Abstract
Objective
Autotaxin (ATX) is an adipocyte-derived lysophospholipase that generates the lipid signaling molecule lysophosphatidic acid (LPA). The aim of this study was to determine the relationship between serum ATX and non-alcoholic fatty liver disease (NAFLD) in obese females.
Design and Methods
102 obese, nondiabetic women (age: 31.5-55.8 years; BMI: 35.0-64.5kg/m2) were classified as having NAFLD (36.3%) or not having NAFLD (63.7%) based on the degree of hepatic steatosis on abdominal CT. Subjects were characterized for metabolic phenotype including measures of energy, glucose, and lipid homeostasis. Fasting serum adipokines and inflammatory markers were determined by ELISA. Linear regression analysis was used to determine features independently associated with NAFLD.
Results
Subjects with and without NAFLD differed in several key features of metabolic phenotype including BMI, waist circumference, fasting glucose and insulin, HOMA-IR, VLDL, triglycerides, and ALT. Serum adipokines, including ATX and leptin, were higher in subjects with NAFLD. Serum ATX was significantly correlated with alkaline phosphatase, fasting glucose, fasting insulin, and HOMA-IR. Linear regression analysis revealed that serum triglycerides and log-transformed ATX were independently associated with hepatic steatosis.
Conclusions
Serum ATX may be a potential pathogenic factor and/or biomarker for NAFLD in obese, nondiabetic women.
Keywords: Autotaxin, ENPP2, adipokines, insulin resistance, lysophosphatidic acid, nonalcoholic fatty liver disease, triglycerides
Introduction
Nonalcoholic fatty liver disease (NAFLD) is a leading cause of chronic liver disease, with 20-30% of adults affected worldwide. While the majority of patients with NAFLD experience a benign course characterized by macrovesicular steatosis, up to 30% develop nonalcoholic steatohepatitis (NASH), cirrhosis, or hepatocellular carcinoma (1). Although age, gender, diet, and genetic factors influence the pathogenesis of NAFLD, obesity and insulin resistance (IR) are the strongest risk factors (1, 2). Obesity is characterized by excess accumulation of adipose tissue, a key endocrine organ that secretes numerous bioactive factors (adipokines) that are critical for metabolic homeostasis and systemic inflammation. In animal models of NAFLD, adipokines such as leptin, adiponectin, and apelin influence hepatic lipid disposal and insulin sensitivity (1, 2, 3). Furthermore, nonparenchymal components of adipose tissue secrete cytokines that regulate inflammation and fibrogenesis in the steatotic liver (1, 2, 3). Human studies of NAFLD have corroborated these findings, since levels of circulating adipokines reflect histologic markers of disease severity (4).
Autotaxin (ATX) [ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (ENPP2)] is an adipokine with lysophospholipase D activity that hydrolyzes lysophosphatidylcholine (LPC) to lysophosphatidic acid (LPA) (5). LPA is a potent lipid mediator that acts through at least six known G-coupled protein receptors to influence numerous biological processes (5). Recently, the ATX-LPA pathway has been implicated in obesity and IR (6). In obese mice, both adipocyte-specific ATX deletion and pharmacologic inhibition improve glucose tolerance, whereas administration of LPA impairs glucose tolerance (7, 8). However, the in vivo metabolic effects and underlying mechanisms in murine models remain controversial (9). In obese humans, ATX mRNA expression in visceral adipose tissue is associated with impaired glucose tolerance (10). Furthermore, ATX-LPA signaling has been implicated in hepatic fibrogenesis, as LPA stimulates rat hepatic stellate cell proliferation and contractility (11). Finally, human studies of chronic hepatitis C reveal strong correlations between circulating ATX, LPA levels, and serum and histologic markers of hepatic fibrosis (11). Together these studies suggest that the ATX-LPA pathway may play a role in the pathogenesis of both obesity-related IR and hepatic injury. However, the relationship between serum ATX and obesity-associated metabolic and hepatic phenotypes remains unknown.
The goal of the present study was to determine the relationship between serum ATX and NAFLD in obese women. We hypothesized that serum ATX would be associated with NAFLD. To test this hypothesis, we assessed hepatic steatosis as well as other key metabolic parameters in severely obese, nondiabetic women. We then determined serum ATX and assessed its relationship with hepatic steatosis compared to other known adipokines. We found that serum ATX is higher in severely obese, nondiabetic women with NAFLD compared to those without NAFLD and is independently associated with hepatic steatosis in this population.
Methods
Study Design and Participation
The current analysis was performed in a subset of participants previously enrolled in a randomized control trial of weight-loss interventions for severe obesity (RENEW, clinicaltrials.gov Trial Registration Identifier: NCT00712127) (12). The study was approved by the Institutional Review Board (IRB) at the University of Pittsburgh, and all subjects provided written informed consent prior to participation. From February 2007 to March 2009, women between 30 and 55 years of age were enrolled in a randomized, single-blind, control trial designed to assess the effects of weight loss and physical activity on obesity-related health risks. Inclusion criteria included body mass index (BMI) ≥ 35 kg/m2, ability to walk without assistance, and ability to obtain medical clearance for dietary and physical activity interventions. Exclusion criteria included diagnosis of cancer within 5 years of enrollment, history of coronary artery disease, prior participation in a weight-loss program within one year of enrollment, previous bariatric surgery, and history of uncontrolled hypertension, diabetes mellitus, or pregnancy within 6 months of enrollment. Participants with liver enzyme levels greater than 30% above the upper limit of normal laboratory ranges were excluded.
Demographic and Clinical Evaluation
Participant race and ethanol use were self-reported. For the 12-month period prior to enrollment, all subjects were asked to quantify both the average frequency of drinking episodes and average number of drinks per episode. The Cut down/Annoyed/Guilty/Eye-Opener (CAGE) questionnaire was used to screen for ethanol dependence (Table S1). Subjects underwent measurements of height and weight to calculate BMI. Resting systolic (SBP) and diastolic blood pressures (DBP) were determined using an automated arm sphygmomanometer. Clinical laboratory parameters were determined in serum derived from 12-h fasted subjects using the University of Pittsburgh core laboratory and included the following: AST, ALT, alkaline phosphatase, creatinine, cholesterol (total, VLDL, LDL, HDL), triglycerides, glucose, and insulin. The homeostatic model assessment of insulin resistance (HOMA-IR) index was calculated as glucose (mg/dl) × insulin (mU/L)/405.
Determination of Metabolic Syndrome
The presence of metabolic syndrome (MetS) was determined using the 2004 National Cholesterol Education Program Adult Treatment Panel III guidelines (13). Components of MetS in women included waist circumference ≥ 88 cm, SBP ≥ 130 mm Hg or DSP ≥ 85 mm Hg or use of antihypertensive medications, fasting triglyceride level ≥ 150 mg/dl or on drug treatment for elevated triglycerides, HDL < 50 mg/dl or on drug treatment of low HDL, and fasting glucose ≥ 100 mg/dl. MetS is defined as the presence of three or more criteria.
Hepatic Fat Content Measurement
Hepatic steatosis was assessed using hepatic and splenic attenuation data from unenhanced abdominal CT scans as previously described (12). The liver:spleen attenuation ratio (L/S ratio) was calculated as the primary measure of hepatic fat content (14). Shores et al. (15) demonstrated that L/S ratio strongly correlates with both histologic degree of steatosis (r = −0.89, p < 0.0001) and hepatic triglyceride content (r = −0.80, p < 0.001) in severely obese patients. Histologic diagnosis of NAFLD requires at least 5% steatosis (1), and multiple studies have demonstrated that a CT-determined L/S ratio less than 1.1 is strongly predictive of moderate (at least 30%) macrovesicular steatosis by liver biopsy (14, 16). Therefore, NAFLD was defined as an L/S ratio of less than 1.1.
Serum adipokine measurement
The current study was performed using previously collected specimens from the RENEW trial. Serum was isolated by centrifugation, aliquoted, and immediately stored at −80° C for future use. Serum levels of IL-6, CRP, and leptin were previously measured as part of the RENEW trial (12), while serum ATX and adiponectin levels were assayed by ELISA (R&D Systems). ATX protein strongly correlates with ATX activity (17).
Statistical Analysis
Statistical analysis was performed with Stata version 11 (StataCorp), and GraphPad Prism version 6.0 (GraphPad Software). Clinical and demographic characteristics were reported with absolute frequencies, percentages, medians, and interquartile ranges (IQR). Comparisons between groups were performed with Fisher's exact test for categorical variables or the Mann Whitney U test for continuous variables. Outliers were identified as serum ATX greater than 2 quartiles above the median, resulting in exclusion of one participant. Adipokines were assessed for normal distribution using the Shapiro-Wilk test, and, as all adipokines failed normality testing, log-transformation was applied. Among the log-transformed adipokines, only log-transformed ATX exhibited normal distribution, and hence log-normalized ATX levels were compared between groups by Welch's t-tests. All other adipokine levels were compared using the Mann-Whitney U test. Correlation between variables was determined using partial Spearman's rank correlation coefficient analysis with adjustment for race. Linear regression was used to determine predictors of L/S ratio, and patient characteristics with p < 0.2 in univariate models were included with age in the multivariate analysis. To create the most parsimonious model, a backward stepwise elimination algorithm was used to select regressors for the multivariable model. Variance inflation factors were checked in the final model to ensure no collinearity between explanatory variables, and both graphical methods as well as Cameron-Trivedi tests were performed to ensure homoskedacity of the multivariate model.
Results
Clinical and Demographic Features of Participants
Clinical and demographic characteristics are summarized in Table 1. Among 101 severely obese women, 37 (36.6%) exhibited CT evidence of NAFLD by L/S ratio of less than 1.1. Self-reported patterns of alcohol use were similar among subjects with and without NAFLD, and no study participants exhibited alcohol dependence by CAGE questionnaire (Table S1). Furthermore, although serum ALT was higher in subjects with NAFLD, the ALT as well as other liver function tests (AST, alkaline phosphatase) were within the normal range for all subjects. There were no differences in ethnic distribution, antihypertensive medication use, lipid-lowering medication use, or tobacco use between subjects with and without NAFLD. The median BMI of the study population was 42.9 (IQR 40.1-46.1). Subjects with NAFLD had higher measures of weight/adiposity including BMI and waist circumference. Blood pressure and serum cholesterol (total, HDL, and LDL) did not differ between groups. However, both serum triglycerides as well as measures of glucose homeostasis (fasting glucose, insulin, and HOMA-IR) were higher in participants with NAFLD. Overall, 59% of the study population had MetS, but there were no differences in either the prevalence of MetS or the number of MetS features between subjects with and without NAFLD. These findings demonstrate that among nondiabetic women with severe obesity, IR and hypertriglyceridemia are cardinal features of NAFLD.
Table 1.
Baseline characteristics of patients stratified by liver:spleen attenuation ratio of 1.1 on abdominal CT. All values are expressed as medians and IQRs unless otherwise specified. Continuous variables were compared with Mann-Whitney U test. Categorical variables were compared with Fisher's exact test. Significant differences between groups (p<0.05) are highlighted in bold. ALT, alanine transaminase; AST, aspartate transaminase; HDL, high-density lipoprotein; HOMA-IR, homeostatic model assessment insulin resistance; LDL, low-density lipoprotein; VLDL, very low-density lipoprotein; WHO, World Health Organization. Clinical and Demographic Characteristics of Participants
| Characteristics | Total (N=101) | NAFLD (N=37) | No NAFLD (N=64) | P Value |
|---|---|---|---|---|
| Age | 47.5 (41.4-52.1) | 46.8 (39.8-51.0) | 47.7 (41.7-53.2) | 0.390 |
| African American, n (%) | 37 (36.6%) | 11 (29.8%) | 26 (40.6%) | 0.289 |
| Tobacco use, n (%) | 11 (10.9%) | 4 (10.8%) | 7 (10.9%) | 1.000 |
| Antihypertensive medication use, n (%) | 35 (34.6%) | 15 (40.5%) | 20 (31.3%) | 0.387 |
| Lipid-lowering medication use, n (%) | 9 (8.9%) | 1 (2.7%) | 8 (12.5%) | 0.150 |
| Weight, kg | 113.2 (103.8-126.3) | 123.0 (108.4-132.7) | 111.3 (101.3-121.0) | 0.004 |
| Body Mass Index, kg/m2 | 42.9 (40.0-46.1) | 44.5 (41.8-49.0) | 41.7 (38.3-44.1) | <0.001 |
| WHO Obesity Class II | 25 (24.8%) | 4 (10.8%) | 21 (32.8%) | 0.017 |
| Waist circumference, cm | 120.2 (113.1-128.6) | 127.0 (115.2-133.7) | 117.6 (112.1-126.4) | 0.002 |
| Systolic blood pressure, mm Hg | 136 (127-146) | 136 (129-144) | 136 (126-148) | 0.910 |
| Diastolic blood pressure, mm Hg | 78 (72-83) | 77 (73-81) | 80 (70-83) | 0.716 |
| Glucose, mg/dl | 94.0 (83.5-100.0) | 95.0 (88.3-103.0) | 90.0 (82.0-96.0) | 0.006 |
| Insulin, mU/L | 14.78 (9.01-19.63) | 17.41 (11.56-21.36) | 13.28 (8.89-18.78) | 0.016 |
| HOMA-IR Index | 3.30 (2.07-4.75) | 4.07 (2.64-5.23) | 2.73 (1.81-4.11) | 0.004 |
| Total cholesterol, mg/dl | 192.0 (166.5-207.5) | 187 (168-211) | 193 (165-207) | 0.902 |
| HDL, mg/dl | 48 (41-56) | 46 (41-52.5) | 50 (41-58) | 0.112 |
| LDL, mg/dl | 121.0 (105.5-137.0) | 121 (108-136) | 125 (104-137) | 0.947 |
| VLDL, mg/dl | 17 (14-24) | 19 (15-25) | 16 (13-22) | 0.023 |
| Triglycerides, mg/dl | 103 (86.0-141.5) | 118 (92-155) | 97 (79-131) | 0.015 |
| Number of metabolic syndrome features | 3 (2-3) | 3 (2-4) | 3 (2-3) | 0.111 |
| Metabolic syndrome, n (%) | 59 (58.4%) | 26 (70.3%) | 33 (51.6%) | 0.093 |
| AST, U/L | 24 (21-27) | 24 (21-28) | 24 (21-27) | 0.541 |
| ALT, U/L | 28 (24-35) | 33 (26-37) | 26 (22-33) | 0.008 |
| Alkaline phosphatase, U/L | 85 (74-98) | 87 (77-103) | 83 (72-97) | 0.231 |
| Creatinine, mg/dl | 0.8 (0.7-0.9) | 0.8 (0.7-0.9) | 0.8 (0.7-0.9) | 0.128 |
Serum ATX is higher in NAFLD and is positively correlated with IR in severely obese women
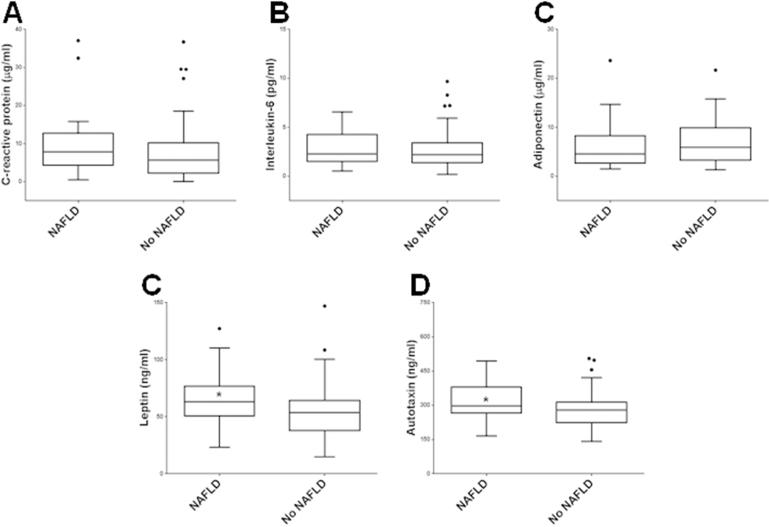
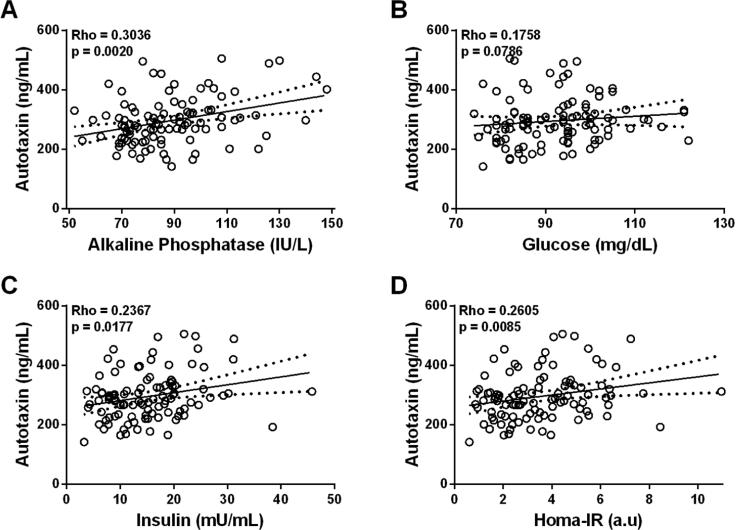
Since adipokines modulate hepatic metabolism in animal and human studies of NAFLD (2, 3), we measured serum ATX and other key adipokines (Figure 1, Table S2). Interestingly, serum adipokines traditionally associated with more advanced NAFLD, including elevated CRP (18, 19) and IL-6 (20, 21) and decreased adiponectin (4, 21), were not observed in subjects with NAFLD compared to those without NAFLD, reflecting early disease in this study cohort. Only serum leptin [63.00 ng/ml (IQR, 50.55-76.80) vs. 53.60 ng/ml (37.95-63.58), p = 0.019)] and ATX [298.04 ng/ml (266.72-379.46) vs. 279.04 ng/ml (223.70-317.96), p = 0.022] were significantly higher in subjects with NAFLD, indicating that ATX may provide additional sensitivity for early identification of hepatic steatosis in severely obese women. Next, we determined race-adjusted partial correlations between serum ATX and patient characteristics (Figure 2). Only alkaline phosphatase and markers of IR (fasting blood glucose, fasting serum insulin, and HOMA-IR) exhibited significant, positive correlations with serum ATX. On the other hand, neither weight nor BMI were correlated with serum ATX concentrations, suggesting that serum ATX levels are primarily related to IR in severely obese women. Finally, medication use did not influence serum ATX, as ATX levels were unchanged regardless of antihypertensive medication use [287.38 ng/ml (222.88-353.69) with use vs. 279.04 ng/ml (227.34-305.94) without, p = 0.251] or lipid-lowering agent use [357.14 ng/ml (230.02-297.54) with use vs. 280.31 ng/ml (222.88-313.57.91) without, p = 0.743].
Figure 1.
Tukey box whisker plots of circulating concentrations of C-reactive protein, interleukin-6, adiponectin, leptin, and ATX in subjects with and without NAFLD. The Tukey method of box whisker plot was used to generate the box whisker plot: the difference between the 25th and 75th percentiles was defined as the interquartile range (IQR). The upper and lower limits of the whiskers were defined using the 75th percentile plus 1.5IQR and 25th percentile plus 1.5IQR, respectively, in comparison with the largest and smallest data set values. *, p < 0.05.
Figure 2.
Race-adjusted Spearman partial correlations of serum ATX with alkaline phosphatase, glucose, insulin, and HOMA-IR.
Liver:spleen ratio is correlated with serum ATX and multiple features of MetS
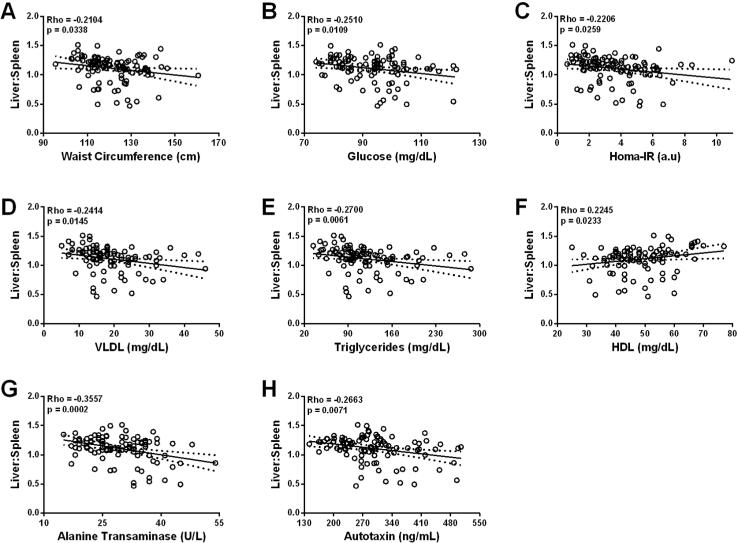
Since L/S ratio is a continuous estimation of hepatic steatosis, we used race-adjusted partial Spearman correlation analysis to identify relationships between L/S ratio and subject characteristcs. As expected, multiple features of MetS were significantly related to L/S ratio (Figure 3): waist ciracumference, glucose, HOMA-IR, VLDL, and triglycerides were negatively correlated with L/S ratio, whereas HDL was positively correlated with L/S ratio. Serum ALT was also negatively correlated with L/S ratio, consistent with early liver dysfunction with hepatic steatosis. Interestingly, serum ATX demonstrated a similar relationship and was the only measured adipokine significantly correlated with L/S ratio, thus highlighting a potential association between ATX and severity of steatosis.
Figure 3.
Race-adjusted Spearman partial correlations of L/S ratio with waist circumference, glucose, HOMA-IR, VLDL, triglycerides, HDL, ALT, and serum ATX.
Serum ATX and triglycerides are independently associated with hepatic steatosis in severely obese women
To identify characteristics associated with hepatic steatosis, we used linear regression analysis. Regression variables with p < 0.2 in univariate analyses (Table 2) were included with demographic features in a backward stepwise elimination algorithm to generate the final multivariable model (Table 3). While BMI and blood pressure were not associated with L/S ratio in the univariate analysis (at p<0.05), other markers of the metabolic syndrome, including waist circumference, HOMA-IR, serum triglycerides, and serum HDL exhibited significant univariate relationships with hepatic steatosis. Among serologic biomarkers, ATX and log-transformed ATX were associated with hepatic steatosis in univariate models. Multivariable regression analysis revealed that log-transformed ATX and serum triglycerides were independently associated with hepatic steatosis.
Table 2.
Univariate linear regression analysis of features associated with hepatic steatosis as measured by L/S ratio. HOMA-IR, homeostatic model assessment insulin resistance; HDL, high-density lipoprotein; LDL, low-density lipoprotein; VLDL, very low-density lipoprotein. Features associated with L/S ratio at p<0.05 are highlighted in bold. Features with p<0.2 were included in the multivariable analysis. Nonstandardized regression coefficients (B) are reported.
| Characteristics | Univariate B (95% CI) | P Value |
|---|---|---|
| Age | 0.003 (−0.003 – 0.010) | 0.313 |
| African American ethnicity | −0.078 (−0.166 – 0.011) | 0.085 |
| Smoking | −0.012 (−0.152 – 0.127) | 0.864 |
| Weight | −0.002 (−0.005 – 0.001) | 0.205 |
| Body mass index | −0.006 (−0.014 – 0.002) | 0.157 |
| Waist circumference | −0.004 (−0.008 – −0.001) | 0.034 |
| Systolic blood pressure | −0.001 (−0.004 – 0.002) | 0.420 |
| Diastolic blood pressure | −0.003 (−0.008 – 0.002) | 0.265 |
| HOMA-IR | −0.026 (−0.048 – 0.003) | 0.026 |
| HDL | 0.004 (0.001 – 0.008) | 0.023 |
| LDL | −0.001 (−0.002 – 0.001) | 0.652 |
| VLDL | −0.005 (−0.009 – −0.001) | 0.014 |
| Triglycerides | −0.001 (−0.002 – −0.001) | 0.006 |
| Creatinine | 0.271 (−0.035 – 0.579) | 0.082 |
| C-reactive protein | −0.003 (−0.008 – 0.003) | 0.368 |
| Interleukin-6 | 0.008 (−0.015 – −0.031) | 0.500 |
| Leptin | −0.001 (−0.003 – 0.001) | 0.459 |
| Adiponectin | 0.007 (−0.003 – 0.017) | 0.153 |
| Autotaxin | −0.001 (−0.001 – −0.001) | 0.003 |
| Log Autotaxin | −0.234 (−0.389 – −0.079) | 0.004 |
Table 3.
Multivariable linear regression analysis of features associated with hepatic steatosis. The model was determined using automated backward stepwise elimination. Nonstandardized regression coefficients (B) are reported. Multivariable linear regression model of features associated with hepatic steatosis
| Characteristics | Multivariate B (95% CI) | P Value |
|---|---|---|
| Race | −0.060 (−0.143 – 0.024) | 0.161 |
| Waist circumference | −0.003 (−0.007 – 0.0003) | 0.079 |
| Triglycerides | −0.001 (−0.001 – −0.0001) | 0.018 |
| Log Autotaxin | −0.173 (−0.327 – −0.018) | 0.029 |
Although BMI was not significantly associated with L/S ratio in univariate analysis, we re-analyzed the final multivariable model with BMI to determine its effect on the other model regressors; the conclusions of the model were qualitatively unchanged after inclusion of BMI (Table S3).
Discussion
ATX is an adipocyte-secreted lysophospholipase D recently implicated in the pathogenesis of obesity and/or IR in rodents and humans (6). Since both obesity and IR are strong risk factors for NAFLD, we measured serum ATX along with other adipokines as well as hepatic, metabolic, and anthropometric characteristics to determine the relationship between ATX and hepatic steatosis in a cohort of severely obese, nondiabetic women. Four important observations were noted. First, severely obese women with CT evidence of moderate hepatic steatosis exhibited a metabolic phenotype characterized by greater IR and hypertriglyceridemia. Second, among measured adipokines, serum ATX and leptin were higher in subjects with NAFLD. Next, markers of IR (fasting glucose, insulin, and HOMA-IR) exhibited significant, positive correlations with serum ATX levels. Finally, linear regression analysis revealed that log-transformed serum ATX and serum triglycerides were independently associated with the NAFLD in severely obese women.
Even among severely obese women without overt diabetes, NAFLD was present in 36.6% of participants. While significantly lower than the 50-90% reported prevalence in previous studies of severe obesity (22, 23, 24, 25, 26), this figure is concerning, since NAFLD is associated with greater liver-related and cardiovascular morbidity and mortality (27, 28). We suspect disparities in reported prevalence are due to differences in cohorts, since previous NAFLD prevalence studies derived data from bariatric surgery patients who typically exhibit more advanced metabolic derangements. In the current study, although the prevalence of metabolic syndrome and the number of metabolic syndrome features were similar between groups, individuals with NAFLD were more insulin resistant (by HOMA-IR) than those without NAFLD.
Given the close association between greater adiposity, adipocyte dysfunction, and NAFLD, the relationships between several serum adipokines and hepatic steatosis have been evaluated (25, 29, 30, 31). In the present study, serum ATX and leptin were higher in individuals with CT evidence of hepatic steatosis, whereas liver function tests and other adipokines traditionally associated with NAFLD (2, 3) did not differ between groups. Although alterations in these factors are described in NAFLD, serum concentrations were most strongly associated with steatohepatitis, fibrosis, and cirrhosis (19, 29, 32). Therefore, while CT assessment cannot definitively diagnose NAFLD severity (33), the absence of significant changes in serum IL-6, CRP, and adiponectin likely indicate milder disease (simple steatosis) in this cohort. Together, this adipokine pattern suggests that serum ATX may provide enhanced discrimination of early NAFLD in severely obese women.
Because serum ATX was higher in subjects with NAFLD, we examined correlations between serum ATX and markers of metabolic dysfunction to identify factors influencing ATX elevation in this cohort. Serum ATX levels were positively correlated with alkaline phosphatase, fasting blood glucose, fasting serum insulin, and HOMA-IR. Interestingly, although ATX is produced and secreted by adipocytes (34, 35), serum ATX was not correlated with markers of adiposity including weight, BMI, or waist circumference. The lack of a relationship with the latter is notable because prior work has demonstrated higher ATX mRNA expression in visceral fat of obese human subjects (10). One possible explanation is inclusion of only women with extreme obesity in the present study. Boucher, et al., likewise failed to demonstrate a correlation between adipose tissue ATX mRNA expression and BMI in a population of similarly obese women (36), whereas a recent study of a more diverse population revealed a negative correlation between BMI and both serum ATX and subcutaneous adipose tissue ATX mRNA expression (9). In contrast, serum ATX levels were positively correlated with multiple measures of IR in the present study, suggesting that circulating ATX has a greater relationship with obesity-related derangements in glucose homeostasis and/or insulin action rather than adiposity per se.
In addition to the above, serum ATX may be influenced by hepatic clearance, since serum ATX was also positively correlated with alkaline phosphatase in this cohort. While the precise fate of circulating ATX remains unclear, both human and animal studies suggest that ATX is metabolized by the liver (11). In a murine study of ATX catabolism, hepatic sinusoidal endothelial cells were shown to rapidly bind and eliminate circulating ATX (37). In rat models of chronic liver injury, serum ATX and LPA concentrations were higher despite unchanged hepatic ATX mRNA expression (38). In addition, rodents undergoing 70% partial hepatectomy exhibited a rapid rise in circulating ATX and LPA, suggesting decreased hepatic clearance (38). These observations are further corroborated by human studies of liver disease, where both serum LPA and ATX increased with advanced histologic stage in patients with chronic hepatitis C (17). As subjects in the current study did not exhibit overt clinical evidence of liver injury, observed serum ATX elevations in patients with NAFLD may highlight the role of ATX as an early marker of subclinical hepatic dysfunction.
An important question to consider is whether serum ATX might contribute mechanistically to the pathogenesis of NAFLD. The ATX-LPA system influences adipocyte biology and whole body lipid partitioning (6). Specifically, in cultured adipocytes, manipulation of LPA or its receptor (LPAR1) inhibits adipocyte differentiation, in part via down-regulation of PPARγ2 (39). Conversely, in rodent models, adipocyte-specific disruption of ATX promotes adipose tissue expansion / diet-induced obesity but improves glucose tolerance (7). Although a recent study suggests a more complex role of ATX in adipocyte biology and obesity (9), these findings nonetheless suggest a potential model in which adipocyte-secreted ATX increases LPA, which in turn negatively regulates adipocyte differentiation and energy storage capacity, thereby impairing glucose tolerance and promoting ectopic lipid accumulation. These phenomena may contribute to hepatic steatosis. Indeed, results from our multivariate linear regression analysis support this hypothesis, since we identified log-transformed ATX and serum triglycerides as independent regressors for hepatic steatosis. A second multivariable analysis including BMI did not change the conclusions of the model, indicating that the findings were independent of obesity status. Hypertriglyceridemia has been previously reported to increase the risk for developing NAFLD (1). The greater serum triglyceride pool may reflect enhanced hepatic de novo lipogenesis and hepatic VLDL secretion occurring in response to increased hepatic fatty acid influx often associated with IR. (31, 40). Thus, the association of serum ATX with hepatic steatosis may be due to ATX-mediated adipocyte dysfunction and impaired glucose homeostasis, but further studies are required to confirm this.
A few limitations of our study are noted. First, because this was a retrospective analysis of prospectively collected data, causal relationships could not be ascertained. Second, the study population was limited to severely obese women (obesity class II and III), thereby limiting the generalizability of our findings. Third, our characterization of NAFLD was limited to CT L/S ratio and liver function tests. Greater discrimination of the relationship between NAFLD and serum ATX could be determined with a wider spectrum and more precise histological confirmation of NALFD severity. Finally, although ATX is elevated in patients with chronic hepatitis C (17), serologic testing for viral hepatitis was not performed. However, the probability of viral hepatitis in this population is low since participants with liver enzyme levels 30% or more above the upper limit of normal range were excluded from the study. Despite these limitations, examining of this high-risk cohort with relatively mild metabolic dysfunction identified ATX as a marker of early NAFLD.
In summary, this study demonstrates for the first time that serum ATX is significantly higher with early or mild NAFLD and is independently associated with hepatic steatosis in severely obese, nondiabetic females. These findings suggest that serum ATX may be a potential pathogenic factor and/or useful biomarker for NAFLD in this population. Future studies are required to further define the relationship between serum ATX, LPA signaling and hepatic steatosis as well as to determine potential mechanistic links between the ATX-LPA system and both hepatic and metabolic disease.
Supplementary Material
Key points.
What is already known about this subject:
Autotaxin (ATX) [ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (ENPP2)] is an adipocyte secreted protein responsible for generating the lipid signaling molecule lysophosphatidic acid (LPA).
The ATX-LPA pathway has recently been implicated in adipocyte biology as well as glucose homeostasis / insulin action in cell and animal models.
The relationship between serum ATX and obesity-associated metabolic complications such as non-alcoholic fatty liver disease (NAFLD) in humans remains poorly understood.
What this study adds:
In severely obese, nondiabetic women, serum ATX and leptin are higher in subjects with NAFLD compared to those without NAFLD.
In severely obese, nondiabetic women, serum ATX positively correlates with markers of insulin resistance (fasting glucose, insulin, and HOMA-IR).
In severely obese, nondiabetic women, log-transformed serum ATX and serum triglycerides are independently associated with hepatic steatosis.
Acknowledgements
We acknowledge: Bret Goodpaster, John Jakicic and the members of the Department of Health and Physical Activity, Physical Activity and Weight Management Research Center for their contributions to the original RENEW study.
Funding Agencies: NIH R01 DK090166, Howard Hughes Medical Institute Physician-Scientist Early Career Award (EEK); NIH T32 DK007052 (VLR); NIH T32 DK063922 (VPR); NIH T35 DK065521 (JST). The Pennsylvania State Department of Health funded the original RENEW study.
Abbreviations
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- ATX
autotaxin
- BMI
body mass index
- CRP
C-reactive protein
- HDL
high-density lipoprotein
- HOMA-IR
homeostatic model assessment-insulin resistance
- IL-6
interleukin-6
- IR
insulin resistance
- LDL
low-density lipoprotein
- LPA
lysophosphatidic acid
- L/S ratio
liver:spleen attenuation ratio
- MetS
metabolic syndrome
- NAFLD
nonalcoholic fatty liver disease
- VLDL
very low-density lipoprotein
Footnotes
Competing interests: None.
Author contributions: VPR, VLR, and EEK were project leaders and contributed to all aspects of this work. JA, RCW, and JST contributed to data assembly and analysis. JPD was a member of the original RENEW study, provided data/samples, and performed experiments. PCK contributed intellectual and practical ATX expertise. All authors contributed intellectually to this work/manuscript. EEK is the guarantor of this work and takes responsibility for the integrity of the data and data analysis.
References
- 1.Farrell GC, Larter CZ. Nonalcoholic fatty liver disease: from steatosis to cirrhosis. Hepatology (Baltimore, Md) 2006;43:S99–S112. doi: 10.1002/hep.20973. [DOI] [PubMed] [Google Scholar]
- 2.Bugianesi E, McCullough AJ, Marchesini G. Insulin resistance: a metabolic pathway to chronic liver disease. Hepatology (Baltimore, Md) 2005;42:987–1000. doi: 10.1002/hep.20920. [DOI] [PubMed] [Google Scholar]
- 3.Marra F, Bertolani C. Adipokines in liver diseases. Hepatology (Baltimore, Md) 2009;50:957–969. doi: 10.1002/hep.23046. [DOI] [PubMed] [Google Scholar]
- 4.Lemoine M, Ratziu V, Kim M, Maachi M, Wendum D, Paye F, et al. Serum adipokine levels predictive of liver injury in non-alcoholic fatty liver disease. Liver international : official journal of the International Association for the Study of the Liver. 2009;29:1431–1438. doi: 10.1111/j.1478-3231.2009.02022.x. [DOI] [PubMed] [Google Scholar]
- 5.Nakanaga K, Hama K, Aoki J. Autotaxin--an LPA producing enzyme with diverse functions. Journal of biochemistry. 2010;148:13–24. doi: 10.1093/jb/mvq052. [DOI] [PubMed] [Google Scholar]
- 6.Rancoule C, Dusaulcy R, Treguer K, Gres S, Attane C, Saulnier-Blache JS. Involvement of autotaxin/lysophosphatidic acid signaling in obesity and impaired glucose homeostasis. Biochimie. 2014;96:140–143. doi: 10.1016/j.biochi.2013.04.010. [DOI] [PubMed] [Google Scholar]
- 7.Dusaulcy R, Rancoule C, Gres S, Wanecq E, Colom A, Guigne C, et al. Adipose-specific disruption of autotaxin enhances nutritional fattening and reduces plasma lysophosphatidic acid. Journal of lipid research. 2011;52:1247–1255. doi: 10.1194/jlr.M014985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rancoule C, Attane C, Gres S, Fournel A, Dusaulcy R, Bertrand C, et al. Lysophosphatidic acid impairs glucose homeostasis and inhibits insulin secretion in high-fat diet obese mice. Diabetologia. 2013;56:1394–1402. doi: 10.1007/s00125-013-2891-3. [DOI] [PubMed] [Google Scholar]
- 9.Nishimura S, Nagasaki M, Okudaira S, Aoki J, Ohmori T, Ohkawa R, et al. ENPP2 contributes to adipose tissue expansion in diet-induced obesity. Diabetes. 2014 doi: 10.2337/db13-1694. [DOI] [PubMed] [Google Scholar]
- 10.Rancoule C, Dusaulcy R, Treguer K, Gres S, Guigne C, Quilliot D, et al. Depot-specific regulation of autotaxin with obesity in human adipose tissue. Journal of physiology and biochemistry. 2012;68:635–644. doi: 10.1007/s13105-012-0181-z. [DOI] [PubMed] [Google Scholar]
- 11.Ikeda H, Yatomi Y. Autotaxin in liver fibrosis. Clinica chimica acta; international journal of clinical chemistry. 2012;413:1817–1821. doi: 10.1016/j.cca.2012.07.014. [DOI] [PubMed] [Google Scholar]
- 12.Goodpaster BH, Delany JP, Otto AD, Kuller L, Vockley J, South-Paul JE, et al. Effects of diet and physical activity interventions on weight loss and cardiometabolic risk factors in severely obese adults: a randomized trial. JAMA : the journal of the American Medical Association. 2010;304:1795–1802. doi: 10.1001/jama.2010.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112:2735–2752. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 14.Iwasaki M, Takada Y, Hayashi M, Minamiguchi S, Haga H, Maetani Y, et al. Noninvasive evaluation of graft steatosis in living donor liver transplantation. Transplantation. 2004;78:1501–1505. doi: 10.1097/01.tp.0000140499.23683.0d. [DOI] [PubMed] [Google Scholar]
- 15.Shores NJ, Link K, Fernandez A, Geisinger KR, Davis M, Nguyen T, et al. Non-contrasted computed tomography for the accurate measurement of liver steatosis in obese patients. Digestive diseases and sciences. 2011;56:2145–2151. doi: 10.1007/s10620-011-1602-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Park SH, Kim PN, Kim KW, Lee SW, Yoon SE, Park SW, et al. Macrovesicular hepatic steatosis in living liver donors: use of CT for quantitative and qualitative assessment. Radiology. 2006;239:105–112. doi: 10.1148/radiol.2391050361. [DOI] [PubMed] [Google Scholar]
- 17.Watanabe N, Ikeda H, Nakamura K, Ohkawa R, Kume Y, Aoki J, et al. Both plasma lysophosphatidic acid and serum autotaxin levels are increased in chronic hepatitis C. Journal of clinical gastroenterology. 2007;41:616–623. doi: 10.1097/01.mcg.0000225642.90898.0e. [DOI] [PubMed] [Google Scholar]
- 18.Yoneda M, Mawatari H, Fujita K, Iida H, Yonemitsu K, Kato S, et al. High-sensitivity C-reactive protein is an independent clinical feature of nonalcoholic steatohepatitis (NASH) and also of the severity of fibrosis in NASH. Journal of gastroenterology. 2007;42:573–582. doi: 10.1007/s00535-007-2060-x. [DOI] [PubMed] [Google Scholar]
- 19.Targher G. Relationship between high-sensitivity C-reactive protein levels and liver histology in subjects with non-alcoholic fatty liver disease. Journal of hepatology. 2006;45:879–881. doi: 10.1016/j.jhep.2006.09.005. author reply 881-872. [DOI] [PubMed] [Google Scholar]
- 20.Abiru S, Migita K, Maeda Y, Daikoku M, Ito M, Ohata K, et al. Serum cytokine and soluble cytokine receptor levels in patients with non-alcoholic steatohepatitis. Liver international : official journal of the International Association for the Study of the Liver. 2006;26:39–45. doi: 10.1111/j.1478-3231.2005.01191.x. [DOI] [PubMed] [Google Scholar]
- 21.Haukeland JW, Damas JK, Konopski Z, Loberg EM, Haaland T, Goverud I, et al. Systemic inflammation in nonalcoholic fatty liver disease is characterized by elevated levels of CCL2. Journal of hepatology. 2006;44:1167–1174. doi: 10.1016/j.jhep.2006.02.011. [DOI] [PubMed] [Google Scholar]
- 22.Sorrentino P, Tarantino G, Conca P, Perrella A, Terracciano ML, Vecchione R, et al. Silent non-alcoholic fatty liver disease-a clinical-histological study. Journal of hepatology. 2004;41:751–757. doi: 10.1016/j.jhep.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 23.Shimada M, Kawahara H, Ozaki K, Fukura M, Yano H, Tsuchishima M, et al. Usefulness of a combined evaluation of the serum adiponectin level, HOMA-IR, and serum type IV collagen 7S level to predict the early stage of nonalcoholic steatohepatitis. The American journal of gastroenterology. 2007;102:1931–1938. doi: 10.1111/j.1572-0241.2007.01322.x. [DOI] [PubMed] [Google Scholar]
- 24.Boza C, Riquelme A, Ibanez L, Duarte I, Norero E, Viviani P, et al. Predictors of nonalcoholic steatohepatitis (NASH) in obese patients undergoing gastric bypass. Obesity surgery. 2005;15:1148–1153. doi: 10.1381/0960892055002347. [DOI] [PubMed] [Google Scholar]
- 25.Wolf AM, Busch B, Kuhlmann HW, Beisiegel U. Histological changes in the liver of morbidly obese patients: correlation with metabolic parameters. Obesity surgery. 2005;15:228–237. doi: 10.1381/0960892053268408. [DOI] [PubMed] [Google Scholar]
- 26.Ong JP, Elariny H, Collantes R, Younoszai A, Chandhoke V, Reines HD, et al. Predictors of nonalcoholic steatohepatitis and advanced fibrosis in morbidly obese patients. Obesity surgery. 2005;15:310–315. doi: 10.1381/0960892053576820. [DOI] [PubMed] [Google Scholar]
- 27.Ong JP, Pitts A, Younossi ZM. Increased overall mortality and liver-related mortality in non-alcoholic fatty liver disease. Journal of hepatology. 2008;49:608–612. doi: 10.1016/j.jhep.2008.06.018. [DOI] [PubMed] [Google Scholar]
- 28.Dunn W, Xu R, Wingard DL, Rogers C, Angulo P, Younossi ZM, et al. Suspected nonalcoholic fatty liver disease and mortality risk in a population-based cohort study. The American journal of gastroenterology. 2008;103:2263–2271. doi: 10.1111/j.1572-0241.2008.02034.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Targher G, Bertolini L, Rodella S, Zoppini G, Scala L, Zenari L, et al. Associations between plasma adiponectin concentrations and liver histology in patients with nonalcoholic fatty liver disease. Clinical endocrinology. 2006;64:679–683. doi: 10.1111/j.1365-2265.2006.02527.x. [DOI] [PubMed] [Google Scholar]
- 30.Hui JM, Hodge A, Farrell GC, Kench JG, Kriketos A, George J. Beyond insulin resistance in NASH: TNF-alpha or adiponectin? Hepatology (Baltimore, Md) 2004;40:46–54. doi: 10.1002/hep.20280. [DOI] [PubMed] [Google Scholar]
- 31.Uygun A, Kadayifci A, Yesilova Z, Erdil A, Yaman H, Saka M, et al. Serum leptin levels in patients with nonalcoholic steatohepatitis. The American journal of gastroenterology. 2000;95:3584–3589. doi: 10.1111/j.1572-0241.2000.03297.x. [DOI] [PubMed] [Google Scholar]
- 32.Wieckowska A, Papouchado BG, Li Z, Lopez R, Zein NN, Feldstein AE. Increased hepatic and circulating interleukin-6 levels in human nonalcoholic steatohepatitis. The American journal of gastroenterology. 2008;103:1372–1379. doi: 10.1111/j.1572-0241.2007.01774.x. [DOI] [PubMed] [Google Scholar]
- 33.Torres DM, Harrison SA. Diagnosis and therapy of nonalcoholic steatohepatitis. Gastroenterology. 2008;134:1682–1698. doi: 10.1053/j.gastro.2008.02.077. [DOI] [PubMed] [Google Scholar]
- 34.Ferry G, Tellier E, Try A, Gres S, Naime I, Simon MF, et al. Autotaxin is released from adipocytes, catalyzes lysophosphatidic acid synthesis, and activates preadipocyte proliferation. Up-regulated expression with adipocyte differentiation and obesity. The Journal of biological chemistry. 2003;278:18162–18169. doi: 10.1074/jbc.M301158200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gesta S, Simon MF, Rey A, Sibrac D, Girard A, Lafontan M, et al. Secretion of a lysophospholipase D activity by adipocytes: involvement in lysophosphatidic acid synthesis. Journal of lipid research. 2002;43:904–910. [PMC free article] [PubMed] [Google Scholar]
- 36.Boucher J, Quilliot D, Praderes JP, Simon MF, Gres S, Guigne C, et al. Potential involvement of adipocyte insulin resistance in obesity-associated up-regulation of adipocyte lysophospholipase D/autotaxin expression. Diabetologia. 2005;48:569–577. doi: 10.1007/s00125-004-1660-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jansen S, Andries M, Vekemans K, Vanbilloen H, Verbruggen A, Bollen M. Rapid clearance of the circulating metastatic factor autotaxin by the scavenger receptors of liver sinusoidal endothelial cells. Cancer letters. 2009;284:216–221. doi: 10.1016/j.canlet.2009.04.029. [DOI] [PubMed] [Google Scholar]
- 38.Watanabe N, Ikeda H, Nakamura K, Ohkawa R, Kume Y, Tomiya T, et al. Plasma lysophosphatidic acid level and serum autotaxin activity are increased in liver injury in rats in relation to its severity. Life sciences. 2007;81:1009–1015. doi: 10.1016/j.lfs.2007.08.013. [DOI] [PubMed] [Google Scholar]
- 39.Simon MF, Daviaud D, Pradere JP, Gres S, Guigne C, Wabitsch M, et al. Lysophosphatidic acid inhibits adipocyte differentiation via lysophosphatidic acid 1 receptor-dependent down-regulation of peroxisome proliferator-activated receptor gamma2. The Journal of biological chemistry. 2005;280:14656–14662. doi: 10.1074/jbc.M412585200. [DOI] [PubMed] [Google Scholar]
- 40.Chitturi S, Farrell G, Frost L, Kriketos A, Lin R, Fung C, et al. Serum leptin in NASH correlates with hepatic steatosis but not fibrosis: a manifestation of lipotoxicity? Hepatology (Baltimore, Md) 2002;36:403–409. doi: 10.1053/jhep.2002.34738. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.