstinence. The similarity of our findings regarding topiramate’s effects on drinking quantities to those from longer clinical trials, however, suggests this limitation is unlikely to affect interpretation of the current results. Second, our sample was comprised of nontreatment-seeking heavy drinkers, so there may be limits to generalizability. Third, we tested the effects of topiramate on craving, stimulation, and sedation early in the ascending limb of drinking episodes when blood alcohol levels were relatively low. These findings may not generalize to the corresponding descending limb or to higher blood alcohol levels in the ascending limb. Finally, we examined only a select subset of possible mechanisms and other possible mechanisms may be involved.
In conclusion, this study is the first to demonstrate that topiramate’s effects on craving while drinking mediate its effects on daily volumes of alcohol use. This finding is consistent with the hypothesis that topiramate inhibits dopamine release in the midbrain while drinking (Johnson et al. 2003), and that this mechanism may be responsible for its beneficial effects. Our results provide the first prospective evidence that alcohol-elicited craving is an important treatment target for pharmacotherapy.
Acknowledgments
The National Institute on Alcohol Abuse and Alcoholism at the National Institutes of Health supported this work (AA07850, AA017273, AA019681) as well as a Senior Research Career Scientist Award from the Department of Veterans Affairs.
Footnotes
Results do not change if all drinking episodes remain in the analysis, regardless of when begin-drink reports were initiated.
Including these two participants in analyses of the effects of topiramate on alcohol use did not change the results.
Authors Contribution
All authors were responsible for the study concept and design. RM, JM, and AB were responsible for the acquisition of study data. TC and RS conducted medical exams on study applicants and provided medical coverage for this study. RM provided clinical coverage during alcohol cue reactivity assessments. RM, HT, and AB were responsible for data analysis. All authors contributed to the interpretation of findings. RM, JM, and HT drafted the manuscript and all authors critically reviewed content and approved the final version for publication.
Disclosure/Conflict of Interest
RS is a consultant for D&A Pharma, CT San Remo and Transcept Pharmaceuticals. Otherwise, the authors have no conflicts to disclose.
References
- Baltieri DA, Daró FR, Ribeiro PL, de Andrade AG. Comparing topiramate with naltrexone in the treatment of alcohol dependence. Addiction. 2008;103:2035–2044. doi: 10.1111/j.1360-0443.2008.02355.x. [DOI] [PubMed] [Google Scholar]
- Carney MA, Tennen H, Affleck G, Del Boca FK, Kranzler HR. Levels and patterns of alcohol consumption using timeline follow-back, daily diaries and real-time “electronic interviews”. J Stud Alcohol. 1998;59:447–454. doi: 10.15288/jsa.1998.59.447. [DOI] [PubMed] [Google Scholar]
- Corrao G, Bagnardi V, Zambon A, La Vecchia C. A meta-analysis of alcohol consumption and the risk of 15 diseases. Prev Med. 2004;38:613–619. doi: 10.1016/j.ypmed.2003.11.027. [DOI] [PubMed] [Google Scholar]
- Davidson D, Camara P, Swift R. Behavioral effects and pharmacokinetics of low dose intravenous alcohol in humans. Alcohol Clin Exp Res. 1997;21:1294–1299. [PubMed] [Google Scholar]
- Del Boca FK, Kranzler HR, Brown J, Korner PF. Assessment of medication compliance in alcoholics through UV light detection of a riboflavin tracer. Alcohol Clin Exp Res. 1996;20:1412–1417. doi: 10.1111/j.1530-0277.1996.tb01142.x. [DOI] [PubMed] [Google Scholar]
- Engeli S, Jordan J. Novel metabolic drugs and blood pressure: implications for the treatment of obese hypertensive patients. Curr Hypertens Rep. 2013;15:470–474. doi: 10.1007/s11906-013-0374-z. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. User’s guide for the Structured Clinical Interview for DSM-IV Axis I disorders (SCID-I), Clinician Version, User’s Guide. New York State Psychiatric Institute, Biometrics Research Department; New York: 1997. [Google Scholar]
- Gibbons RD, Hedeker D, DuToit S. Advances in analysis of longitudinal data. Annu Rev Clin Psychol. 2010;6:79–107. doi: 10.1146/annurev.clinpsy.032408.153550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilig M, Egli M. Pharmacological treatment of alcohol dependence: target symptoms and target mechanisms. Pharmacol Ther. 2006;111:855–876. doi: 10.1016/j.pharmthera.2006.02.001. [DOI] [PubMed] [Google Scholar]
- Hustad JT, Carey KB. Using calculations to estimate blood alcohol concentrations for naturally occurring drinking episodes: a validity study. J Stud Alcohol. 2005;66:130–138. doi: 10.15288/jsa.2005.66.130. [DOI] [PubMed] [Google Scholar]
- Johnson BA, Ait-Daoud N, Bowden CL, DiClemente CC, Roache JD, Lawson K, Javors MA, Ma JZ. Oral topiramate for treatment of alcohol dependence: a randomised controlled trial. Lancet. 2003;361:1677–1685. doi: 10.1016/S0140-6736(03)13370-3. [DOI] [PubMed] [Google Scholar]
- Johnson BA, Ait-Daoud N, Roache JD. The COMBINE SAFTEE: a structured instrument for collecting adverse events adapted for clinical studies in the alcoholism field. J Stud Alcohol Suppl. 2005;15:157–167. doi: 10.15288/jsas.2005.s15.157. [DOI] [PubMed] [Google Scholar]
- Johnson BA, Rosenthal N, Capece JA, Wiegand F, Mao L, Beyers K, McKay A, Ait-Daoud N, Anton RF, Ciraulo DA, Kranzler HR, Mann K, O’Malley SS, Swift RM. Topiramate for treating alcohol dependence: a randomized controlled trial. JAMA. 2007;289:1641–1651. doi: 10.1001/jama.298.14.1641. [DOI] [PubMed] [Google Scholar]
- Kanny D, Liu Y, Brewer RD, Lu H. Binge drinking – United States, 2011. Morbidity and Mortality Weekly Report, Center for Disease Control and Prevention. 2013;62(Suppl):77–80. [Google Scholar]
- Kranzler HR, Armeli S, Feinn R, Tennen H, Gelernter J, Covault J. GRIK1 genotype moderates topiramate’s effects on daily drinking level, expectations of alcohol’s positive effects and desire to drink. Int J Neuropsychoph. 2014b doi: 10.1017/S1461145714000510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kranzler HR, Covault J, Feinn R, Armeli S, Tennen H, Arias AJ, Gelernter J, Pond T, Oncken C, Kampman KM. Topiramate treatment for heavy drinkers: moderation by a GRIK1 polymorphism. Am J Psychiatry. 2014a;171:445–452. doi: 10.1176/appi.ajp.2013.13081014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine J, Schooler N. SAFTEE: a technique for the systematic assessment of side effects in clinical trials. Psychopharmacol Bull. 1986;22:343–381. [PubMed] [Google Scholar]
- Likhitsathian S, Uttawichai K, Booncharoen H, Wittayanookulluk A, Angkurawaranon C, Srisurapanont M. Topiramate treatment for alcoholic outpatients recently receiving residential treatment programs: a 12-week, randomized, placebo-controlled trial. Drug Alcohol Depend. 2013;133:440–446. doi: 10.1016/j.drugalcdep.2013.06.032. [DOI] [PubMed] [Google Scholar]
- Martin CS, Earleywine M, Musty RE, Perrine MW, Swift RM. Development and validation of the Biphasic Alcohol Effects Scale. Alcohol Clin Exp Res. 1993;17:140–146. doi: 10.1111/j.1530-0277.1993.tb00739.x. [DOI] [PubMed] [Google Scholar]
- Matthews DB, Miller WR. Estimating blood alcohol concentration: two computer programs and their applications in therapy and research. Addict Behav. 1979;4:55–60. doi: 10.1016/0306-4603(79)90021-2. [DOI] [PubMed] [Google Scholar]
- Miranda R, MacKillop M, Monti PM, Rohsenow DJ, Tidey J, Gwaltney C, Swift R, Ray L, McGeary J. Effects of topiramate on urge to drink and the subjective effects of alcohol: a preliminary laboratory study. Alcohol Clin Exp Res. 2008;32:489–497. doi: 10.1111/j.1530-0277.2007.00592.x. [DOI] [PubMed] [Google Scholar]
- Miranda R, Monti PM, Ray L, Treloar HR, Reynolds EK, Ramirez J, Chun T, Gwaltney CJ, Justus A, Tidey J, Blanchard A, Magill M. Characterizing subjective responses to alcohol among adolescent problem drinkers. J Abnorm Psychol. 2014;123:117–129. doi: 10.1037/a0035328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda R, Ray L, Blanchard A, Reynolds EK, Monti PM, Chun T, Justus A, Swift RM, Tidey J, Gwaltney CJ, Ramirez J. Effects of naltrexone on adolescent alcohol cue reactivity and sensitivity: an initial randomized trial. Addict Biol. 2013;13:1–14. doi: 10.1111/adb.12050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monti PM, Binkoff JA, Zwick WR, Abrams DB, Nirenberg TD, Liepman MR. Reactivity of alcoholics and nonalcoholics to drinking cues. J Abnorm Psychol. 1987;96:122–126. doi: 10.1037//0021-843x.96.2.122. [DOI] [PubMed] [Google Scholar]
- Muthén BO. [Accessed 26 July 2014];Bayesian analysis In Mplus: A brief introduction. 2010 Available at: http://www.statmodel.com/download/IntroBayesVersion%203.pdf.
- Muthén LK, Muthén BO. Mplus user’s guide. 7. Los Angeles, CA: Muthén & Muthén; 1998–2013. [Google Scholar]
- National Institute on Alcohol Abuse and Alcoholism. NIH Publication No. 07-3769. Bethesda, MD: Author; 2005. Helping patients who drink too much: a clinician’s guide. [Google Scholar]
- Paparrigopoulos T, Tzavellas E, Karaiskos D, Kourlaba G, Liappas I. Treatment of alcohol dependence with low-dose topiramate: an open-label controlled study. BMC Psychiatry. 2011;11:41. doi: 10.1186/1471-244X-11-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piasecki TM, Wood PK, Shiffman S, Sher KJ, Heath AC. Responses to alcohol and cigarette use during ecologically assessed drinking episodes. Psychopharmacology. 2012;223:331–344. doi: 10.1007/s00213-012-2721-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preacher KJ, Curran PJ, Bauer DJ. Computational tools for probing interaction effects in multiple linear regression, multilevel modeling, and latent curve analysis. J Educ Behav Stat. 2006;31:437–448. [Google Scholar]
- Preacher KJ, Zyphur MJ, Zhang Z. A general multilevel SEM framework for assessing multilevel mediation. Psychol Methods. 2010;15:209–233. doi: 10.1037/a0020141. [DOI] [PubMed] [Google Scholar]
- Ramirez J, Miranda R. Alcohol craving in adolescents: bridging the laboratory and natural environment. Psychopharmacology. 2014;231:1841–1851. doi: 10.1007/s00213-013-3372-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raudenbush SW, Bryk AS. Hierarchical linear modeling: applications and data analysis methods. 2. Thousand Oaks, CA: Sage Publications, Inc; 2002. [Google Scholar]
- Ray LA, Miranda R, Tidey J, McGeary JE, MacKillop J, Gwaltney C, Rohsenow DJ, Swift RM, Monti PM. Polymorphisms of the μ-opioid receptor and dopamine D4 receptor genes and subjective responses to alcohol in the natural environment. J Abnorm Psychol. 2010;119:115–125. doi: 10.1037/a0017550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubio G, Martinez-Gras I, Manzanares J. Modulation of impulsivity by topiramate: implications for the treatment of alcohol dependence. J Clin Psychopharmacol. 2009;29:584–589. doi: 10.1097/JCP.0b013e3181bfdb79. [DOI] [PubMed] [Google Scholar]
- Rubio G, Ponce G, Jiménez-Arriero MA, Palomo T, Manzanares J, Ferre F. Effects of topiramate in the treatment of alcohol dependence. Pharmacopsychiatry. 2004;37:37–40. doi: 10.1055/s-2004-815473. [DOI] [PubMed] [Google Scholar]
- Rueger SY, McNamara PJ, King AC. Expanding the utility of the biphasic alcohol effects scale (BAES) and initial psychometric support for the brief-BAES (B-BAES) Alcohol Clin Exp Res. 2009;33:916–924. doi: 10.1111/j.1530-0277.2009.00914.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer JD, Willett JB. Applied longitudinal data analysis: Modeling change and event occurrence. New York, NY: Oxford University Press; 2003. [Google Scholar]
- Smith GS, Branas CC, Miller TR. Fatal nontraffic injuries involving alcohol: a metaanalysis. Ann of Emer Med. 1999;33:659–668. [PubMed] [Google Scholar]
- Sobell LD, Sobell MD. Timeline follow-back: a technique for assessing self-reported alcohol consumption. In: Litten R, Allen K, editors. Measuring Alcohol Consumption. Human Press; Clifton, NJ: 1992. pp. 41–65. [Google Scholar]
- Sullivan JT, Sykora K, Schneiderman J, Naranjo CA, Sellers EM. Assessment of alcohol withdrawal: the revised clinical institute withdrawal assessment for alcohol scale (CIWA-Ar) Brit J Addict. 1989;84:1353–1357. doi: 10.1111/j.1360-0443.1989.tb00737.x. [DOI] [PubMed] [Google Scholar]
- Tidey JW, Monti PM, Rohsenow DJ, Gwaltney CJ, Miranda R, McGeary JE, MacKillop J, Swift RM, Abrams DB, Shiffman S, Paty JA. Moderators of naltrexone’s effects on drinking, urge, and alcohol effects in non-treatment seeking heavy drinkers in the natural environment. Alcohol Clin Exp Res. 2008;32:58–66. doi: 10.1111/j.1530-0277.2007.00545.x. [DOI] [PMC free article] [PubMed] [Google Scholar]