Abstract
Vascular calcification is highly prevalent and, when present, is associated with major adverse cardiovascular events. Vascular smooth muscle cells play an integral role in mediating vessel calcification by undergoing differentiation to osteoblast-like cells and generating matrix vesicles that serve as a nidus for calcium-phosphate deposition in the vessel wall. Once believed to be a passive process, it is now recognized that vascular calcification is a complex and highly regulated process that involves activation of cellular signaling pathways, circulating inhibitors of calcification, genetic factors, and hormones. This review will examine several of the key mechanisms linking vascular smooth muscle cells to vessel calcification that may be targeted to reduce vessel wall mineralization and, thereby, reduce cardiovascular risk.
Keywords: calcification inhibitors, matrix vesicles, vascular smooth muscle cells, vascular calcification
Introduction
Ectopic calcification is a highly prevalent vascular pathophenotype that has been associated with aging, atherothrombotic cardiovascular disease, diabetes mellitus, and chronic kidney disease. When present, it portends a worse clinical outcome and predicts major adverse cardiovascular events as shown in several population-based studies. In the Multi-Ethnic Study of Atherosclerosis (MESA) study, which included 6,814 community-dwelling participants, the prevalence of vascular calcification was assessed by computed tomography (CT) scanning. This revealed that the prevalence of coronary calcification for men when stratified by Caucasian, African-American, Hispanic, and Chinese ethnicity was 70.4%, 52.0 %, 56.6 %, and 59.2%, respectively, and for women was 44.7%, 37.0%, 34.8%, and 41.9% respectively. The risk for a major coronary event, defined as a myocardial infarction or death from coronary disease, for individuals with a coronary artery calcium score of >300 was 6.84 (95% CI: 2.93-15.99), which was significantly higher than that observed for individuals with a calcium score of 1-100 (HR=3.89; 95% CI: 1.72—8.79).1, 2 For those individuals that did not have evidence of vascular calcification at the index study, 18% developed coronary artery calcification over the mean 3-year follow-up period.3
The prevalence of calcification is known to increase with age with evidence of vascular calcification present in ≥ 90% of men and ≥ 67% of women over the age of 70 years.1, 4 In younger adults, age 33-45 years evaluated in the Coronary Artery Risk Development in Young Adults (CARDIA) study, there was concordance between the presence of coronary artery calcification and the Framingham Risk Score. For individuals with a risk score >10%, 17.2% of individuals were found to have a coronary artery calcium score of ≥ 100% while only 1.3% of participants with the lowest risk score (0-2.5%) had high coronary artery calcium scores. When stratified by sex, a similar pattern was found; however, when stratified by race, the prevalence of coronary artery calcification was higher in Caucasian than African- American participants although African-Americans were found to have a higher overall burden of vascular calcification. 5
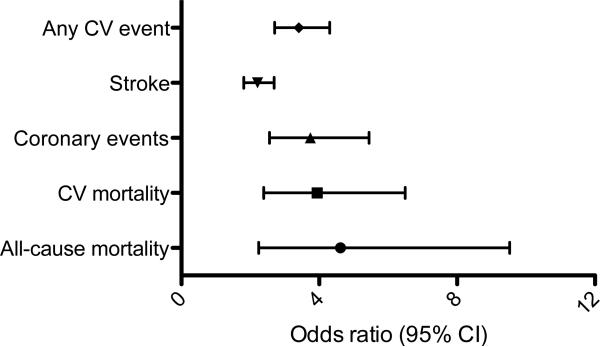
The prevalence of vascular calcification was also examined in a comprehensive meta-analysis that included 218,080 patients from 30 studies that were followed for a mean of 10.1 years. This study revealed that the odds ratio for cardiovascular mortality based on the presence of calcification versus no calcification was 3.94 (95% CI: 2.39-6.50), although there was heterogeneity among the studies included. For example, the risk of cardiovascular mortality was high (OR=18.38; 95% CI: 4.30- 78.47) in one included study of patients with renal insufficiency as compared to another study that evaluated asymptomatic individuals (OR=2.31; 95% CI: 1.60- 3.34) (Fig. 1). Subgroup analysis further revealed that the odds ratio for any cardiovascular event was significantly higher in individuals with renal insufficiency (OR=6.22; 95% CI: 2.73, 14.14) while cardiovascular mortality was higher in patients with diabetes mellitus (OR=2.27; 95% CI: 1.07-3.04). This suggests that the high prevalence of vascular calcification in patients with chronic kidney disease and diabetes mellitus may explain, in part, their increased risk for adverse cardiovascular events. 6
Figure 1. Vascular calcification predicts cardiovascular events.
In a meta- analysis, risk for major adverse events was determined for patients with cardiovascular calcification versus those without calcification. Data from 218,080 patients from 30 studies was examined to determine the odds ratio (OR) for all-cause and cardiovascular (CV) mortality as well as any CV event, stroke, or coronary events. Patients included in these studies ranged from asymptomatic individuals to those with diabetes mellitus, chronic kidney disease, chest pain and multiple risk factors.
Adapted with permission fromRennenberg, et al. Vascular calcifications as a marker of increased cardiovascular risk: a meta-analysis. Vasc Health Risk Manage 2009;5:185-97. (6)
Vascular smooth muscle cells are involved in intimal and medial vessel calcification
Vascular calcification may be localized to atherosclerotic plaques where it is occurs as dispersed punctate or patchy crystals.7 Calcification in the atherosclerotic neointima is detected as microcalcifications (range: ≥0.5 to ⍰ 15 μm) in its early stages that may progress to calcific nodules or areas of actual bone formation. Microcalcifications are believed to originate from apoptotic smooth muscle cells (SMC) or matrix vesicles that are released by these cells and occur near the internal elastic lamina. Atherosclerotic vascular calcification is also associated with lipid deposition and inflammation in the neointima (reviewed in 7).
Vascular calcification may also occur in the medial layer of the vessel (Monckeberg's medial sclerosis) where it is present surrounding smooth muscle cells (SMC) and along the elastic lamellae.8 Medial calcification decreases vessel compliance and is prevalent in aging, diabetes mellitus, and chronic kidney disease.8 The pathogenesis of vascular medial calcification is incompletely characterized however, similar to neointimal calcification, the process is believed to recapitulate skeletal bone formation.8 Studies from monogenetic disease states and animal models of human disease associated with precocious or significant vascular calcification have shown that this is a complex and highly regulated process. Several overlapping mechanisms involving SMCs have been implicated in the pathogenesis of vascular intimal and medial calcification, including reprogramming and differentiation of SMC to an osteoblast-like phenotype and deposition of SMC-generated calcifying matrix vesicles in the vessel wall, These processes are facilitated by loss of calcification inhibitors, increased SMC oxidant and/or endoplasmic reticulum stress, DNA damage response signaling, apoptosis, and disorders of calcium-phosphate homeostasis that may occur as a result of perturbed hormonal regulation of the system.
Differentiation of vascular smooth muscle cells to an osteoblast-like phenotype
Vascular SMC have been shown to differentiate to osteoblast-like cells and enact a cellular program that mediates deposition of bone matrix in blood vessels. In calcified blood vessels, bone-related transcription factors, including Msx2, Sox9, Runx2, and osterix, which upregulate bone and chondrocyte proteins, have been detected in populations of SMC. These transcription factors regulate key processes important for osteoblast differentiation and phenotype. Pro-osteogenic factors such as the bone morphogenetic proteins and inflammatory mediators such as tumor necrosis factor-α (TNF- α) can activate Msx2 and Wnt signaling to upregulate expression of the transcription factors Runx2 and osterix. 8-11 Runx2, in turn, increases expression of the bone-related proteins osteocalcin, sclerostin, and receptor activator of nuclear factor-kappaβ ligand (RANKL) 12. Osterix, which is downstream from Runx2, increases expression of other bone-related proteins including bone sialoprotein and alkaline phosphatase. 8,13, 14
Differentiation of vascular SMC to osteoblast-like cells that express osteoblast transcription factors and bone matrix-related proteins has been confirmed in vitro and in vivo Aortic myofibroblasts exposed to TNF- α demonstrate increased expression of Msx2 that is associated with upregulation of Wnt3a, Wnt7a, and alkaline phosphatase that promotes vascular calcification.15 This was confirmed in a diabetic low-density lipoprotein receptor knockout mouse model of vascular calcification. In this model, a high-fat diet increased serum TNF- α levels, which was associated with increased aortic expression of bone morphogenetic protein-2, Msx2, Wnt3a and Wnt7a as well as aortic calcification.16 Vascular SMC exposed to procalcifying levels of phosphate, akin to what may occur in patients with CKD, lose expression of the smooth muscle contractile proteins SM22α and SM α-actin and express the bone markers Runx2, osteopontin, osteocalcin, and alkaline phosphatase.17 Definitive proof that SMCs undergo this process in vivo was provided by lineage tracing studies performed in a matrix Gla protein (MGP)-knockout mouse model, which is lacking the calcification inhibitor MGP and is prone to early and severe vascular calcification. In this study, SMC differentiation to osteoblast-like cells was confirmed by downregulation of myocardin and increased Runx2 expression that occurred prior to the deposition of calcium in the vasculature. Furthermore, it was determined that it was the increase in Runx2 expression, and not downregulation of myocardin and SMC contractile proteins, that was important for differentiation to osteoblast-like cells and calcification. These studies also confirmed that SMCs, and not bone marrow-derived progenitor cells, that were responsible for vascular calcification.18
Several mechanisms underlying the genetic reprogramming of SMCs to osteoblast- like cells have been identified, although this remains an area of active investigation. Phosphorylation and activation of Erk signaling is important for osteoblast differentiation and has been shown to occur in SMC prior to myocardin downregulation.18 The transcription factor kruppel-like factor 4 also contributes to differentiation of SMCs exposed to high phosphate by suppressing SMC contractile genes. Elevated levels of phosphate induce kruppel-like factor 4, which, in turn, binds to the promoter of genes that encode the contractile proteins SM22α and SM α-actin to repress transcription.19
MicroRNAs (miRs) have emerged as key regulators of SMC differentiation to osteoblast-like cells by regulating gene expression under procalcifying conditions. MicroRNAs are ~22 nucleotide small non-coding RNAs that bind to complementary seed sequences in the 3’-untranslated region of target mRNA to silence gene expression by degrading the mRNA or limiting translation. MicroRNAs typically regulate the expression of many genes and it has been suggested that in disease states these genes may be associated with a common signaling pathway. Collectively, early studies have reported increased expression of miRs that target smooth muscle contractile proteins and decreased expression of miRs that target osteoblast differentiation markers (reviewed in 20). For example, under calcifying conditions, the miR-143/145 complex, which regulates expression of SMC differentiation markers and kruppel-like factor 4 is downregulated. Other studies demonstrated that downregulation of miR-204, miR-205, miR-133a, or miR-30b/c in SMCs, occurs prior to calcification and upregulates Runx2 expression.21, 22 MicroRNA-125b, which targets Ets1 and osterix was found to be downregulated 21 days after exposure of SMCs to osteogenic medium.23 Another series of miRs, miR- 135a(*), miR-762, miR-714, miR-712(*), which target the calcium efflux proteins NCX1, PMCA1, NCKX4, have also been implicated in SMC calcification.21 Although these early studies indicate the importance of miRs in SMC differentiation to an osteoblast-like phenotype by showing the association between one miR and one protein target, it is more likely that this process is associated with changes in expression of a panel of miRs that target several proteins important for calcification.
Matrix vesicles, exosomes, and calciprotein complexes
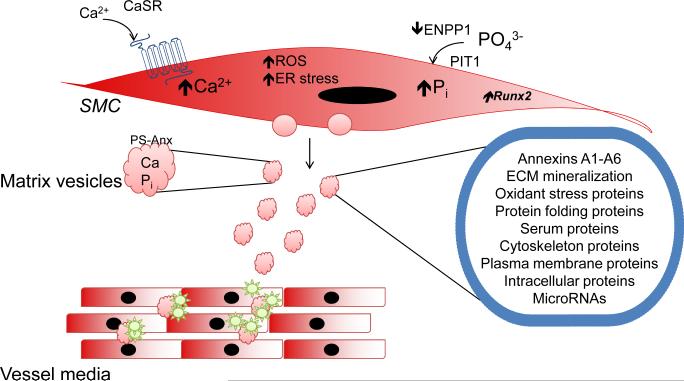
Vascular SMC-generated small extracellular membranous bodies, or matrix vesicles, serve as mineral nucleation sites and are responsible for the initial deposition of calcium and phosphate in blood vessels (Fig. 2). These matrix vesicles originate from dedifferentiated or calcifying SMCs, possibly as a mechanism to decrease high levels of intracellular calcium.24 High cytosolic calcium levels promote translocation of annexins, predominantly annexin 6, to the plasma membrane. This triggers the release of vesicles and converts them to mineralization competent matrix vesicles with a Ca2+-Pi-phosphatidylserine complex as the nucleation core.25 Studies have shown that microtubule dynamics also play a role in SMC matrix vesicle release. In murine SMC exposed to high Pi, microtubule stabilization with paclitaxel was shown to prevent the release of matrix vesicles and calcification.26
Figure 2. Matrix vesicles initiate vascular calcification.
In vascular smooth muscle cells (SMC) exposed to high levels of phosphate, which is taken up by the phosphate transporter PIT1, SMC undergo differentiation to osteoblast-like cells and express the master osteoblast transcription factor Runx2. These SMC generate matrix vesicles that deposit in the vessel wall and serve as a site for nucleation and vascular medial calcification. These membrane-bound matrix vesicles include proteins for calcium and phosphate import and related to extracellular (ECM) mineralization, cytoskeleton, cellular stress and other intracellular proteins.
Adapted with permission from Kapustin, et al. Calcium regulates key components of vascular smooth muscle cell-derived matrix vesicles to enhance mineralization. Circ Res 2011;109:e1-12. (27)
Matrix vesicles also package cargo and mass spectrometry has identified 79 proteins in SMC-derived matrix vesicles, including proteins related to calcification, extracellular matrix, and calcium channels; matrix biogenesis, trafficking, and cytoskeletal proteins; oxidant and endoplasmic stress-related proteins; and other serum proteins.27 Matrix vesicles also possess increased expression and activity of transglutaminase 2, which is a calcium-dependent enzyme that promotes extracellular matrix crosslinking, and matrix metalloproteinase-2 27, 28. The presence of these extracellular matrix-modifying enzymes indicates that matrix vesicles are involved in the disruption of normal vessel architecture in addition to serving as the nidus for calcification.
Accumulating evidence indicates that matrix vesicles are secreted from multivesicular bodies and are enriched with the exosome markers CD63, CD9, CD81, and MHC I. Secretion of these exosome-like structures is regulated by sphingomyelin phosphodiesterase 3 and inhibition of this enzyme prevents vascular calcification. Exosomes generated by SMC under calcifying conditions were found to contain amorphous calcium phosphphate crystals and have been detected in vessels at the site of calcification.29
Calciprotein complexes are distinct from matrix vesicles and exosomes and high circulating levels of these complexes have been detected when pathological vascular calcification is present.30 Calciprotein particles are 50-300 nm and contain the liverderived protein fetuin A, which is a mineral carrier protein that binds calcium, phosphate, and ultimately acidic proteins such as albumin to stabilize supersaturated fetuin A as a colloid.31 Although high levels of calciprotein complexes are associated with vascular calcification, is not known if these calciprotein complexes contribute directly to vascular calcification.
Cellular processes that regulate smooth muscle cell calcification
The cellular and systemic conditions that are permissive for SMC differentiation to osteoblast-like cells and generation of matrix vesicles are multifactorial. At a cellular level, procalcifying conditions may occur as a result of factors that increase cellular stress responses. Similarly, systemic factors such as a loss of circulating inhibitors of calcification or changes in levels of hormonal regulators of calcium and phosphate homeostasis also facilitate SMC differentiation and vascular calcification. Based on the complexity of the systems that regulate vascular calcification, is likely that many of these factors are operative simultaneously.
Oxidant stress and endoplasmic reticulum stress
Oxidant stress and endoplasmic reticulum stress have both been implicated in vascular calcification and shown to promote SMC differentiation. Increased activity of NADPH oxidase and elevated levels of hydrogen peroxide initiate SMC differentiation by upregulating Runx2 expression.32, 33 Similarly, the pro-inflammatory receptor for advanced glycation end-products (RAGE) increases NADPH oxidase activity and SMC oxidant stress to increase Runx2 and alkaline phosphatase expression and SMC transition to an osteoblast-like phenotype. Inhibition of RAGE using an anti-RAGE antibody was found to decrease oxidant stress through a mechanism that involved decreased oxidant stress and Runx2 expression.34 Other studies found that pyridoxamine and alagebrium, inhibitors of advanced glycation end-products, prevented experimental diabetes-associated vascular calcification in a rat model. 35 Constitutive activation of the parathryroid hormone receptor (PTH1R) in the vasculature has also been shown to limit calcification, in part, by decreasing oxidant stress. In a diabetic mouse model with vascular specific expression of constitutively active vascular PTH1R, aorta superoxide levels were decreased compared to controls and this was associated with a decrease in aorta wall thickness, collagen, and vascular calcification. 36 Saturated fatty acids also stimulate vascular calcification through a mechanism that involves increased oxidant stress. Mice fed a palmitic acid-enriched diet demonstrated increased vascular medial calcification that was limited by coadministration of the antioxidant apocynin. In vitro studies performed in SMCs confirmed that palmitate increased reactive oxygen species production and calcification. 37
Oxidant stress can activate endoplasmic reticulum (ER) stress, which is another mechanism by which SMCs undergo differentiation to osteoblast-like cells. In human SMC, bone morphogenetic protein-2 has been shown to increase NADPH oxidase activity and reactive oxygen species production to activate ER stress. Endoplasmic reticulum stress increased expression of the transcription factor XBP-1 that was shown to bind to the Runx2 promoter, initiate SMC differentiation, and increase SMC calcification.38 Other studies found an increase in the ER stress protein activating transcription factor 4 (ATF4) in SMCs and calcified aortas from experimental models. In this study, siRNA knockdown of ATF4 decreased ER stress, apoptosis, and SMC calcification.39 Tumor necrosis factor-α has also been shown to induce vascular calcification through activation of ER stress and the PERK-eIF2α-ATF4-CHOP signaling pathways.40 In patients with renal insufficiency who have elevated serum levels of oxysterols, which can activate ER stress, treatment with simvastatin and ezetamide decreased ER stress and limited calcification.41
DNA damage response, autophagy, and apoptosis
Prolonged cellular stress may activate homeostatic repair processes or cells may undergo apoptosis when overwhelmed by the stress. One repair mechanism, the DNA damage response signaling pathway, has been linked to SMC calcification. Prelamin A, the precursor protein of lamin A, induces SMC senescence and DNA damage by inhibiting mitosis. Prelamin A accumulates in calcifying SMC with a concomitant increase in DNA damage and downregulation of the ataxia- telangiectasia-mutated pathway, which regulates an arm of the DNA damage response pathway. DNA damage response signaling was found to stimulate SMC osteogenic differentiation and p16-induced cellular senescence. This occurred, in part, when SMC acquired a senescence-associated secretory phenotype and increased alkaline phosphatase activity.42
Autophagy, a catabolic process that can be an adaptive response to cell stress, was found to limit SMC calcification by inhibiting matrix vesicle release. Under high phosphate (3 mmol/L) conditions, pharmacological or molecular inhibition of autophagy resulted in increased SMC calcium deposition. Downregulation of autophagy was also associated with loss of SMC contractile proteins but not SMC differentiation to an osteogenic phenotype; however, inhibition of autophagy did increase the release of procalcific matrix vesicles with high levels of alkaline phosphatase activity.43 Thus, factors that interfere with autophagy are likely to increase SMC and vascular calcification.
Apoptosis has also been linked to SMC calcification. Early studies revealed that SMCs might release apoptotic bodies that are similar to matrix vesicles and can concentrate calcium.44 Conversely, calcium-phosphate crystals may also cause SMC cell death with their potency determined by their size and crystal composition; those crystals that are ≤1 μm lead to rapid increases in intracellular calcium and apoptosis through a mechanism that may involve crystal dissolution within lysosomes.45
Inhibitors of ectopic vascular calcification
Ectopic vascular calcification is prevented by the presence of local and circulating inhibitors that limit mineralization of vessels. Decreased expression or activity of any of these inhibitors, genetic or acquired, creates a milieu that favors vascular calcification. Matrix-Gla protein is an N-terminal γ-carboxylated protein that inhibits calcification by limiting precipitation of calcium-phosphate in the blood vessel wall. Matrix-Gla protein requires phosphorylation of 3 serine residues for secretion and γ-carboxylation of 5 glutamate residues for both secretion and activation. The γ-carboxylation process is dependent upon vitamin K as a cofactor and, therefore, explains why vitamin K deficiency or the administration of high doses of the vitamin K antagonist warfarin is associated with vascular calcification.46 Vascular SMC express MGP; when MGP is deficient, as in MGP knockout mouse models, there is severe vascular calcification and early demise. Matrix vesicles generated by SMC were found to lack MGP suggesting that the absence of this inhibitor increased their procalcifying potential. 25, 27 There is also evidence in CKD that MGP is dephosphorylated and under-carboxylated rendering this inhibitor less effective.47
Another key inhibitor is extracellular pyrophosphate that is generated by ecto-ATPase nucleotide pyrophosphatase/phosphodiesterase 1 (ENPP1) and prevents hydroxyapatite formation and propagation. The importance of ENPP1 for limiting vascular calcification was demonstrated by transplanting aortas from Enpp1 knockout mice into wild type mice and vice versa. This revealed that normal levels of extracellular pyrophosphate were sufficient to prevent calcification (Enpp1 aorta in WT mouse) while systemic Enpp1 deficiency was able to produce vascular calcification even on the background of normal vascular pyrophosphate production (WT aorta in Enpp1 knockout mouse).48 Similarly, defects in pyrophosphate metabolism have also been identified as a mechanism underlying vascular calcification in Hutchinson-Gilford progeria syndrome.49
Missense mutations identified in the ATP-binding cassette subfamily C 6 (ABCC6) gene, which is liked to pseudoxanthoma elasticum, have been associated with connective tissue calcification. In Abcc6 knockout mice, arterial calcium accumulation was 1.5-2.0-fold higher than that observed in wild-type mice and vascular expression of the osteogenic and chondrogenic markers Runx2 and Sox9 was present in calcified vessels.50 In rat models of CKD, there was evidence of decreased Abcc6 protein levels suggesting that CKD induces an acquired Abcc6 transporter deficiency.51 The gene NT5E has also has been related to vascular calcification. This gene encodes the glycosyl-phosphatidylinositol-linked plasma membrane CD73 ectoenzyme. CD73 lies downstream of ENPP1 and hydrolyzes AMP to adenosine. Adenosine importantly regulates expression of alkaline phosphatase that is necessary for hydroxyapatite formation and mineralization of vessels.52
Fetuin A is a member of the cystatin superfamily. This cysteine protease inhibitor is synthesized by the liver and is typically regarded as a circulating inhibitor of calcification. Fetuin A forms calciprotein complexes that physically inhibit calcium apatite formation.53 Osteoprotegerin is a member of the tumor necrosis factor-α superfamily and inhibits osteoclast differentiation to inhibit bone remodeling and release of calcium and phosphate into the circulation. Osteoprotegerin is a decoy receptor for receptor activator of nuclear factor kappa B (RANK) ligand and decreases activation of RANK signaling. Mice that are deficient in osteoprotegerin develop spontaneous arterial calcification through a mechanism that involves activation of bone morphogenetic protein-4 and alternative NF-kB signaling as well as RANK ligand-interleukin-6 signaling in SMCs.54, 55
Hormonal regulation of smooth muscle calcification
Vascular calcification is also subject to hormonal regulation and sex steroids appear to play a role in this process. Estrogens inhibit vascular calcification and aortic SMC isolated from female pigs exposed to calcifying conditions are resistant to mineralization owing to higher osteoprotegerin levels compared to SMC isolated from ovariectomized pigs.56 Estrogens were also shown to inhibit RANK ligand signaling in human SMC via estrogen receptor-α signaling. In ovariectomized apoE knockout mice that received estrogen replacement, vascular calcification was diminished as a result of an estrogen-mediated inhibition of bone morphogenetic protein and Smad 1/5/8 signaling as well as an increase in the calcification inhibitor MGP.57 The contribution of testosterone to vascular calcification is more controversial. One study found a 3-4-fold increase in atherosclerotic vascular calcification in mice that was associated with upregulation of the androgen receptor and occurred independent of sex.58 By contrast, another study reported that testosterone inhibited phosphate-stimulated SMC apoptosis and calcification by androgen receptor-mediated transactivation of growth arrest-specific gene 6.59
The role of the calciotropic parathyroid hormone (PTH) in vascular calcification has also been controversial. Early studies performed in a rat model of CKD that underwent parathyroidectomy followed by PTH replacement therapy demonstrated significant aortic medial calcification suggesting that PTH has a direct role in promoting vascular calcification.60 Conversely, other studies that examined activation of the PTH1 receptor, which is highly expressed in SMCs, suggested that PTH prevented vascular calcification. To examine the consequences of SMC PTH1 receptor activation for vascular calcification, a novel transgenic mouse model with SMC-restricted expression of a constitutively active form of PTH1 receptor [SM22-PTH1R(H223R);LDLR−/−] was studied. Compared to nontransgenic siblings, these mice exhibited reduced aortic oxidant stress, Wnt/β-catenin signaling vascular calcification.36 Independently, other investigators found that pulsatile administration of PTH(1-34) reduced vascular calcification in a rat model of uremia.61
The relationship between vitamin D status and vascular calcification appears to be dose-related with physiological levels appearing to be protective while pharmacological levels induce vascular calcification. In SMCs, which express vitamin D receptors, vitamin D is believed to inhibit matrix mineraliza SMC differentiation to osteoblast-like cells. In SMC, 1,2,5(OH)2D (calcitriol) may either increase or decrease vascular calcification, depending upon which cellular processes are involved. Calcitriol regulates calcium and phosphate levels as well as SMC expression of osteoblast genes that supports vascular calcification but its anti-inflammatory effects that may prevent SMC mineralization. In the vitamin D receptor knockout mouse, impaired vitamin D signaling in SMCs was associated with SMC differentiation and increased vascular calcification, although studies have suggested that this occurs due to systemic, and not local, vitamin D receptor signaling in other tissues.48, 62
Klotho is a transmembrane protein that is expressed by the kidney and blood vessels and has been associated with vascular calcification.63 Klotho functions as a co-receptor for fibroblast growth factor-23 (FGF23) and maintains the balance of circulating calcium and phosphate.64 Activation of the vitamin D receptor increases expression of klotho and FGF23 to increase renal phosphate excretion by downregulating the sodium phosphate transporters Slc34A1/NaPi-2a and Slc34A3/NaPi-2c. Klotho inhibits vascular calcification by preventing SMC differentiation while disruption of klotho-FGF23 signaling results in hyperphosphatemia with ectopic calcification.64 Studies in klotho hypomorphic mice (kl/kl) found that these mice had hyperaldosteronism. In vitro, aldosterone increased SMC expression of the phosphate transporter PIT-1 and SMC expression of osteoblast genes, all of which was inhibitable by treatment with spironolactone.65 This is not surprising, as aldosterone has been shown previously to upregulate alkaline phosphatase activity in calcifying SMC and to increase expression of tumor necrosis factor-α, which has been implicated in SMC differentiation via NF-kB p65 and Msx2 to induce expression of Runx2, Wnt/β-catenin signaling, and alkaline phosphatase.65, 66
Conclusion
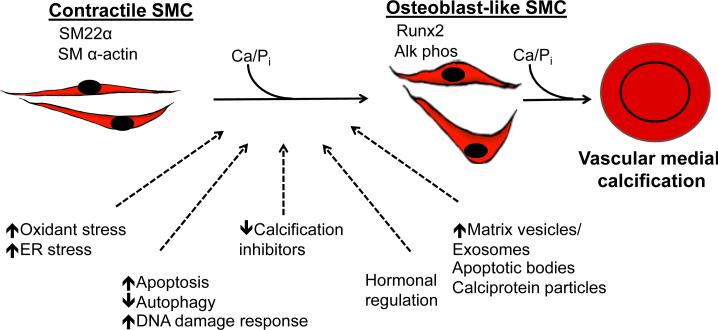
Vascular SMCs play an integral role in ectopic arterial calcification (Fig. 3). Under procalcifying conditions, SMCs undergo differentiation to osteoblast-like cells and express the master osteoblast transcription factor Runx2 along with other bone- related proteins with concomitant downregulation of SMC contractile proteins. These reprogrammed SMC generate matrix vesicles and exosomes that initiate the mineralization process and form bone matrix within the vessel wall. Genetic, metabolic, and hormonal signaling regulates SMC calcification processes, although the exact signaling pathways and interactions between these regulators remain incompletely characterized. Future efforts will likely focus on identifying key regulatory points and interactions between mechanisms linked to SMC differentiation and mineralization processes that can be targeted to reduce calcification, and, thereby, improve vascular compliance and reduce cardiovascular risk.
Figure 3. Mechanisms of vascular medial calcification.
Contractile vascular smooth muscle cells undergo differentiation to osteoblast-like cells when exposed to high levels of phosphate and other cellular and/or systemic processes that facilitate vascular calcification. These osteoblast-like cells participate in vascular medial calcification.
Acknowledgements
This work was funded by NIH/NHLBI R01 105301.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affct the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bild DE, Detrano R, Peterson D, Guerci A, Liu K, Shahar E, et al. Ethnic differences in coronary calcification: the Multi-Ethnic Study of Atherosclerosis (MESA). Circulation. 2005;111:1313–20. doi: 10.1161/01.CIR.0000157730.94423.4B. [DOI] [PubMed] [Google Scholar]
- 2.Detrano R, Guerci AD, Carr JJ, Bild DE, Burke G, Folsom AR, et al. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. New Engl J Med. 2008;358:1336–45. doi: 10.1056/NEJMoa072100. [DOI] [PubMed] [Google Scholar]
- 3.DeFilippis AP, Blaha MJ, Ndumele CE, Budoff MJ, Lloyd-Jones DM, McClelland RL,N, et al. The association of Framingham and Reynolds risk scores with incidence and progression of coronary artery calcification in MESA (Multi-Ethnic Study of Atherosclerosis). J Am Coll Card. 2011;58:2076–83. doi: 10.1016/j.jacc.2011.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wong ND, Kouwabunpat D, Vo AN, Detrano RC, Eisenberg H, Goel M, et al. Coronary calcium and atherosclerosis by ultrafast computed tomography in asymptomatic men and women: relation to age and risk factors. Am Heart J. 1994;127:422–30. doi: 10.1016/0002-8703(94)90133-3. [DOI] [PubMed] [Google Scholar]
- 5.Okwuosa TM, Greenland P, Burke GL, Eng J, Cushman M, Michos ED, et al. Prediction of coronary artery calcium progression in individuals with low Framingham Risk Score: the Multi-Ethnic Study of Atherosclerosis. JACC CV Imag. 2012;5:144–53. doi: 10.1016/j.jcmg.2011.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rennenberg RJ, Kessels AG, Schurgers LJ, van Engelshoven JM, de Leeuw PW, Kroon AA. Vascular calcifications as a marker of increased cardiovascular risk: a meta-analysis. Vasc Health Risk Manage. 2009;5:185–97. doi: 10.2147/vhrm.s4822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Otsuka F, Sakakura K, Yahagi K, Joner M, Virmani R. Has our understanding of calcification in human coronary atherosclerosis progressed? Arterioscler Thromb Vasc Biol. 2014;34:724–36. doi: 10.1161/ATVBAHA.113.302642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bostrom KI, Rajamannan NM, Towler DA. The regulation of valvular and vascular sclerosis by osteogenic morphogens. Circ Res. 2011;109:564–77. doi: 10.1161/CIRCRESAHA.110.234278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Westendorf JJ, Kahler RA, Schroeder TM. Wnt signaling in osteoblasts and bone diseases. Gene. 2004;341:19–39. doi: 10.1016/j.gene.2004.06.044. [DOI] [PubMed] [Google Scholar]
- 10.Hill TP, Spater D, Taketo MM, Birchmeier W, Hartmann C. Canonical Wnt/beta-catenin signaling prevents osteoblasts from differentiating into chondrocytes. Develop Cell. 2005;8:727–38. doi: 10.1016/j.devcel.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 11.Bodine PV, Komm BS. Wnt signaling and osteoblastogenesis. Rev End Met Disord. 2006;7:33–9. doi: 10.1007/s11154-006-9002-4. [DOI] [PubMed] [Google Scholar]
- 12.Lian JB, Stein GS, Javed A, van Wijnen AJ, Stein JL, et al. Networks and hubs for the transcriptional control of osteoblastogenesis. Rev End Met Disord. 2006;7:1–16. doi: 10.1007/s11154-006-9001-5. [DOI] [PubMed] [Google Scholar]
- 13.Nakashima K, Zhou X, Kunkel G, Zhang Z, Deng JM, Behringer RR, et al. The novel zinc finger-containing transcription factor osterix is required for osteoblast differentiation and bone formation. Cell. 2002;108:17–29. doi: 10.1016/s0092-8674(01)00622-5. [DOI] [PubMed] [Google Scholar]
- 14.Zhu F, Friedman MS, Luo W, Woolf P, Hankenson KD. The transcription factor osterix (SP7) regulates BMP6-induced human osteoblast differentiation. J Cell Physiol. 2012;227:2677–85. doi: 10.1002/jcp.23010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shao JS, Cheng SL, Pingsterhaus JM, Charlton-Kachigian N, Loewy AP, Towler DA. Msx2 promotes cardiovascular calcification by activating paracrine Wnt signals. J Clin Invest. 2005;115:1210–20. doi: 10.1172/JCI24140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Al-Aly Z, Shao JS, Lai CF, Huang E, Cai J, Behrmann A, et al. Aortic Msx2-Wnt calcification cascade is regulated by TNF-alpha-dependent signals in diabetic Ldlr−/− mice. Arterioscler Thromb Vasc Biol. 2007;27:2589–96. doi: 10.1161/ATVBAHA.107.153668. [DOI] [PubMed] [Google Scholar]
- 17.Steitz SA, Speer MY, Curinga G, Yang HY, Haynes P, Aebersold R, et al. Smooth muscle cell phenotypic transition associated with calcification: upregulation of Cbfa1 and downregulation of smooth muscle lineage markers. Circ Res. 2001;89:1147–54. doi: 10.1161/hh2401.101070. [DOI] [PubMed] [Google Scholar]
- 18.Speer MY, Yang HY, Brabb T, Leaf E, Look A, Lin WL, et al. Smooth muscle cells give rise to osteochondrogenic precursors and chondrocytes in calcifying arteries. Circ Res. 2009;104:733–41. doi: 10.1161/CIRCRESAHA.108.183053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yoshida T, Yamashita M, Hayashi M. Kruppel-like factor 4 contributes to high phosphate-induced phenotypic switching of vascular smooth muscle cells into osteogenic cells. J Biol Chem. 2012;287:25706–14. doi: 10.1074/jbc.M112.361360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leopold JA. MicroRNAs regulate vascular medial calcification. Cells. 2014 doi: 10.3390/cells3040963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goettsch C, Hutcheson JD, Aikawa E. MicroRNA in cardiovascular calcification: focus on targets and extracellular vesicle delivery mechanisms. Circ Res. 2013;112:1073–84. doi: 10.1161/CIRCRESAHA.113.300937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Balderman JA, Lee HY, Mahoney CE, Handy DE, White K, Annis S, et al. Bone morphogenetic protein-2 decreases microRNA-30b and microRNA-30c to promote vascular smooth muscle cell calcification. JAHA. 2012;1:e003905. doi: 10.1161/JAHA.112.003905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goettsch C, Rauner M, Pacyna N, Hempel U, Bornstein SR, Hofbauer LC. miR-125b regulates calcification of vascular smooth muscle cells. Am J Pathol. 2011;179:1594–600. doi: 10.1016/j.ajpath.2011.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Demer LL, Tintut Y. Inflammatory, metabolic, and genetic mechanisms of vascular calcification. Arterioscler Thromb Vasc Biol. 2014;34:715–23. doi: 10.1161/ATVBAHA.113.302070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kapustin AN, Shanahan CM. Calcium regulation of vascular smooth muscle cell-derived matrix vesicles. Trends CV Med. 2012;22:133–7. doi: 10.1016/j.tcm.2012.07.009. [DOI] [PubMed] [Google Scholar]
- 26.Lee K, Kim H, Jeong D. Microtubule stabilization attenuates vascular calcification through the inhibition of osteogenic signaling and matrix vesicle release. Biochem Biophys Res Commun. 2014;451:436–41. doi: 10.1016/j.bbrc.2014.08.007. [DOI] [PubMed] [Google Scholar]
- 27.Kapustin AN, Davies JD, Reynolds JL, McNair R, Jones GT, Sidibe A, et al. Calcium regulates key components of vascular smooth muscle cell-derived matrix vesicles to enhance mineralization. Circ Res. 2011;109:e1–12. doi: 10.1161/CIRCRESAHA.110.238808. [DOI] [PubMed] [Google Scholar]
- 28.Chen NX, O'Neill K, Chen X, Kiattisunthorn K, Gattone VH, Moe SM. Transglutaminase 2 accelerates vascular calcification in chronic kidney disease. Am J Nephrol. 2013;37:191–8. doi: 10.1159/000347031. [DOI] [PubMed] [Google Scholar]
- 29.Kapustin A, Chatrou M, Kalra S, Drozdov I, Soong D, Furmanik M, et al. Regulated exosome secretion by vascular smooth muscle cells mediates vascular calcification. Heart. 2014;100(Suppl 3):A93–4. [Google Scholar]
- 30.Herrmann M, Schafer C, Heiss A, Graber S, Kinkeldey A, Buscher A, et al. Clearance of fetuin-A--containing calciprotein particles is mediated by scavenger receptor-A. Circ Res. 2012;111:575–84. doi: 10.1161/CIRCRESAHA.111.261479. [DOI] [PubMed] [Google Scholar]
- 31.Jahnen-Dechent W, Heiss A, Schafer C, Ketteler M. Fetuin-A regulation of calcified matrix metabolism. Circ Res. 2011;108:1494–509. doi: 10.1161/CIRCRESAHA.110.234260. [DOI] [PubMed] [Google Scholar]
- 32.Byon CH, Javed A, Dai Q, Kappes JC, Clemens TL, Darley-Usmar VM, et al. Oxidative stress induces vascular calcification through modulation of the osteogenic transcription factor Runx2 by AKT signaling. J Biol Chem. 2008;283:15319–27. doi: 10.1074/jbc.M800021200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sutra T, Morena M, Bargnoux AS, Caporiccio B, Canaud B, Cristol JP. Superoxide production: a procalcifying cell signalling event in osteoblastic differentiation of vascular smooth muscle cells exposed to calcification media. Free Rad Res. 2008;42:789–97. doi: 10.1080/10715760802400766. [DOI] [PubMed] [Google Scholar]
- 34.Wei Q, Ren X, Jiang Y, Jin H, Liu N, Li J. Advanced glycation end products accelerate rat vascular calcification through RAGE/oxidative stress. BMC Cardiovasc Disord. 2013;13:13. doi: 10.1186/1471-2261-13-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brodeur MR, Bouvet C, Bouchard S, Moreau S, Leblond J, Deblois D, et al. Reduction of advanced-glycation end products levels and inhibition of RAGE signaling decreases rat vascular calcification induced by diabetes. PloS One. 2014;9:e85922. doi: 10.1371/journal.pone.0085922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cheng SL, Shao JS, Halstead LR, Distelhorst K, Sierra O, Towler DA. Activation of vascular smooth muscle parathyroid hormone receptor inhibits Wnt/beta-catenin signaling and aortic fibrosis in diabetic arteriosclerosis. Circ Res. 2010;107:271–82. doi: 10.1161/CIRCRESAHA.110.219899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brodeur MR, Bouvet C, Barrette M, Moreau P. Palmitic acid increases medial calcification by inducing oxidative stress. J Vasc Res. 2013;50:430–41. doi: 10.1159/000354235. [DOI] [PubMed] [Google Scholar]
- 38.Liberman M, Johnson RC, Handy DE, Loscalzo J, Leopold JA. Bone morphogenetic protein-2 activates NADPH oxidase to increase endoplasmic. Biochem Biophys Res Commun. 2011;413:436–41. doi: 10.1016/j.bbrc.2011.08.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Duan XH, Chang JR, Zhang J, Zhang BH, Li YL, Teng X, et al. Activating transcription factor 4 is involved in endoplasmic reticulum stress-mediated apoptosis contributing to vascular calcification. Apoptosis. 2013;18:1132–44. doi: 10.1007/s10495-013-0861-3. [DOI] [PubMed] [Google Scholar]
- 40.Masuda M, Miyazaki-Anzai S, Levi M, Ting TC, Miyazaki M. PERK-eIF2alpha-ATF4-CHOP signaling contributes to TNFalpha-induced vascular calcification. JAHA. 2013;2:e000238. doi: 10.1161/JAHA.113.000238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Miyazaki-Anzai S, Masuda M, Demos-Davies KM, Keenan AL, Saunders SJ, Masuda R, et al. Endoplasmic reticulum stress effector CCAAT/enhancer-binding protein homologous protein (CHOP) regulates chronic kidney disease-induced vascular calcification. JAHA. 2014;3:e000949. doi: 10.1161/JAHA.114.000949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu Y, Drozdov I, Shroff R, Beltran LE, Shanahan CM. Prelamin A accelerates vascular calcification via activation of the DNA damage response and senescence-associated secretory phenotype in vascular smooth muscle cells. Circ Res. 2013;112:e99–109. doi: 10.1161/CIRCRESAHA.111.300543. [DOI] [PubMed] [Google Scholar]
- 43.Dai XY, Zhao MM, Cai Y, Guan QC, Zhao Y, Guan Y, et al. Phosphate-induced autophagy counteracts vascular calcification by reducing matrix vesicle release. Kid Int. 2013;83:1042–51. doi: 10.1038/ki.2012.482. [DOI] [PubMed] [Google Scholar]
- 44.Proudfoot D, Skepper JN, Hegyi L, Bennett MR, Shanahan CM, Weissberg PL. Apoptosis regulates human vascular calcification in vitro: evidence for initiation of vascular calcification by apoptotic bodies. Circ Res. 2000;87:1055–62. doi: 10.1161/01.res.87.11.1055. [DOI] [PubMed] [Google Scholar]
- 45.Ewence AE, Bootman M, Roderick HL, Skepper JN, McCarthy G, Epple M, et al. Calcium phosphate crystals induce cell death in human vascular smooth muscle cells: a potential mechanism in atherosclerotic plaque destabilization. Circ Res. 2008;103:e28–34. doi: 10.1161/CIRCRESAHA.108.181305. [DOI] [PubMed] [Google Scholar]
- 46.Schurgers LJ, Spronk HM, Skepper JN, Hackeng TM, Shanahan CM, Vermeer C, et al. Post-translational modifications regulate matrix Gla protein function: importance for inhibition of vascular smooth muscle cell calcification. J Thromb Haemost. 2007;5:2503–11. doi: 10.1111/j.1538-7836.2007.02758.x. [DOI] [PubMed] [Google Scholar]
- 47.Lomashvili KA, Wang X, Wallin R, O'Neill WC. Matrix Gla protein metabolism in vascular smooth muscle and role in uremic vascular calcification. J Biol Chem. 2011;286:28715–22. doi: 10.1074/jbc.M111.251462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lomashvili KA, Narisawa S, Millan JL, O'Neill WC. Vascular calcification is dependent on plasma levels of pyrophosphate. Kid Int. 2014;85:1351–6. doi: 10.1038/ki.2013.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Villa-Bellosta R, Rivera-Torres J, Osorio FG, Acin-Perez R, Enriquez JA, Lopez-Otin C, et al. Defective extracellular pyrophosphate metabolism promotes vascular calcification in a mouse model of Hutchinson-Gilford progeria syndrome that is ameliorated on pyrophosphate treatment. Circulation. 2013;127:2442–51. doi: 10.1161/CIRCULATIONAHA.112.000571. [DOI] [PubMed] [Google Scholar]
- 50.Kauffenstein G, Pizard A, Le Corre Y, Vessieres E, Grimaud L, Toutain B, et al. Disseminated arterial calcification and enhanced myogenic response are associated with abcc6 deficiency in a mouse model of pseudoxanthoma elasticum. Arterioscler Thromb Vasc Biol. 2014;34:1045–56. doi: 10.1161/ATVBAHA.113.302943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lau WL, Liu S, Vaziri ND. Chronic Kidney Disease Results in Deficiency of ABCC6, the Novel Inhibitor of Vascular Calcification. Am J Nephrol. 2014;40:51–5. doi: 10.1159/000365014. [DOI] [PubMed] [Google Scholar]
- 52.Nitschke Y, Baujat G, Botschen U, Wittkampf T, du Moulin M, Stella J, et al. Generalized arterial calcification of infancy and pseudoxanthoma elasticum can be caused by mutations in either ENPP1 or ABCC6. Am J Hum Gen. 2012;90:25–39. doi: 10.1016/j.ajhg.2011.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Westenfeld R, Schafer C, Smeets R, Brandenburg VM, Floege J, Ketteler M, et al. Fetuin-A (AHSG) prevents extraosseous calcification induced by uraemia and phosphate challenge in mice. Nephrol Dial Trans. 2007;22:1537–46. doi: 10.1093/ndt/gfm094. [DOI] [PubMed] [Google Scholar]
- 54.Callegari A, Coons ML, Ricks JL, Rosenfeld ME, Scatena M. Increased calcification in osteoprotegerin-deficient smooth muscle cells: Dependence on receptor activator of NF-kappaB ligand and interleukin 6. J Vasc Res. 2014;51:118–31. doi: 10.1159/000358920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Panizo S, Cardus A, Encinas M, Parisi E, Valcheva P, Lopez-Ongil S, et al. RANKL increases vascular smooth muscle cell calcification through a RANK-BMP4-dependent pathway. Circ Res. 2009;104:1041–8. doi: 10.1161/CIRCRESAHA.108.189001. [DOI] [PubMed] [Google Scholar]
- 56.Rzewuska-Lech E, Jayachandran M, Fitzpatrick LA, Miller VM. Differential effects of 17beta-estradiol and raloxifene on VSMC phenotype and expression of osteoblast-associated proteins. Am J Physiol. 2005;289:E105–12. doi: 10.1152/ajpendo.00366.2004. [DOI] [PubMed] [Google Scholar]
- 57.Osako MK, Nakagami H, Koibuchi N, Shimizu H, Nakagami F, Koriyama H, et al. Estrogen inhibits vascular calcification via vascular RANKL system: common mechanism of osteoporosis and vascular calcification. Circ Res. 2010;107:466–75. doi: 10.1161/CIRCRESAHA.110.216846. [DOI] [PubMed] [Google Scholar]
- 58.McRobb L, Handelsman DJ, Heather AK. Androgen-induced progression of arterial calcification in apolipoprotein E-null mice is uncoupled from plaque growth and lipid levels. Endocrinology. 2009;150:841–8. doi: 10.1210/en.2008-0760. [DOI] [PubMed] [Google Scholar]
- 59.Son BK, Akishita M, Iijima K, Ogawa S, Maemura K, Yu J, et al. Androgen receptor-dependent transactivation of growth arrest-specific gene 6 mediates inhibitory effects of testosterone on vascular calcification. J Biol Chem. 2010;285:7537–44. doi: 10.1074/jbc.M109.055087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Neves KR, Graciolli FG, dos Reis LM, Graciolli RG, Neves CL, Magalhaes AO, et al. Vascular calcification: contribution of parathyroid hormone in renal failure. Kid Int. 2007;71:1262–70. doi: 10.1038/sj.ki.5002241. [DOI] [PubMed] [Google Scholar]
- 61.Sebastian EM, Suva LJ, Friedman PA. Differential effects of intermittent PTH(1-34) and PTH(7-34) on bone microarchitecture and aortic calcification in experimental renal failure. Bone. 2008;43:1022–30. doi: 10.1016/j.bone.2008.07.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hruska KA, Mathew S, Lund RJ, Memon I, Saab G. The pathogenesis of vascular calcification in the chronic kidney disease mineral bone disorder: the links between bone and the vasculature. Sem Nephrol. 2009;29:156–65. doi: 10.1016/j.semnephrol.2009.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lim K, Lu TS, Molostvov G, Lee C, Lam FT, Zehnder D, et al. Vascular Klotho deficiency potentiates the development of human artery calcification and mediates resistance to fibroblast growth factor 23. Circulation. 2012;125:2243–55. doi: 10.1161/CIRCULATIONAHA.111.053405. [DOI] [PubMed] [Google Scholar]
- 64.Norman PE, Powell JT. Vitamin D and cardiovascular disease. Circ Res. 2014;114:379–93. doi: 10.1161/CIRCRESAHA.113.301241. [DOI] [PubMed] [Google Scholar]
- 65.Voelkl J, Alesutan I, Leibrock CB, Quintanilla-Martinez L, Kuhn V, Feger M, et al. Spironolactone ameliorates PIT1-dependent vascular osteoinduction in klotho-hypomorphic mice. J Clin Invest. 2013;123:812–22. doi: 10.1172/JCI64093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jaffe IZ, Tintut Y, Newfell BG, Demer LL, Mendelsohn ME. Mineralocorticoid receptor activation promotes vascular cell calcification. Arterioscler Thromb Vasc Biol. 2007;27:799–805. doi: 10.1161/01.ATV.0000258414.59393.89. [DOI] [PubMed] [Google Scholar]