Abstract
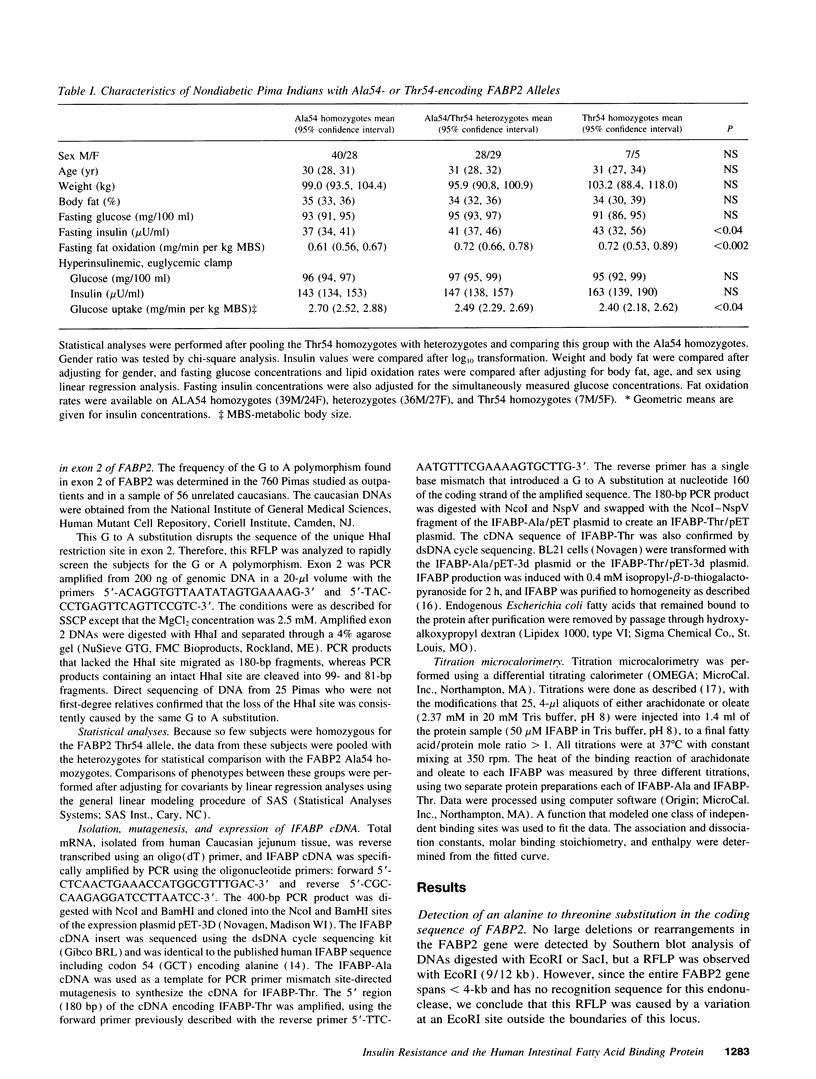
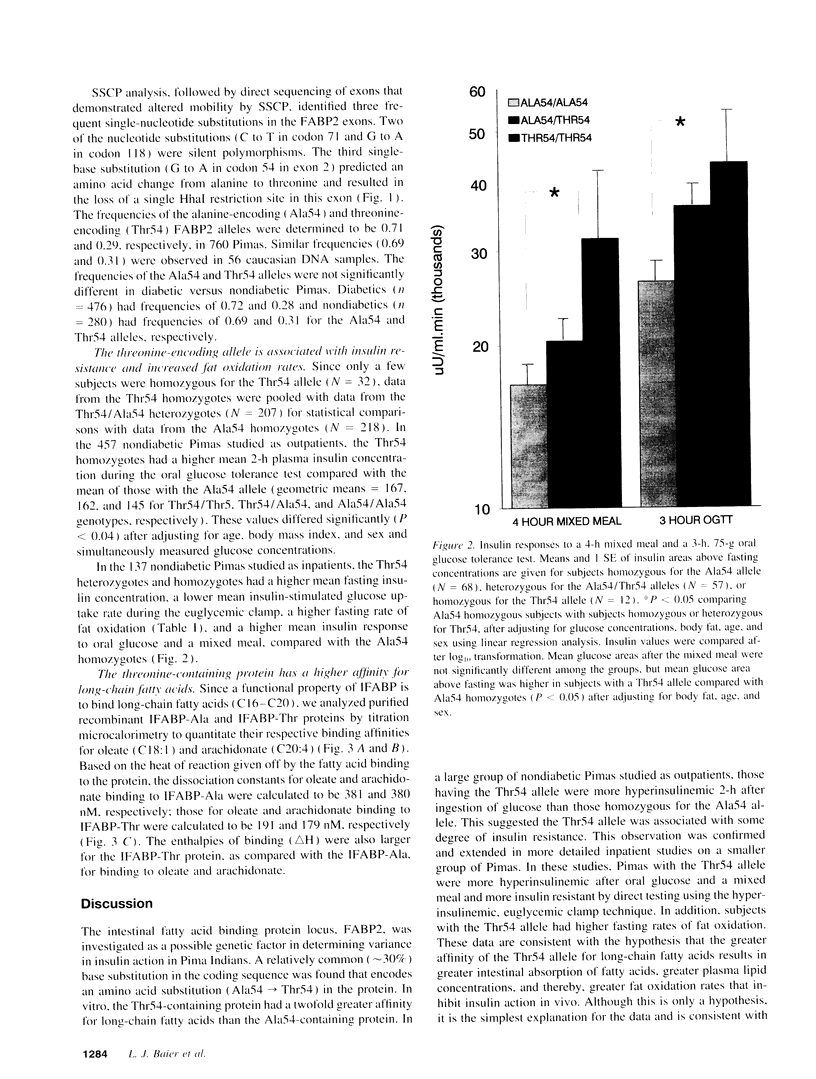
The intestinal fatty acid binding protein locus (FABP2) was investigated as a possible genetic factor in determining insulin action in the Pima Indian population. A polymorphism at codon 54 of FABP2 was identified that results in an alanine-encoding allele (frequency 0.71) and a threonine-encoding allele (frequency 0.29). Pimas who were homozygous or heterozygous for the threonine-encoding allele were found to have a higher mean fasting plasma insulin concentration, a lower mean insulin-stimulated glucose uptake rate, a higher mean insulin response to oral glucose and a mixed meal, and a higher mean fat oxidation rate compared with Pimas who were homozygous for the alanine-encoding allele. Since the FABP2 threonine-encoding allele was found to be associated with insulin resistance and increased fat oxidation in vivo, we further analyzed the FABP2 gene products for potential functional differences. Titration microcalorimetry studies with purified recombinant protein showed that the threonine-containing protein had a twofold greater affinity for long-chain fatty acids than the alanine-containing protein. We conclude that the threonine-containing protein may increase absorption and/or processing of dietary fatty acids by the intestine and thereby increase fat oxidation, which has been shown to reduce insulin action.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boden G., Jadali F., White J., Liang Y., Mozzoli M., Chen X., Coleman E., Smith C. Effects of fat on insulin-stimulated carbohydrate metabolism in normal men. J Clin Invest. 1991 Sep;88(3):960–966. doi: 10.1172/JCI115399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonadonna R. C., Zych K., Boni C., Ferrannini E., DeFronzo R. A. Time dependence of the interaction between lipid and glucose in humans. Am J Physiol. 1989 Jul;257(1 Pt 1):E49–E56. doi: 10.1152/ajpendo.1989.257.1.E49. [DOI] [PubMed] [Google Scholar]
- Cohn S. M., Simon T. C., Roth K. A., Birkenmeier E. H., Gordon J. I. Use of transgenic mice to map cis-acting elements in the intestinal fatty acid binding protein gene (Fabpi) that control its cell lineage-specific and regional patterns of expression along the duodenal-colonic and crypt-villus axes of the gut epithelium. J Cell Biol. 1992 Oct;119(1):27–44. doi: 10.1083/jcb.119.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felley C. P., Felley E. M., van Melle G. D., Frascarolo P., Jéquier E., Felber J. P. Impairment of glucose disposal by infusion of triglycerides in humans: role of glycemia. Am J Physiol. 1989 Jun;256(6 Pt 1):E747–E752. doi: 10.1152/ajpendo.1989.256.6.E747. [DOI] [PubMed] [Google Scholar]
- Felley C. P., Kleiber H., van Melle G. D., Frascarolo P., Jéquier E., Felber J. P. Resistance to insulin mediated glucose disposal in obese subjects: respective effect of lipid metabolism and glycemia. Int J Obes Relat Metab Disord. 1992 Mar;16(3):185–191. [PubMed] [Google Scholar]
- Humphreys P., McCarthy M., Tuomilehto J., Tuomilehto-Wolf E., Stratton I., Morgan R., Rees A., Owens D., Stengård J., Nissinen A. Chromosome 4q locus associated with insulin resistance in Pima Indians. Studies in three European NIDDM populations. Diabetes. 1994 Jun;43(6):800–804. doi: 10.2337/diab.43.6.800. [DOI] [PubMed] [Google Scholar]
- Jakoby M. G., Miller K. R., Toner J. J., Bauman A., Cheng L., Li E., Cistola D. P. Ligand-protein electrostatic interactions govern the specificity of retinol- and fatty acid-binding proteins. Biochemistry. 1993 Jan 26;32(3):872–878. doi: 10.1021/bi00054a019. [DOI] [PubMed] [Google Scholar]
- Kelley D. E., Mokan M., Simoneau J. A., Mandarino L. J. Interaction between glucose and free fatty acid metabolism in human skeletal muscle. J Clin Invest. 1993 Jul;92(1):91–98. doi: 10.1172/JCI116603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleiber H., Munger R., Jallut D., Tappy L., Felley C., Golay A., Frascarolo P., Jéquier E., Felber J. P. Interaction of lipid and carbohydrate metabolism after infusions of lipids or of lipid lowering agents: lack of a direct relationship between free fatty acid concentrations and glucose disposal. Diabete Metab. 1992 Mar-Apr;18(2):84–90. [PubMed] [Google Scholar]
- Knowler W. C., Pettitt D. J., Saad M. F., Bennett P. H. Diabetes mellitus in the Pima Indians: incidence, risk factors and pathogenesis. Diabetes Metab Rev. 1990 Feb;6(1):1–27. doi: 10.1002/dmr.5610060101. [DOI] [PubMed] [Google Scholar]
- Lillioja S., Mott D. M., Spraul M., Ferraro R., Foley J. E., Ravussin E., Knowler W. C., Bennett P. H., Bogardus C. Insulin resistance and insulin secretory dysfunction as precursors of non-insulin-dependent diabetes mellitus. Prospective studies of Pima Indians. N Engl J Med. 1993 Dec 30;329(27):1988–1992. doi: 10.1056/NEJM199312303292703. [DOI] [PubMed] [Google Scholar]
- Lillioja S., Mott D. M., Zawadzki J. K., Young A. A., Abbott W. G., Knowler W. C., Bennett P. H., Moll P., Bogardus C. In vivo insulin action is familial characteristic in nondiabetic Pima Indians. Diabetes. 1987 Nov;36(11):1329–1335. doi: 10.2337/diab.36.11.1329. [DOI] [PubMed] [Google Scholar]
- Lillioja S., Nyomba B. L., Saad M. F., Ferraro R., Castillo C., Bennett P. H., Bogardus C. Exaggerated early insulin release and insulin resistance in a diabetes-prone population: a metabolic comparison of Pima Indians and Caucasians. J Clin Endocrinol Metab. 1991 Oct;73(4):866–876. doi: 10.1210/jcem-73-4-866. [DOI] [PubMed] [Google Scholar]
- Lowe J. B., Sacchettini J. C., Laposata M., McQuillan J. J., Gordon J. I. Expression of rat intestinal fatty acid-binding protein in Escherichia coli. Purification and comparison of ligand binding characteristics with that of Escherichia coli-derived rat liver fatty acid-binding protein. J Biol Chem. 1987 Apr 25;262(12):5931–5937. [PubMed] [Google Scholar]
- Martin B. C., Warram J. H., Krolewski A. S., Bergman R. N., Soeldner J. S., Kahn C. R. Role of glucose and insulin resistance in development of type 2 diabetes mellitus: results of a 25-year follow-up study. Lancet. 1992 Oct 17;340(8825):925–929. doi: 10.1016/0140-6736(92)92814-v. [DOI] [PubMed] [Google Scholar]
- Martin B. C., Warram J. H., Rosner B., Rich S. S., Soeldner J. S., Krolewski A. S. Familial clustering of insulin sensitivity. Diabetes. 1992 Jul;41(7):850–854. doi: 10.2337/diab.41.7.850. [DOI] [PubMed] [Google Scholar]
- Popp-Snijders C., Schouten J. A., Heine R. J., van der Meer J., van der Veen E. A. Dietary supplementation of omega-3 polyunsaturated fatty acids improves insulin sensitivity in non-insulin-dependent diabetes. Diabetes Res. 1987 Mar;4(3):141–147. [PubMed] [Google Scholar]
- Prochazka M., Lillioja S., Tait J. F., Knowler W. C., Mott D. M., Spraul M., Bennett P. H., Bogardus C. Linkage of chromosomal markers on 4q with a putative gene determining maximal insulin action in Pima Indians. Diabetes. 1993 Apr;42(4):514–519. doi: 10.2337/diab.42.4.514. [DOI] [PubMed] [Google Scholar]
- RANDLE P. J., GARLAND P. B., HALES C. N., NEWSHOLME E. A. The glucose fatty-acid cycle. Its role in insulin sensitivity and the metabolic disturbances of diabetes mellitus. Lancet. 1963 Apr 13;1(7285):785–789. doi: 10.1016/s0140-6736(63)91500-9. [DOI] [PubMed] [Google Scholar]
- Sacchettini J. C., Banaszak L. J., Gordon J. I. Expression of rat intestinal fatty acid binding protein in E. coli and its subsequent structural analysis: a model system for studying the molecular details of fatty acid-protein interaction. 1990 Oct 15-Nov 8Mol Cell Biochem. 98(1-2):81–93. doi: 10.1007/BF00231371. [DOI] [PubMed] [Google Scholar]
- Sacchettini J. C., Gordon J. I. Rat intestinal fatty acid binding protein. A model system for analyzing the forces that can bind fatty acids to proteins. J Biol Chem. 1993 Sep 5;268(25):18399–18402. [PubMed] [Google Scholar]
- Schumacher M. C., Hasstedt S. J., Hunt S. C., Williams R. R., Elbein S. C. Major gene effect for insulin levels in familial NIDDM pedigrees. Diabetes. 1992 Apr;41(4):416–423. doi: 10.2337/diab.41.4.416. [DOI] [PubMed] [Google Scholar]
- Storlien L. H., Jenkins A. B., Chisholm D. J., Pascoe W. S., Khouri S., Kraegen E. W. Influence of dietary fat composition on development of insulin resistance in rats. Relationship to muscle triglyceride and omega-3 fatty acids in muscle phospholipid. Diabetes. 1991 Feb;40(2):280–289. doi: 10.2337/diab.40.2.280. [DOI] [PubMed] [Google Scholar]
- Sweetser D. A., Birkenmeier E. H., Klisak I. J., Zollman S., Sparkes R. S., Mohandas T., Lusis A. J., Gordon J. I. The human and rodent intestinal fatty acid binding protein genes. A comparative analysis of their structure, expression, and linkage relationships. J Biol Chem. 1987 Nov 25;262(33):16060–16071. [PubMed] [Google Scholar]




