Abstract
Objective
Maternal obesity is associated with increased risk of large-for-gestational-age (LGA) and small-for-gestational-age (SGA) births. Both are related to childhood obesity. We considered that patterns of gestational weight gain (GWG) may help to disentangle these competing risks.
Design and methods
Patterns of GWG were characterized among a cohort of overweight or obese women (n=651). Polytomous logistic regression models tested for associations between GWG patterns and birthweight outcomes: SGA (<10th) and LGA (>90th percentile).
Results
Rates of SGA were higher than those for LGA (14.9% vs. 7.8%). Four GWG patterns were identified: consistently high (29%), early adequate/late high (33%), consistently adequate (18%), and consistently low (20%). Risk of LGA was highest in women with consistently high GWG (adjusted odds ratio [OR] 4.62 [1.53, 13.96]), and risk was elevated, but with lower magnitude, among women with early adequate/late high gains (OR 3.07 [1.01, 9.37]). High GWG before 20 weeks, regardless of later gain, was related to LGA. Low gain before 20 weeks accompanied by high gain later may be associated with reduced SGA risk (0.55 [0.29, 1.07]).
Conclusions
The pattern of weight gain during pregnancy may be an important contributor to or marker of abnormal fetal growth among overweight and obese women.
Keywords: Gestational weight gain, obesity, macrosomia, growth restriction
Introduction
Childhood obesity rates have tripled over the past 30 years, and risk is particularly high among infants born to overweight or obese mothers.1 Childhood obesity has immediate and long-term health consequences, including elevated blood pressure, elevated cholesterol, impaired glucose tolerance, insulin resistance, type 2 diabetes, and cardiovascular disease.2,3 Increasing obesity in young children is a multifactorial problem with intergenerational, intrauterine, environmental, and behavioral components. While maternal obesity increases the risk of gestational diabetes and large-for-gestational age (LGA) infants, it is also associated with hypertension in pregnancy, placental dysfunction, and fetal growth restriction, resulting in small-for-gestational age (SGA) newborns. Both LGA and SGA infants have excess risk of childhood obesity;4-6 gestational weight gain (GWG) may help to differentiate these outcomes among overweight and obese women.
For more than 20 years, the Institute of Medicine (IOM) has recommended lower ranges of GWG for mothers with higher pre-pregnancy body-mass index (BMI) to balance risk for SGA, LGA, and other adverse pregnancy outcomes.7 Pregnancy weight gain above the IOM recommendation is associated with increased risk of macrosomia in offspring, and low gestational gain is associated with growth restriction, even among overweight and obese women.8 Recent evidence suggests that weight gain in the first half of pregnancy may be more strongly related than weight gain in the second half of pregnancy to higher newborn body fat and offspring BMI at ages 7 and 9.9-11 These studies have been conducted in predominantly normal weight women, and the relationship between the trajectory of GWG and fetal growth among overweight and obese women is not well understood. This is important as the offspring of overweight and obese women are at high risk for childhood obesity.
Racial or ethnic differences in weight-related pregnancy outcomes are also well established. African American women have a greater risk for low GWG and SGA, despite having higher rates of pre-pregnancy obesity compared to White women.12 The 1990 IOM Committee encouraged pregnant African American women to gain at the upper end of the recommended ranges, but insufficient evidence that this recommendation improved pregnancy weight gain or SGA led the 2009 IOM Committee to abandon this race-specific guidance. A recent report evaluating several adverse pregnancy outcomes in normal-weight women did not find evidence that optimal GWG among African American women should be higher than that recommended for their Caucasian counterparts, despite the fact that low GWG was associated with higher SGA risk among African American women.13
The effects of race, BMI, and GWG on fetal growth are complex, especially in women who are overweight or obese prior to pregnancy. We considered that the pattern of GWG in early and late gestation may help to clarify these associations. We examined the association between GWG patterns and birth weight outcomes (SGA or LGA infants) in a cohort of 651 predominantly overweight and obese women. We hypothesized that weight gain in the first half of pregnancy is more strongly associated with abnormalities of fetal growth at delivery than weight gained in the second half of pregnancy.
Methods
The Pregnancy Evaluation and Preeclampsia Prevention-3 (PEPP-3) study recruited women from the outpatient clinic at Magee-Womens Hospital, Pittsburgh, PA, before 16 weeks' gestation and followed them through delivery. PEPP-3 was designed to study how pre-pregnancy adiposity is associated with preeclampsia risk; thus, predominantly overweight and obese women were enrolled, with a small number of normal weight women included as controls (approximately 1:6 ratio). By design, nulliparous women were oversampled. A total of 875 women enrolled in PEPP-3, and 651 (74%) delivered and were included in this analysis. Not included were those with a pregnancy loss (n=75), lost to follow up (n=56), those who planned to deliver at another hospital (n=71) or were ineligible due to preexisting diabetes, hypertension or renal disease, or multiple gestation (n=21). The study was approved by the University of Pittsburgh Institutional Review Board, and all participants provided written informed consent.
Enrollment occurred, on average, at 9.2 ± 2.6 weeks' gestation, and the women completed a structured interview that included self-reported pre-pregnancy weight. Height and weight were measured at this enrollment visit, following a research protocol, and weight, assessed at every prenatal visit, was gathered from the medical record. There were, on average, 10.5 ± 2.6 weights available for each participant (range 3-21). Pre-pregnancy self-reported weight was highly correlated with the first measured weight in pregnancy (r=0.99, mean difference 1.8 ± 6.7 pounds) and was used to calculate GWG. Maternal delivery weight was abstracted from the medical record, and when missing, the last prenatal weight prior to delivery was used (if less than 27 days prior to delivery). Pre-pregnancy BMI [self-reported pregravid weight (kg)/measured height (m)2] was categorized as normal weight (18.5-24.9), overweight (25-29.9), obese I (30-34.9), obese II (35-39.9) and obese III (>=40).
Assessment of Gestational Weight Gain
Adequacy of GWG at delivery (delivery weight minus pre-pregnancy weight) was classified as low, adequate, or high, based on the 2009 IOM recommendations at the gestational age of delivery.7 These recommendations are specific to a woman's pre-pregnancy BMI and were projected to gestational age at delivery using a recommended gain of 1.1-4.4 pounds in the first trimester with recommended weekly gain(s) in the second and third trimesters of 1 pound/week (range 1.0-1.3 pounds) for underweight, 1 pound/week (range 0.8-1.0 pounds) for normal-weight, 0.6 pounds/week (range 0.5-0.7 pounds) for overweight, and 0.5 pounds/week (range 0.4-0.6 pounds) for obese women. Adequacy of weight gain in the first and second halves of pregnancy was classified using these recommended ranges applied to gain up to 20 weeks' gestational age, and again from 20 weeks' to delivery. We explored several approaches to combining these two components of weight gain to characterize observed patterns. We examined all combinations of weight gain in the first and second halves of pregnancy (Figure 1) and detected four dominant patterns (consistently low, consistently adequate [including women who, early, gained low and, then, adequately], early adequate or low/late high, and consistently high). There were small numbers of women who gained more rapidly in the first compared to the second half of gestation (from adequate to low or from high to low/adequate, n=39). These were grouped on the basis of delivery weight gain status as the numbers were too small to analyze separately. Results were replicated after removing this group to ensure that their patterns did not bias the results.
Figure 1. Study participants according to GWG in the first (up to 20 weeks') and second half of pregnancy (20 weeks to delivery), Pregnancy Evaluation and Preeclampsia Prevention Study, 2008-2013.
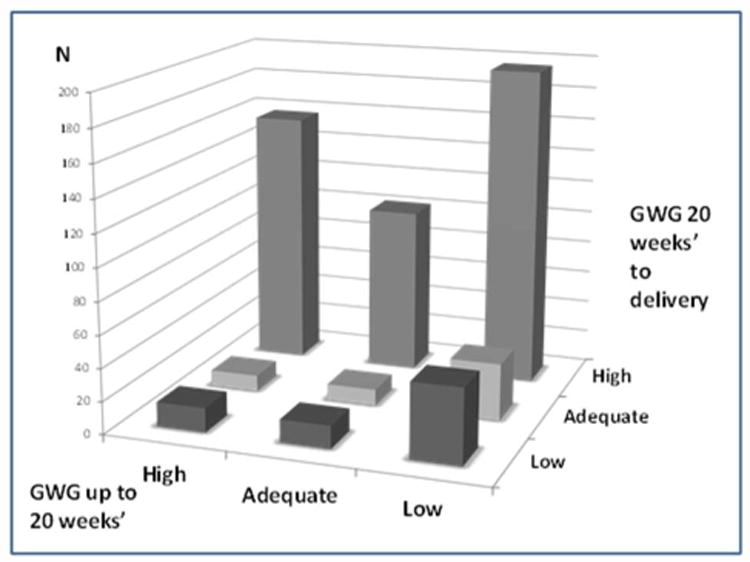
For a clear graphical representation of weight-gain trends across gestation, we calculated standardized GWG values specific to pre-pregnancy BMI, using reference ranges published by Hutcheon et al.14 Zero reflects GWG equivalent to the expected gain; a positive standardized GWG reflects gain above that expected, while a negative value reflects gain below that expected. We calculated this value for each visit and then selected the nearest observation to each 4-week interval from 12 through 40 weeks to create a plot. Women with preterm delivery contributed observations until the last prenatal visit prior to delivery. We also used this standardized GWG approach to examine patterns across gestation. The patterns identified were not different from those identified when combining early and late gestational weight gain, and we opted to present results using the early/late gain as these may be more clinically meaningful.
Outcomes were SGA (<10th percentile) or LGA (greater than the 90th percentile), using a global reference that relies upon the fetal-weight reference developed by Hadlock,15 which was then customized to U.S. White and African American mean ± SD birth weights from 1995-2004.16,17 Adequate-for-gestational-age infants (AGA; 10th-90th percentile) were the referent group.
Covariates collected at the enrollment interview were maternal age; race/ethnicity (self-reported as African American, Caucasian, or other); married or living with a partner; education (less than high school, high school or equivalent, college or more); income (less than $20K, $20K to less than $50K, more than $50K, unknown or refused); insurance status at the enrollment visit (private or Veterans Administration, Medicaid, no insurance), smoking at enrollment visit (any vs. none), and nulliparity. Factors abstracted from the medical record included gestational age at delivery (based predominantly on ultrasound), birth weight, infant sex, and diagnosis of gestational diabetes mellitus (GDM) using the Carpenter and Couston criteria.18 Preeclampsia was defined as new hypertension in pregnancy and proteinuria after 20 weeks, and gestational hypertension was defined as new hypertension in pregnancy without proteinuria.
Statistical analysis
Maternal characteristics were evaluated according to SGA, AGA, and LGA outcomes and to GWG patterns as mean ± SD for continuous data and percentages for categorical variables. Polytomous logistic regression (also known as multinomial logistic regression) is an extension of the typical binary logistic regression model to a dependent variable with more than 2 possible responses (in this case, SGA, AGA, and LGA birth weight). Polytomous logistic regression models were used to describe the relationship between each of the GWG patterns and risk of SGA or LGA. We then modeled the risk of these outcomes relative to GWG in the first 20 weeks, adjusted for gain in the second half of pregnancy. Covariates having known associations with fetal growth—e.g., age, race/ethnicity, pre-pregnancy BMI, parity, smoking, preeclampsia, and GDM—were selected a priori. We also tested for effect measure modification by maternal race/ethnicity and pre-pregnancy BMI by incorporating interaction terms for each potential effect modifier and GWG pattern (using indicator variables for each potential level of each variable).
Results
Overall, the cohort was predominantly African American, low income, publicly insured, nulliparous, and obese prior to pregnancy, with high rates of smoking (Table 1). There were 97 SGA births (14.9%), 51 LGA births (7.8%) and 503 AGA births. Women with SGA births tended to be younger, delivered infants, on average, 1 week earlier, and were more likely to develop preeclampsia than women with AGA births. Women with LGA births were more likely to have a higher pre-pregnancy BMI and were on average older than women with AGA births. Women with consistently low gain were more likely to be African American, publicly insured, multiparous, and heavier prior to pregnancy (Table 2). Women with consistently high gain were more likely to be White, nulliparous and to have high rates of preterm delivery.
Table 1. Maternal characteristic according to small-, adequate-, and large-for-gestational-age births.
| Characteristic | Total (N=651) | SGA (N=97) | AGA (N=503) | LGA (N=51) | p-value |
|---|---|---|---|---|---|
| Age (Years)* | 23.7, 4.2 | 22.9, 4.4 | 23.7, 4.1 | 25.0, 4.6 | 0.015 |
| Race, % | 0.392 | ||||
| African American | 63.3 | 70.1 | 61.4 | 68.6 | |
| White | 34.4 | 26.8 | 36.4 | 29.4 | |
| Other | 2.3 | 3.1 | 2.2 | 2.0 | |
| Married or living with partner, % | 36.3 | 28.9 | 38.2 | 31.4 | 0.163 |
| Education, % | 0.245 | ||||
| Less Than HS | 9.2 | 11.3 | 8.5 | 11.8 | |
| HS Diploma or GED | 49.6 | 56.7 | 49.3 | 39.2 | |
| College Education | 39.0 | 28.9 | 40.4 | 45.1 | |
| Graduate or Professional | 2.2 | 3.1 | 1.8 | 3.9 | |
| Income, % | 0.240 | ||||
| Less Than $20,000 | 52.1 | 51.5 | 52.3 | 51 | |
| $20,000-$49,999 | 20.6 | 13.4 | 21.7 | 23.5 | |
| More Than $50,000 | 5.7 | 4.1 | 6.0 | 5.9 | |
| Don't Know/Refused | 21.7 | 30.9 | 20.1 | 19.6 | |
| Insurance, % | 0.903 | ||||
| Private | 10.2 | 7.4 | 10.4 | 13.7 | |
| Medical Assistance | 57.7 | 60.6 | 57.2 | 56.9 | |
| No Insurance | 31.2 | 30.9 | 31.4 | 29.4 | |
| Unknown | 0.5 | 0.0 | 0.6 | 0.0 | |
| Smoking, % | 44.5 | 46.4 | 44.5 | 41.2 | 0.831 |
| Nulliparous, % | 77.6 | 87.6 | 75.7 | 76.5 | 0.036 |
| Prepregnancy BMI (kg/m2)* | 32.3, 8.0 | 32.2, 7.6 | 32.1, 8.0 | 34.7, 8.6 | 0.080 |
| Prepregnancy BMI Category, % | 0.821 | ||||
| Normal | 17.1 | 17.5 | 17.3 | 13.7 | |
| Overweight | 24.0 | 23.7 | 24.7 | 17.6 | |
| Class 1 Obese | 28.1 | 27.8 | 28.2 | 27.5 | |
| Class 2 Obese | 15.4 | 17.5 | 14.7 | 17.6 | |
| Class 3 Obese | 15.5 | 13.4 | 15.1 | 23.5 | |
| Gestational Age, Enrollment (wks)* | 9.2, 2.8 | 9.6, 2.8 | 9.1, 2.8 | 9.1, 2.7 | 0.315 |
| Gestational Age, Delivery (wks)* | 39.0, 2.4 | 38.3, 3.8 | 39.1, 2.1 | 38.9, 2.2 | 0.007 |
| Preterm Birth (< 37 Weeks), % | 11.1 | 15.5 | 9.9 | 13.7 | 0.232 |
| Preeclampsia, % | 8.3 | 19.6 | 6.2 | 7.8 | <.001 |
| Gestational Hypertension, % | 11.1 | 11.3 | 11.2 | 9.8 | 0.953 |
| Gestational Diabetes, % | 2.8 | 0.0 | 3.4 | 2.0 | 0.164 |
Mean, SD [standard deviation]
Table 2. Maternal characteristics according to gestational weight gain patterns.
| Characteristic | Low (N=130) | Adequate (N=119) | Consistently High (N=187) | Early Adequate/Late High (N=215) | p-value |
|---|---|---|---|---|---|
| Age (Years)* | 24.3, 4.7 | 23.6, 4.0 | 24.0, 4.5 | 23.1, 3.7 | 0.037 |
| Race, % | |||||
| African American | 71.3 | 68.9 | 56.1 | 62.1 | 0.091 |
| White | 27.1 | 27.7 | 41.7 | 35.5 | |
| Other | 1.6 | 3.4 | 2.1 | 2.3 | |
| Married or living with partner, % | 34.9 | 31.9 | 35.3 | 40.2 | 0.462 |
| Education, % | |||||
| Less Than HS | 10.9 | 10.9 | 9.6 | 7.0 | 0.704 |
| HS Diploma or GED | 51.9 | 44.5 | 48.1 | 52.3 | |
| College Education | 34.9 | 41.2 | 39.6 | 39.7 | |
| Graduate or Professional | 2.3 | 3.4 | 2.7 | 0.9 | |
| Income, % | |||||
| Less Than $20,000 | 52.7 | 56.3 | 46.5 | 54.2 | 0.566 |
| $20,000-$49,999 | 17.8 | 20.2 | 22.5 | 20.6 | |
| More Than $50,000 | 3.9 | 5 | 5.9 | 7 | |
| Don't Know/Refused | 25.6 | 18.5 | 25.1 | 18.2 | |
| Insurance, % | |||||
| Private | 7.1 | 5.9 | 13.5 | 11.8 | 0.026 |
| Medical Assistance | 64.6 | 63.0 | 55.1 | 52.4 | |
| No Insurance | 28.3 | 29.4 | 29.7 | 35.4 | |
| Unknown | 0.0 | 0.0 | 1.6 | 0.0 | |
| Smoking, % | 45.7 | 38.7 | 51.9 | 41.1 | 0.078 |
| Nulliparous, % | 67.4 | 73.9 | 81.8 | 82.2 | 0.004 |
| Prepregnancy BMI (kg/m2)* | 36.5, 9.0 | 31.3, 7.1 | 31.3, 6.4 | 31.3, 8.3 | <.001 |
| Prepregnancy BMI Category, % | |||||
| Normal | 10.1 | 20.2 | 15.0 | 21.0 | <.001 |
| Overweight | 14.0 | 26.9 | 26.7 | 26.2 | |
| Class 1 Obese | 24.0 | 25.2 | 34.2 | 26.6 | |
| Class 2 Obese | 20.2 | 13.4 | 16.0 | 13.1 | |
| Class 3 Obese | 31.8 | 14.3 | 8.0 | 13.1 | |
| Gestational Age, Delivery (wks)* | 38.8, 2.8 | 38.9, 2.5 | 38.8, 2.6 | 39.3, 1.8 | 0.119 |
| Preterm Birth (<37 weeks), % | 11.6 | 8.4 | 16.0 | 7.9 | 0.052 |
| Preeclampsia, % | 5.4 | 8.4 | 8.6 | 9.9 | 0.553 |
| Gestational Hypertension, % | 12.4 | 5.0 | 13.4 | 11.7 | 0.124 |
| Gestational Diabetes, % | 3.9 | 2.5 | 3.8 | 0.9 | 0.250 |
Mean, SD [standard deviation]
The prevalence of GWG patterns in the first and second halves of pregnancy are shown in Figure 1. Almost half of this predominantly overweight/obese cohort of pregnant women gained less than the recommended amount of weight before 20 weeks. Only 22.0% gained in the recommended range and 30.7% gained above the recommended range in the first half of pregnancy. By delivery, these numbers were reversed with 20.0% having low GWG, 18.2% adequate GWG, and the majority (61.8%) having high GWG (n=402). Of the women with high gain, there were two dominant patterns: those with consistently high gain across pregnancy and those with low or adequate gain for the first 20 weeks and high gain later (Figure 2).
Figure 2. Patterns of gestational weight gain across pregnancy, Pregnancy Evaluation and Preeclampsia Prevention Study, 2008-2013.
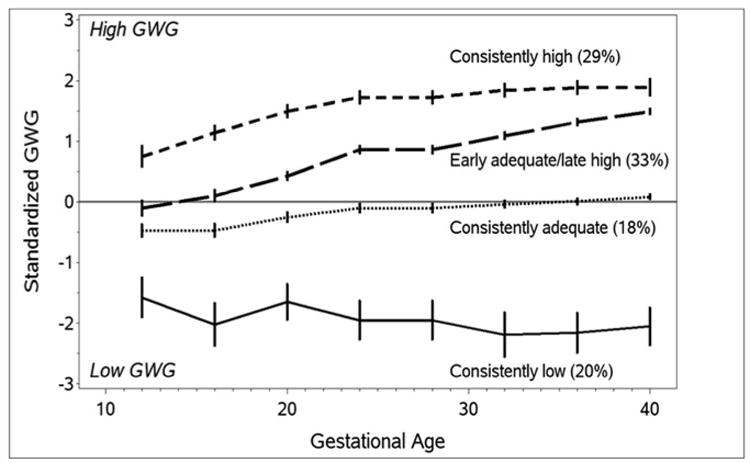
The prevalence of SGA and LGA differed in our cohort according to these patterns of weight gain (Table 3). As expected, the group with consistently low gain had the highest rate of SGA (20.0%) and lowest rate of LGA (0.7%). Women with high weight gain across gestation had the highest rate of LGA births (13.4%). The early adequate/late high gain group had the lowest rate of SGA (11.6%) and a similar rate of LGA (9.8%). After accounting for maternal age, race, pre-pregnancy BMI, parity, smoking, preeclampsia, and GDM, women with consistently high GWG had a very high risk of LGA (OR 4.62, 95% CI 1.53, 13.96) compared to those with adequate gain. LGA risk was also high for the early adequate/late high gainers (OR 3.07, 95% CI 1.01, 9.37) and SGA risk was lowest and borderline significant compared to women with adequate gain (OR 0.55, 95% CI 0.29, 1.07). Results were similar after excluding women with who gained more rapidly in the first compared to the second half of gestation (Table 4) and also after excluding women with preterm delivery, gestational diabetes, or preeclampsia.
Table 3. The association between GWG patterns and birth weight outcomes (SGA/LGA).
| SGA | LGA | |||||||
|---|---|---|---|---|---|---|---|---|
|
|
||||||||
| n (%) | Odds Ratio | 95% CI | n (%) | Odds Ratio | 95% CI | |||
| Lower | Upper | Lower | Upper | |||||
| Low (n=130) | 26 (20.0) | 1.21 | 0.62 | 2.39 | 1 (0.7) | 0.15 | 0.02 | 1.42 |
| Adequate (n=119) | 22 (18.5) | 1.00 | (referent) | 4 (3.4) | 1.00 | (referent) | ||
| Consistently High (n=187) | 24 (12.8) | 0.69 | 0.36 | 1.34 | 25 (13.4) | 4.62 | 1.53 | 13.96 |
| Early Adequate/Late High (n=215) | 25 (11.6) | 0.55 | 0.29 | 1.07 | 21 (9.8) | 3.07 | 1.01 | 9.37 |
Model: polytomous logistic regression, exposure: GWG patterns, outcome: birth weight category, referent outcome group: AGA infant. Data are presented as odds ratios (95% confidence intervals). All effects are adjusted for age, race, pre-pregnancy BMI, parity, smoking, preeclampsia, and GDM
Table 4.
The association between GWG patterns and birth weight outcomes (SGA/LGA), excluding 39 women who gained more rapidly in the first compared to the second half of gestation (from adequate to low GWG, or from high to low/adequate GWG).
| SGA | LGA | |||||||
|---|---|---|---|---|---|---|---|---|
|
|
||||||||
| n (%) | Odds Ratio | 95% CI | n (%) | Odds Ratio | 95 % CI | |||
| Lower | Upper | Lower | Upper | |||||
| Low (n=120) | 22 (18.3) | 1.43 | 0.67 | 3.03 | 1 (0.8) | 0.14 | 0.01 | 1.32 |
| Adequate (n=104) | 16 (15.4) | 1.00 | (referent) | 4 (3.9) | 1.00 | (referent) | ||
| Consistently High (n=173) | 22 (12.7) | 0.87 | 0.42 | 1.80 | 25 (14.5) | 4.65 | 1.52 | 14.19 |
| Early Adequate/Late High (n=215) | 25 (11.7) | 0.69 | 0.34 | 1.41 | 21 (9.8) | 2.78 | 0.90 | 8.55 |
Model: polytomous logistic regression, exposure: GWG patterns, outcome: birthweight category, referent outcome group: AGA infant. Data are presented as odds ratios (95% confidence intervals). Model adjusted for age, race, pre-pregnancy BMI, parity, smoking, preeclampsia, and GDM.
We then modeled the association between GWG in the first 20 weeks of pregnancy and SGA or LGA risk, adjusting for later pregnancy weight gain (Table 5). Low GWG in the first 20 weeks was modestly associated with SGA risk but confidence limits were wide (1.46, 95% CI 0.77, 2.77). In contrast, early high GWG was associated with a 2.93-fold excess risk of LGA (95% CI 1.16, 7.41), regardless of gain in the second half of pregnancy. There was no evidence of effect measure modification in any models by race/ethnicity or pre-pregnancy BMI.
Table 5. The association between adequacy of GWG in the first half of pregnancy and birth weight outcomes (SGA/LGA), adjusting for GWG in the second half of pregnancy.
| SGA | LGA | |||||
|---|---|---|---|---|---|---|
| Odds Ratio | 95% CI | Odds Ratio | 95% CI | |||
| Lower | Upper | Lower | Upper | |||
| Low (n=308) | 1.46 | 0.77 | 2.77 | 1.26 | 0.48 | 3.31 |
| Adequate (n=143) | 1.00 | (referent) | 1.00 | (referent) | ||
| High (n=200) | 1.57 | 0.79 | 3.13 | 2.93 | 1.16 | 7.41 |
Model: polytomous logistic regression, exposure: adequacy of GWG in first 20 weeks, outcome: birth weight category, referent outcome group: AGA infant. Data are presented as odds ratios (95% confidence intervals). Model adjusted for age, race, pre-pregnancy BMI, parity, smoking, preeclampsia, GDM, and adequacy of weight gain from 20 weeks to delivery.
Discussion
Our findings reveal four distinct patterns of gestational weight gain among overweight and obese women that may be important contributors to abnormal fetal growth. High gain in the first half of pregnancy occurred in 30% of women and was independently associated with LGA regardless of later gain. Low GWG in the first 20 weeks of pregnancy was also common but may not be associated with SGA when accompanied by high gain later in gestation. Indeed, the pattern associated with similar risk for both SGA and LGA was low early gain accompanied by high gain in the second half of pregnancy.
The mechanisms by which high early pregnancy weight gain may be related to LGA are still not understood. Excess substrate, perhaps related to insulin resistance, could program a fetus to be large, or perhaps the maternal metabolic profile is established early in gestation, thus adversely affecting the likelihood of the fetus gaining soft tissue in the latter half of gestation.19 It is also possible that LGA or fetal overgrowth is driven less by nutrition in the first half of pregnancy than by vascular factors related to placentation that are modulated by maternal adiposity.20
In addition to being less insulin sensitive, obese pregnant women have an impaired cholesterol response,21 higher blood pressure,22 less visceral fat, higher oxidized LDL, and evidence of chronic placental inflammation compared to normal-weight women.23-25, 26, 27, 28 Although pre-gravid weight is the primary determinant of maternal leptin during pregnancy, among obese women the gestational increase in leptin is lower than among normal weight women.29,30 Few studies have related these factors to fetal growth, but triglycerides, HDL-C, and leptin may have unique or opposite effects on fetal growth in obese women than they do in normal-weight women.31, 24, 32,33 It is possible that early GWG may reflect these and other factors such as glucose, adipokines and inflammation. For example, weight gain in the first trimester (but not after) is associated with post-load maternal glucose concentrations,34 and it is well established that excess glucose fuels overgrowth and perhaps induces epigenetic effects that predispose to childhood obesity.35
The link between early pregnancy weight gain and offspring risk for childhood obesity has been reported in at least two longitudinal cohorts.9,11 Others have observed that pre-pregnancy obesity is more importantly related to newborn fatness assessed by skin calipers than GWG.36 While we were unable to directly measure fetal adiposity as in prior studies, our results do suggest that fetal growth may be affected by the time during pregnancy that weight gain occurs.
Our results also suggest that early GWG (before 20 weeks), while strongly related to LGA, is more modestly associated (if at all) with SGA risk unless accompanied by low GWG later on. Of note, the rate of SGA in this cohort was almost two times higher than the rate of LGA, which was unexpected given the excess risk of fetal overgrowth associated with maternal obesity. The pattern of GWG may help explain these outcomes. For example, obese women who lose weight during pregnancy have excess SGA risk,37,38 and GWG below the recommended ranges among obese women increases risk of SGA from 2.7% to 8.8%.39 Factors associated with low GWG and high rates of SGA among overweight and obese women are unknown, but race/ethnicity, stress, inflammation, and preeclampsia are risk factors for poor fetal growth that deserve further examination in studies that link maternal biomarkers, epigenetic changes, and placental function to abnormal fetal growth. Such studies may also shed light on race disparities in pregnancy weight-related outcomes, as African-American women are at highest risk of obesity and low GWG and of delivering SGA infants.
Several limitations of our study deserve mention. We did not have direct measures of soft tissue fat mass in newborns and relied upon birth weight-derived definitions of abnormal fetal growth. We did, however, use a recently developed global reference for fetal-weight which has been shown to improve classification of abnormal newborn size at birth and allows for comparability to other population studies of fetal growth.16 In the absence of guidance as to whether SGA and LGA are associated with equivalent offspring sequelae, we evaluated them as comparable, competing risks. To better understand the long-term consequences associated with the weight gain patterns during pregnancy that we detected, follow-up studies are needed that will evaluate childhood weight gain trajectories and maternal postpartum weight retention among overweight and obese mother-child dyads. For example, there may be metabolic consequences among mothers and their children following the GWG pattern of low early/late high gain. In addition, there were women with more rapid weight gain in the first compared to the second half of pregnancy (6%), and while this may be an informative GWG pattern we were unable to model risk for SGA or LGA in this group due to small numbers. Also, prevalence of adequate GWG was low and there were only 4 LGA events within that group. Strengths of our study included a large, well-characterized prospective cohort of obese and overweight women with universal GDM screening and rigorously adjudicated hypertensive complications.
In summary, our results demonstrate that the pattern of weight gain during pregnancy may be an important contributor to, or marker of, abnormal fetal growth among overweight and obese women. Low weight gain early in pregnancy may not be detrimental when accompanied by higher gain later in pregnancy. In contrast, high gain early in gestation may be importantly related to risk of fetal overgrowth regardless of later gain. How the weight gain patterns we detected may be related to the maternal metabolic milieu during pregnancy and to later-life obesity risk for mother or child requires further study. Recommendations to further lower the target for pregnancy weight gain in obese women are not supported by our findings. Instead, setting targets for the first and second half of pregnancy may be clinically useful, especially for overweight and obese women.
What is already known about this subject.
Offspring of overweight and obese mothers have a high risk of pediatric obesity
Maternal obesity is related to increased risk of both large- and small-for-gestational-age infants, and both are associated with pediatric obesity risk
The pattern of gestational weight gain contributes to offspring obesity in normal-weight women.
What this study adds.
In a cohort of predominantly overweight or obese mothers, there were more small- than large-for-gestational-age births
High gestational weight gain before 20 weeks, regardless of gain later in pregnancy, was related to large-for-gestational-age births
The pattern of weight gain during pregnancy may be an important contributor to or marker of abnormal fetal growth among overweight and obese women
Acknowledgments
The authors would like to thank Bruce R. Campbell, MLS, from the Magee-Womens Research Institute, for his editing assistance in the preparation of this manuscript.
Funding source: This work was supported by the National Institutes of Health grants P01HD030367 (CAH) and UL1RR024153 and UL1TR000005 (University of Pittsburgh Clinical and Translational Science Institute).
IRB# MOD08050339-24/PRO08050339
Footnotes
Author contributions: Janet Catov, Diane Abatemarco, and Carl Hubel performed the research. Janet Catov and Diane Abatemarco designed the study. Carl Hubel, Andrew Althouse, and Esa Davis contributed essential reagents or tools and made scientific contributions to the analysis and interpretation of the data. Janet Catov and Andrew Althouse analyzed the data. Janet Catov wrote the paper. All authors had final approval of the submitted paper.
The authors report no conflicts of interest.
References
- 1.Druet C, Stettler N, Sharp S, et al. Prediction of childhood obesity by infancy weight gain: an individual-level meta-analysis. Paediatr Perinat Epidemiol. 2012 Jan;26(1):19–26. doi: 10.1111/j.1365-3016.2011.01213.x. [DOI] [PubMed] [Google Scholar]
- 2.Freedman DS, Mei ZG, Srinivasan SR, Berenson GS, Dietz WH. Cardiovascular risk factors and excess adiposity among overweight children and adolescents: The Bogalusa Heart Study. J Pediatr-Us. 2007 Jan;150(1):12–17. doi: 10.1016/j.jpeds.2006.08.042. [DOI] [PubMed] [Google Scholar]
- 3.Han JC, Lawlor DA, Kimm SYS. Childhood obesity. Lancet. 2010 May 15;375(9727):1737–1748. doi: 10.1016/S0140-6736(10)60171-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Catalano PM, Hauguel-De Mouzon S. Is it time to revisit the Pedersen hypothesis in the face of the obesity epidemic? Am J Obstet Gynecol. 2011 Jun;204(6):479–487. doi: 10.1016/j.ajog.2010.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ibanez L, Lopez-Bermejo A, Suarez L, Marcos MV, Diaz M, de Zegher F. Visceral Adiposity without Overweight in Children Born Small for Gestational Age. Journal of Clinical Endocrinology and Metabolism. 2008 Jun 1;93(6):2079–2083. doi: 10.1210/jc.2007-2850. [DOI] [PubMed] [Google Scholar]
- 6.Jaquet D, Deghmoun S, Chevenne D, Collin D, Czernichow P, Lévy-Marchal C. Dynamic change in adiposity from fetal to postnatal life is involved in the metabolic syndrome associated with reduced fetal growth. Diabetologia. 2005 May 01;48(5):849–855. doi: 10.1007/s00125-005-1724-4. [DOI] [PubMed] [Google Scholar]
- 7.Rasmussen K, Yaktine A, editors. Weight gain during pregnancy: reexamining the recommendations. Washington, DC: The National Academies Press; 2009. [PubMed] [Google Scholar]
- 8.Catalano PM, Mele L, Landon MB, et al. Inadequate weight gain in overweight and obese pregnant women: what is the effect on fetal growth? Am J Obstet Gynecol. 2014 Feb 11; doi: 10.1016/j.ajog.2014.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Andersen C, Gamborg M, Sorensen T, EA N. Weight gain in different periods of pregnancy and offspring's body mass index at 7 years of age. Int J Pediatr Obes. 2011;6:e179–186. doi: 10.3109/17477166.2010.521560. [DOI] [PubMed] [Google Scholar]
- 10.Davenport M, Ruchat S, Giroux I, Sopper M, Mottola M. Timing of Excessive Pregnancy-Related Weight Gain and Offspring Adiposity at Birth. Obstet Gynecol. 2013;122:255–261. doi: 10.1097/AOG.0b013e31829a3b86. [DOI] [PubMed] [Google Scholar]
- 11.Fraser A, Tilling K, Macdonald-Wallis C, et al. Association of Maternal Weight Gain in Pregnancy With Offspring Obesity and Metabolic and Vascular Traits in Childhood. Circulation. 2010 Jun 15;121(23):2557–U2548. doi: 10.1161/CIRCULATIONAHA.109.906081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davis E, Stange K, Horwitz R. Childbearing, Stress and Obesity Disparities in Women: A Public Health Perspective. Maternal and Child Health Journal. 2012 Jan 01;16(1):109–118. doi: 10.1007/s10995-010-0712-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bodnar LM, Hutcheon JA, Platt RW, Himes KP, Simhan HN, Abrams B. Should gestational weight gain recommendations be tailored by maternal characteristics? Am J Epidemiol. 2011 Jul 15;174(2):136–146. doi: 10.1093/aje/kwr064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hutcheon JA, Platt RW, Abrams B, Himes KP, Simhan HN, Bodnar LM. A weight-gain-for-gestational-age z score chart for the assessment of maternal weight gain in pregnancy. The American Journal of Clinical Nutrition. 2013 May 1;97(5):1062–1067. doi: 10.3945/ajcn.112.051706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hadlock FP, Harrist RB, Martinez-Poyer J. In utero analysis of fetal growth: A sonographic weight standard. Radiology. 1991;181(1):129–133. doi: 10.1148/radiology.181.1.1887021. [DOI] [PubMed] [Google Scholar]
- 16.Ding G, Tian Y, Zhang Y, Pang Y, Zhang JS, Zhang J. Application of A global reference for fetal-weight and birthweight percentiles in predicting infant mortality. BJOG: An International Journal of Obstetrics & Gynaecology. 2013;120(13):1613–1621. doi: 10.1111/1471-0528.12381. [DOI] [PubMed] [Google Scholar]
- 17.Mikolajczyk RT, Zhang J, Betran AP, et al. A global reference for fetal-weight and birthweight percentiles. The Lancet. 377(9780):1855–1861. doi: 10.1016/S0140-6736(11)60364-4. [DOI] [PubMed] [Google Scholar]
- 18.Carpenter M, Coustan D. Criteria for screening tests for gestational diabetes. Am J Obstet Gynecol. 1982;144:768–773. doi: 10.1016/0002-9378(82)90349-0. [DOI] [PubMed] [Google Scholar]
- 19.Catalano PM, Ehrenberg HM. The short- and long-term implications of maternal obesity on the mother and her offspring. Bjog-Int J Obstet Gy. 2006 Oct;113(10):1126–1133. doi: 10.1111/j.1471-0528.2006.00989.x. [DOI] [PubMed] [Google Scholar]
- 20.King JC. Maternal obesity, metabolism, and pregnancy outcomes. Annu Rev Nutr. 2006;26:271–291. doi: 10.1146/annurev.nutr.24.012003.132249. [DOI] [PubMed] [Google Scholar]
- 21.Scifres CM, Catov JM, Simhan HN. The impact of maternal obesity and gestational weight gain on early and mid-pregnancy lipid profiles. Obesity (Silver Spring) 2014 Mar;22(3):932–938. doi: 10.1002/oby.20576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scifres CM, Chen B, Nelson DM, Sadovsky Y. Fatty acid binding protein 4 regulates intracellular lipid accumulation in human trophoblasts. J Clin Endocrinol Metab. 2011 Jul;96(7):E1083–1091. doi: 10.1210/jc.2010-2084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Straughen JK, Trudeau S, Misra VK. Changes in adipose tissue distribution during pregnancy in overweight and obese compared with normal weight women. Nutrition & Diabetes. 2013 Aug 26;3:e84. doi: 10.1038/nutd.2013.25. online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Misra VK, Trudeau S, Perni U. Maternal Serum Lipids During Pregnancy and Infant Birth Weight: The Influence of Prepregnancy BMI. Obesity. 2011;19(7):1476–1481. doi: 10.1038/oby.2011.43. [DOI] [PubMed] [Google Scholar]
- 25.Meyer BJ, Stewart FM, Brown EA, et al. Maternal Obesity Is Associated With the Formation of Small Dense LDL and Hypoadiponectinemia in the Third Trimester. Journal of Clinical Endocrinology & Metabolism. 2013 Feb;98(2):643–652. doi: 10.1210/jc.2012-3481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Becroft DM, Thompson JM, Mitchell EA. Placental villitis of unknown origin: Epidemiologic associations. American Journal of Obstetrics and Gynecology. 2005;192(1):264–271. doi: 10.1016/j.ajog.2004.06.062. [DOI] [PubMed] [Google Scholar]
- 27.Challier JC, Basu S, Bintein T, et al. Obesity in pregnancy stimulates macrophage accumulation and inflammation in the placenta. Placenta. 2008 Mar;29(3):274–281. doi: 10.1016/j.placenta.2007.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Radaelli T, Varastehpour A, Catalano P, Hauguel-de Mouzon S. Gestational Diabetes Induces Placental Genes for Chronic Stress and Inflammatory Pathways. Diabetes. 2003 Dec 1;52(12):2951–2958. doi: 10.2337/diabetes.52.12.2951. [DOI] [PubMed] [Google Scholar]
- 29.Maple-Brown L, Ye C, Hanley A, et al. Maternal Pregravid Weight Is the Primary Determinant of Serum Leptin and Its Metabolic Associations in Pregnancy, Irrespective of Gestational Glucose Tolerance Status. The Journal of Clinical Endocrinology & Metabolism. 2012;97(11):4148–4155. doi: 10.1210/jc.2012-2290. [DOI] [PubMed] [Google Scholar]
- 30.Misra VK, Trudeau S. The Influence of Overweight and Obesity on Longitudinal Trends in Maternal Serum Leptin Levels During Pregnancy. Obesity. 2011;19(2):416–421. doi: 10.1038/oby.2010.172. [DOI] [PubMed] [Google Scholar]
- 31.Harmon KA, Gerard L, Jensen DR, et al. Continuous Glucose Profiles in Obese and Normal-Weight Pregnant Women on a Controlled Diet. Diabetes Care. 2011 Oct;34(10):2198–2204. doi: 10.2337/dc11-0723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Misra VK, Straughen JK, Trudeau S. Maternal serum leptin during pregnancy and infant birth weight: the influence of maternal overweight and obesity. Obesity (Silver Spring) 2013 May;21(5):1064–1069. doi: 10.1002/oby.20128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Farley DM, Choi J, Dudley DJ, et al. Placental Amino Acid Transport and Placental Leptin Resistance in Pregnancies Complicated by Maternal Obesity. Placenta. 2010 Aug;31(8):718–724. doi: 10.1016/j.placenta.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 34.Tomedi L, Simhan H, Chang CC, McTigue K, Bodnar L. Gestational Weight Gain, Early Pregnancy Maternal Adiposity Distribution, and Maternal Hyperglycemia. Maternal and Child Health Journal. 2014 Jul 01;18(5):1265–1270. doi: 10.1007/s10995-013-1361-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lawlor DA. The Society for Social Medicine John Pemberton Lecture 2011. Developmental overnutrition--an old hypothesis with new importance? Int J Epidemiol. 2013 Feb;42(1):7–29. doi: 10.1093/ije/dys209. [DOI] [PubMed] [Google Scholar]
- 36.Waters T, Huston-Presley L, Catalano P. Neonatal Body Composition According to the Revised Institute of Medicine Recommendations for Maternal Weight Gain. The Journal of Clinical Endocrinology & Metabolism. 2012;97(10):3648–3654. doi: 10.1210/jc.2012-1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Beyerlein A, Schiessl B, Lack N, von Kries R. Associations of gestational weight loss with birth-related outcome: a retrospective cohort study. BJOG. 2011 Jan;118(1):55–61. doi: 10.1111/j.1471-0528.2010.02761.x. [DOI] [PubMed] [Google Scholar]
- 38.Blomberg M. Maternal and neonatal outcomes among obese women with weight gain below the new Institute of Medicine recommendations. Obstet Gynecol. 2011 May;117(5):1065–1070. doi: 10.1097/AOG.0b013e318214f1d1. [DOI] [PubMed] [Google Scholar]
- 39.Vesco KK, Sharma AJ, Dietz PM, et al. Newborn size among obese women with weight gain outside the 2009 Institute of Medicine recommendation. Obstet Gynecol. 2011 Apr;117(4):812–818. doi: 10.1097/AOG.0b013e3182113ae4. [DOI] [PubMed] [Google Scholar]


