Abstract
Background
Anticoagulation management is difficult in chronic kidney disease, with frequent supratherapeutic international normalized ratio (INR ≥4) increasing hemorrhagic risk. We evaluated whether the interaction of INR and lower estimated glomerular filtration rate (eGFR) increases hemorrhage risk and whether patients with lower eGFR experience slower anticoagulation reversal.
Study Design
Prospective cohort study.
Setting & Participants
Warfarin pharmacogenetics cohort (WPC) (1273 long-term warfarin users). Warfarin reversal cohort (WRC) (74 warfarin users admitted with INR ≥4).
Predictor
eGFR , INR as time-dependent covariate and their interaction in the pharmacogenetics cohort; eGFR in the reversal cohort.
Outcomes & Measurements
In the pharmacogenetics cohort, hemorrhagic (serious, life-threatening, fatal bleeding) risk was assessed using proportional hazards regression. In the reversal cohort, anticoagulation reversal was assessed from changes in INR, warfarin and metabolite concentrations, clotting factors (II, VII, IX and X), and PIVKA-II (protein induced by vitamin K absence or antagonist II) levels at presentation and after reversal, using linear regression and path analysis.
Results
In the pharmacogenetics cohort, 454 (35.7%) had eGFR<60 mL/min/1.73 m2. There were 137 hemorrhages in 119 patients over 1802 person-years of follow-up (incidence rate, 7.6 [95% CI, 6.4–8.9]/100 person-years). Patients with lower eGFR had higher frequency of INR ≥4 (p<0.001). Risk of hemorrhage was significantly affected by INR-eGFR interaction. At INR<4 there was no difference in hemorrhage risk by eGFR (all p-values ≥0.4). At INR ≥4, patients with eGFR 30–44 and <30 mL/min/1.73 m2 had 2.2-fold (95% CI, 0.8–6.1; p=0.1) and 5.8-fold (95% CI, 2.9–11.4; p<0.001) higher hemorrhage risk, respectively, versus those with eGFR≥60 mL/min/1.73 m2. In the reversal cohort, 35 (47%) had eGFR<45 mL/min/1.73 m2. Patients with eGFR<45 mL/min/1.73 m2experienced slower anticoagulation reversal as assessed by INR (p=0.04) and PIVKA-II level (p=0.008) than those with eGFR≥45 mL/min/1.73 m2.
Limitations
Limited sample size in the reversal cohort, unavailability of antibiotic usage and urine albumin data.
Conclusions
Patients with lower eGFR have differentially higher hemorrhage risk at INR ≥4. Moreover as INR reversal rate is slower, hemorrhage risk is prolonged.
Index words: kidney function, chronic kidney disease (CKD), warfarin, supra-therapeutic international normalized ratio (INR), pharmacokinetics, hemorrhage, reversal of anticoagulation, adverse event
Therapy with warfarin, the most commonly prescribed oral-anticoagulant, is challenging because of the many factors that influence its pharmacokinetics and pharmacodynamics.1 Despite concerted efforts, anticoagulation management remains suboptimal, with frequent supra-therapeutic international normalized ratio (INR) often associated with hemorrhagic complications.2,3 This reality has earned warfarin a consistent ranking among the top ten drugs associated with serious adverse events.4
There has been a growing appreciation that decreased kidney function affects the clearance of (and response to) drugs that are mainly metabolized by the liver, such as warfarin 5–7. Although anticoagulation management among patients with chronic kidney disease (CKD) is particularly challenging, initiation and management of warfarin therapy in CKD patients is similar compared with those in the general medical population.8,9 We have previously reported that patients with CKD require lower warfarin doses to maintain therapeutic INR, have worse anticoagulation control and have a higher risk of hemorrhage, as compared to patients with normal kidney function.10–12
The goal of the present study was to evaluate whether patients with CKD have a differentially higher risk of hemorrhage during episodes of supra-therapeutic INR (INR≥4) in the warfarin pharmacogenetics cohort (WPC), and whether decreased kidney function influences the rate of INR reversal among patients with episodes of supra-therapeutic INR in the warfarin reversal cohort (WRC). Finally, we provide preliminary data on a potential mechanism by which decreased kidney function influences supra-therapeutic INR, facilitated by assessment of PIVKA-II (protein induced by Vitamin K absence or antagonist II) in the WRC.
Methods
Patient Characteristics and Study Design
The warfarin pharmacogenetics cohort (WPC) (institutional review board protocol numbers X030102003 (Pharmacogenetic Optimization of Anticoagulation Therapy) and X080114012 (Genetic and Environmental Determinants of Warfarin) recruited patients aged 20 years or older initiating warfarin therapy with a target INR range of 2–3. The aims of the study were to identify the influence of clinical and genetic factors on warfarin dose and hemorrhage. These data supported evaluating the interaction of kidney function and supra-therapeutic INR (INR≥4) on risk of hemorrhage.
A detailed history documented information including race, demographics, height and weight, indication for warfarin therapy, co-morbid conditions, medications, and socioeconomic factors, in addition to laboratory values (blood urea nitrogen, serum creatinine, hemoglobin and hematocrit) as detailed in recent publications. Genotyping methodology for the cytochrome P450 (CYP) genes CYP2C9 and CYP4F2 and the gene encoding vitamin K oxidoreductase complex subunit 1 (VKORC1) has been reported previously.10,13,14 All patients were followed up at least monthly15 for up to two years from initiation of therapy (or for the duration of therapy if less than 2 years). Variables influencing warfarin response such as warfarin dose, INR, concurrent medications (such as statins, antiplatelet agents and amiodarone), and dietary vitamin K and alcohol intake, and medication adherence were recorded at each visit.
Patients on warfarin with supra-therapeutic INRs reported on admission were identified. The treating physicians were contacted and patients enrolled in the warfarin reversal cohort (WRC) (institutional review board protocol number X090911007) if they were to receive vitamin K to reverse their INR. Warfarin users (n=102; age ≥20 years) hospitalized with supra-therapeutic INRs (INR≥4; visit 1) were recruited prior to administration of vitamin K per guidelines.15 A structured interview form was used at the time of enrollment to obtain a detailed medical lifestyle, social and concomitant medication history as in the other cohort. Patients were followed up until their INR had decreased by >50% from the initial INR (visit 2). Patients who received plasma, or clotting factors (due to medical necessity; n=28) were excluded from the analysis. The remaining 74 patients were followed up until their INR had decreased by >50% from the initial INR (visit 2). Blood samples (DNA, plasma and serum) were collected at both time points. Single-nucleotide polymorphisms (SNPs) in CYP2C9, VKORC1 and γ-glutamyl carboxylase (GGCX, reference SNP identification number rs11676382) were assessed. This supported the assessment of influence of kidney function on anticoagulation reversal among warfarin users hospitalized with supra-therapeutic INR.
All plasma and serum samples were processed within 30 minutes of blood collection and archived at −70°C. For both visits 1 and 2, plasma samples were analyzed for vitamin K–dependent clotting factors (factors II, VII, IX and X; University of Alabama at Birmingham Hospital laboratories) using the coagulation analyzer STAR (Stago, Parsippany, NJ). PIVKA-II was used to assess functional vitamin K status. The PIVKA-II assay was performed on plasma using a murine monoclonal antibody available in an enzyme immunoassay kit (Asserachrom PIVKA-II; Stago, Parsippany, NJ) at the Tufts University -Vitamin K laboratory as previously reported. Serum samples were analyzed to determine total warfarin and metabolite concentrations (see Item S1, available as online supplementary material) at the University of Pittsburgh.
Assessment of Kidney Function
Kidney function was assessed using estimated glomerular filtration rate (eGFR) calculated using the CKD-EPI (CKD Epidemiology Collaboration) creatinine equation.16,17 Serum creatinine was determined by the Jaffe rate method standardized to isotope-dilution mass spectrometry. Patients were categorized into 4 groups based on eGFR: ≥60 (reference group), 45–59 (CKD stage 3a), 30–44 (CKD stage 3b), and <30 ml/min/1.73 m2 (CKD stages 4 and 5). Patients receiving maintenance dialysis were categorized in the latter group. Both studies were conducted under the approval of the Institutional Review Board of the University of Alabama at Birmingham.
Outcome Definitions and Statistical Analysis
Supra-therapeutic INR was defined as an episode of INR ≥4 among patients on warfarin therapy.3,18 Major hemorrhages included serious, life threatening and fatal bleeding episodes.19. For all hemorrhagic events, complication site (e.g. gastrointestinal), gravity of the event (e.g. requiring medical/ surgical intervention), and laboratory findings at the time of the event were objectively documented. Isolated sub-therapeutic or supra-therapeutic INRs in the absence of evidence of bleeding were not classified as events. Minor hemorrhages (nosebleeds, microscopic hematuria, bruising, and mild hemorrhoidal bleeding) were not included.
During the follow-up, all hemorrhagic complications were captured and verified through review of admissions and emergency department visits. Only medically documented events were included in the analyses. The Alabama Center for Health Statistics was queried to verify cause of death for all deceased patients to ensure inclusion of deaths due to hemorrhagic complications. All complications were reviewed and adjudicated by a blinded reviewer.
Statistical Methods
To assess unadjusted between-group differences across eGFR categories in both cohorts, we performed analysis of variance models for continuous variables and χ2 tests for categorical variables. To determine if the proportion of INRs ≥4 across the eGFR categories in the WPC were significantly different, we used generalized estimating equations (GEEs) with the autoregressive lag-1 covariance structure to account for multiple INR measurements from the same patient as the density of the INRs differ across the patients during clinical care.
Incidence rate of hemorrhage and confidence intervals (CIs) were calculated using SAS version 9.3 (SAS Institute Inc, Cary, NC). After adjusting for age, race, gender, genotype, concomitant medications, clinical comorbidity, and INR at the time of the event, the interaction between kidney function and INR (eGFR-INR) was evaluated using multivariable Cox proportional hazards (PH) regression with the counting process format. 20 This allowed us to account for multiple events and account for the INRs as a time-dependent covariate. Departures from the PH assumption were assessed by evaluating interactions of the predictors and a function of survival time.
We calculated rates-of-changes per hour for INR, PIVKA-II, warfarin concentrations and clotting factor levels (II, VII, IX, X) by dividing the difference in measured levels by the interval of time in hours between visit 1 and visit 2 for each participant. To assess differences between time of enrollment (visit 1) and follow-up (visit 2), we performed paired t-tests.
The influence of kidney function on anticoagulation reversal, including rate of change in INR/hour and rate of change in PIVKA-II/ hour, were assessed using multivariable linear regression analyses with adjustment for age, race, gender, BMI, vitamin K dose, and genotype (CYP2C9, VKORC1, CYP4F2 and GGCX). To understand the indirect effect of eGFR on rate of change in INR through rate of change in PIVKA-II after adjusting for gender, race, vitamin K dose, genotype (CYP2C9, VKORC1, and CYP4F2), and change in clotting factor levels (Factor VII, IX, X), we conducted path analysis with nonparametric bootstrap estimates of the adjusted indirect effects and CIs (see Item S1). All analyses were performed using SAS version 9.3 (SAS Institute Inc, Cary, NC) at a non-directional significance level of α = 0.05.
Results
Study Participants
The clinical and genetic characteristics of participants of the warfarin pharmacogenetics cohort (WPC) are presented in Table 1. Among the study participants, 35.7% had eGFR <60 ml/min/1.73 m2, including 17.5%, 9.0%, and 9.1% with eGFR of 45–59, 30–44, and <30 ml/min/1.73m2 or on dialysis, respectively. These levels of eGFR were significantly associated with race, indications for therapy, number of comorbidities, antiplatelet and amiodarone use, and VKORC1 and CYP4F2 genotypes. These variables were included as covariates in subsequent multivariable analyses.
Table 1.
Clinical and Demographic characteristics of 1273 patients receiving long-term warfarin therapy by baseline eGFR category
| Characteristic | eGFR≥60 (n=819) | eGFR=45–59 (n=223) | eGFR=30–44 (n=115) | eGFR<30 (n=116) | P trendb |
|---|---|---|---|---|---|
| Average Follow-up (y) | 1.4 ± 0.9 | 1.5 ± 1.0 | 1.5 ± 0.9 | 1.2 ± 0.8 | 0.03 |
| Age (y) | 57.9 ± 15.8 | 68.4 ± 12.5 | 72.5 ± 12.7 | 57.7 ± 15.9 | 0.5 |
| BMI (kg/m2) | 30.4 ± 7.7 | 29.4 ± 7.2 | 29.3 ± 7.1 | 30.1 ± 6.8 | 0.6 |
|
| |||||
| Race | 0.1 | ||||
| African American | 364 (44.4) | 73 (32.7) | 37 (32.2) | 77 (66.4) | |
| European American | 448 (54.7) | 149 (66.8) | 78 (67.8) | 39 (33.6) | |
| Other c | 7 (0.9) | 1 (0.5) | 0 (0.0) | 0 (0.0) | |
| Female sex | 381 (46.5) | 118 (52.9) | 60 (52.2) | 58 (50.0) | 0.2 |
| Indication for warfarin | 0.2 | ||||
| Atrial Fibrillation | 311 (38.0) | 128 (57.4) | 63 (54.8) | 38 (32.8) | |
| Stroke | 50 (6.1) | 16 (7.2) | 9 (7.8) | 1 (0.9) | |
| Venous Thromboembolism | 382 (46.6) | 65 (29.1) | 33 (28.7) | 60 (51.7) | |
| Otherd | 76 (9.3) | 14 (6.3) | 10 (8.7) | 17 (14.7) | |
| No. of Comorbid Conditionse | <0.001 | ||||
| Low: 0 or 1 | 280 (34.2) | 43 (19.3) | 14 (12.2) | 14 (12.1) | |
| Moderate: 2–4 | 284 (34.7) | 77 (34.5) | 33 (28.7) | 45 (38.8) | |
| High: ≥5 | 255 (31.1) | 103 (46.2) | 68 (59.1) | 57 (49.1) | |
| Concurrent Medicationsf | |||||
| Antiplatelet agents | 431 (53.1) | 147 (65.9) | 78 (67.8) | 61 (52.6) | 0.06 |
| Statins | 176 (21.7) | 57 (25.6) | 27 (23.5) | 29 (25.0) | 0.3 |
| Amiodarone | 57 (7.0) | 30 (13.5) | 17 (14.8) | 10 (8.6) | 0.03 |
| Genotype | |||||
| CYP2C9 variantg | 189 (24.8) | 59 (27.7) | 28 (27.5) | 22 (21.4) | 0.9 |
| VKORC1 varianth | 320 (40.4) | 99 (45.4) | 61 (56.5) | 37 (33.6) | 0.5 |
| CYP4F2 varianti | 259 (37.3) | 88 (44.2) | 36 (37.9) | 23 (26.1) | 0.3 |
BMI, body mass index; eGFR, estimated glomerular filtration rate; rs, reference single-nucleotide polymorphism identification number
Note: All warfarin pharmacogenetics cohort patients on warfarin therapy with target international normalized ratio of 2–3. Values for categorical variables are given as number (percentage); values for continuous variables, as mean ± standard deviation. eGFR categories expressed in mL/min/1.73 m2.
p is significant at α=0.05 and denote differences across kidney function categories
other race includes 3 Asians and 5 Hispanics
other indications include cardiac thrombus, myocardial infarction, peripheral vascular disease, low ejection fraction, etc.
comorbidity was defined as concomitant diseases (e.g. Hypertension, high cholesterol, diabetes, congestive heart failure)
concurrent antiplatelet agents included aspirin, clopidogrel, and dipyridamole as mono or dual therapy. 7 individuals were missing information on concurrent medication for antiplatelet, statins and amiodarone therapy in the eGFR ≥60 category.
variant genotype includes *2, *3 alleles among European Americans and *2, *3, *5, *6 and *11 alleles among African Americans. Samples for 92 patients had not been typed at the time of this analysis: there were 56, 10, 13, and 13 missing genotypes for eGFR ≥60, 45–59, 30–44, and<30 mL/min1.73, respectively.
Variant VKORC1–1173C/T (rs9934438) corresponds to TT or CT. Samples for 44 patients had not been typed at the time of this analysis: there were 26, 5, 7, and 6 missing genotypes for eGFR ≥60, 45–59, 30–44, and<30 mL/min1.73, respectively.
Variant C (rs2108622; V433M) corresponds to GA or AA. Samples for 196 patients had not been typed at the time of this analysis: there were 124,YP4F2 24, 20, and 28 missing genotypes for eGFR ≥60, 45–59, 30–44, and<30 mL/min1.73, respectively.
Decreased kidney function (eGFR <45 ml/min/1.73m2) was associated with an increased frequency of supra-therapeutic INR (p<0.001) and hemorrhage (Table 2). Over the 1802 person-years of follow-up 137 major hemorrhages were encountered in 119 patients (incidence rate. 7.6 [95% CI, 6.4–8.9]/100 person-years). Gastrointestinal hemorrhage was most common (n=82), followed by hematoma (n=25), genitourinary (n=12), intracranial hemorrhage (n=11), and other (n=7). The incidence of hemorrhage in patients with eGFR 45–59 mL/min/1.73 m2 was similar to that of patients with eGFR ≥ 60 mL/min/1.73 m2 (p=0.6). Compared to patients with eGFR ≥60 ml/min/1.73 m2 those with eGFR 30–44 (incidence rate ratio, 1.8; 95% CI, 1.1–3.0; p=0.03) and <30 mL/min/1.73 m2 (incidence rate ratio, 3.5; 95% CI, 2.3–5.4; p<0.001) experienced hemorrhage more frequently.
Table 2.
Frequency of supra-therapeutic INR and hemorrhage among warfarin users by eGFR category
| eGFR>60 (n=819) | eGFR=45–59 (n=223) | eGFR=30–44 (n=115) | eGFR<30 (n=116) | P trend | |
|---|---|---|---|---|---|
| INR ≥4 | |||||
| No. of INRs | 20953 | 6027 | 3286 | 3359 | |
| INRs ≥4 | 1029 (4.9) | 292 (4.8) | 199 (6.1) | 249 (7.4) | <0.001c |
|
| |||||
| Major Hemorrhaged | |||||
| No. of Events | 68 | 22 | 18 | 29 | |
| Person-years | 1160.5 | 331.5 | 169.7 | 140.1 | |
| Incidence rate | 5.6 (4.6–7.4) | 6.6 (4.3–9.9) | 10.6 (6.5–16.4) | 20.7 (14.3–29.3) | |
| INR at event <4 | 51 (75.0) | 19 (86.4) | 11 (61.1) | 10 (37.0) | |
| INR at event ≥4 | 17 (25.0) | 3 (13.6) | 7 (38.9) | 17 (63.0) | <0.001 |
eGFR,estimated glomerular filtation rate; INR: international normalized ratio
Note: All 1,273 warfarin pharmacogenetics cohort patients on warfarin therapy with target INR of 2–3. Incidence rate given as rate (95% confidence interval); INR given as number (percentage). eGFR categories expressed in ml/min/1.73m2.
p is significant at α =0.05 and denote differences across kidney function categories
The p-values were obtained by using the generalized estimating equation with the autoregressive lag-1 covariance structure to account for multiple INR measurements from the same patient as the density of the INRs differ across the patients during clinical care.
137 major hemorrhages (2 INRs not available); 91 occurred at INR <4 and 44 occurred at INR ≥4. INR not available for 2 events. Breslow-Day test for interaction of Rate Ratio over kidney function strata p=0.002. Breslow-Day test for interaction of Risk Difference over kidney function strata p=0.003.
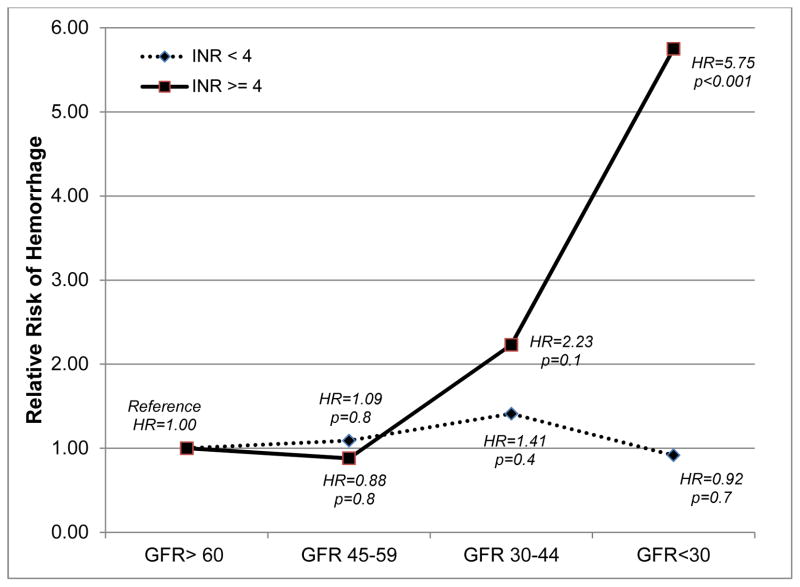
Of the 137 major hemorrhages, INR at the time of event was <4 at 91 events and ≥4 in 44 (Table 2). After adjusting for age, race, gender, genotype, concomitant medications, clinical comorbidity, time in target range and INR at the time of the event, the eGFR-INR interaction was statistically significant (p<0.001; Figure 1).
Figure 1.
Relative risk of hemorrhage among patients with varying kidney function by INR at the time of the event (eGFR ≥60 is the reference group)
Among patients with eGFR≥60 and those with eGFR 45–59 mL/min/1.73 m2, INR did not influence risk of hemorrhage (p=0.8). Among patients with eGFR 30–44 mL/min/1.73 m2, INR≥4 was associated with a 2.2-fold (hazard ratio [HR], 2.2; 95% CI, 0.8–6.1; p=0.1) higher risk of hemorrhage, although this was not statistically significant. Among patients with eGFR <30 mL/min/1.73 m2, INR ≥4 was associated with a 5.8-fold (HR, 5.8; 95% CI, 2.9–11.4; p<0.001) higher risk. This differentially higher risk of hemorrhage among patients with eGFR 30–44 and <30 mL/min/1.73 m2 when INR is ≥4, after adjustment for clinical and genetic factors is illustrated in Figure 1.
Given the significant increase in risk of hemorrhage among patients with eGFR <45 mL/min/1.73 m2 when INR is ≥4 we evaluated the influence of kidney function on anticoagulation reversal among 74 patients (mean age, 61 years; 54% female, 45% African American) who made up the warfarin reversal cohort. In 47.3% of patients, eGFR <60 mL/min/1.73 m2 was present. Venous thromboembolism (46%) was the major indication for warfarin therapy followed by atrial fibrillation (31%). Temporary discontinuation of warfarin was the sole treatment implemented in 31 patients while 43 were treated with vitamin K in addition to temporary discontinuation of warfarin. The institution of vitamin K treatment did not vary by kidney function. Patients with severe CKD (eGFR<30 mL/min/1.73 m2) received higher vitamin K doses, although this finding was not significant (p=0.1; Table 3).
Table 3.
aracteristics of participants in warfarin reversal cohort by eGFR categorya
| Characteristic | eGFR>60 (n=39) | eGFR=45–59 (n=15) | eGFR=30–44 (n=7) | eGFR<30 (n=13) | P trend b |
|---|---|---|---|---|---|
| Age (y) | 57.9 ± 18.9 | 64.3 ± 16.5 | 58.0 ± 15.4 | 69.9 ± 14.5 | 0.1 |
| BMI (kg/m2) | 27.4 ± 5.7 | 26.0 ± 9.5 | 29.4 ± 2.9 | 27.8 ± 7.7 | 0.5 |
| INR at visit 1 | 7.4 ± 2.5 | 6.5 ± 2.3 | 6.1 ± 2.0 | 6.6 ± 2.3 | 0.3 |
| INR at visit 2 | 2.6 ± 1.2 | 3.0 ± 1.0 | 1.8 ± 0.8 | 2.9 ± 1.7 | 0.8 |
| Difference in INR* | 4.8 ± 2.6 | 3.5 ± 1.6 | 4.2 ± 2.2 | 3.7 ± 1.9 | 0.3 |
| Warfarin dose (mg/wk) | 38.4 ± 20.7 | 35.6 ± 15.4 | 30.4 ± 11.7 | 31.5 ± 11.6 | 0.2 |
| Time between visits 1 and 2 (h) | 33.3 [23.3–65.0] | 42.5 [23.7–66.3] | 43.0 [23.3 – 73.9] | 39.4 [22.0–74.8] | 0.9 |
| Vitamin K dose (mg) | 4.8 ± 3.5 | 6.3 ± 3.4 | 2.8 ± 1.5 | 6.6 ± 3.8 | 0.7 |
|
| |||||
| Female sex | 21 (54) | 6 (40) | 3 (43) | 10 (77) | 0.3 |
| African American | 15 (39) | 8 (53) | 2 (29) | 8 (62) | 0.3 |
| Receiving vitamin K | 22 (56) | 7 (47) | 4 (57) | 10 (77) | 0.3 |
| Indication for Warfarin therapy | |||||
| Venous thromboembolism | 20 (51) | 3 (20) | 4 (57) | 7 (54) | |
| Stroke /TIA | 1 (3) | 1 (7) | 0 (0) | 0 (0) | |
| Atrial Fibrillation | 12 (31) | 5 (33) | 3 (43) | 3 (23) | |
| Otherc | 6 (15) | 6 (40) | 0 (0) | 3 (23) | |
| Genotyped | |||||
| CYP2C9 variant | 7 (18) | 1 (7) | 2 (29) | 1 (8) | 0.5 |
| VKORC1 variant | 21 (55) | 7 (47) | 5 (71) | 6 (46) | 0.8 |
| CYP4F2 variant | 10 (26) | 3 (21) | 2 (29) | 3 (23) | 0.9 |
| GGCX variant | 4 (11) | 1 (7) | 1 (14) | 1 (8) | 0.9 |
BMI, body mass index; eGFR, estimated glomerular filtration rate; INR, international normalized ratio; rs, reference single-nulceotide polymorphism identification number;TIA, transient ischemic attack
value at visit 2 less the value at visit 1
Note: All patients on warfarin therapy with target INR of 2–3 were being treated to reverse elevated INR levels by withholding warfarin with or without supplemental vitamin K. Values for categorical variables are given as number (percentage); values for continuous variables, as mean ± standard deviation or median [interquartile range]. eGFR categrories expressed in ml/min/1.73m2.
p is significant at α =0.05 and denote differences across kidney function categories; p-values for continuous variables derived from Kruskal-Wallis test; p-values for categorical variables derived from Chi-Square Test.
other indications include cardiac thrombus, myocardial infarction, peripheral vascular disease, etc.
see notes to Table 1.
The level of anticoagulation, clotting factors, warfarin (and metabolite) concentrations at time of enrollment (visit 1) and follow-up (visit 2) are shown in Table 4. As expected, INR and PIVKA-II levels declined with a parallel increase in clotting factor activity. Similarly, warfarin and metabolite levels decreased, although the change in 7-hydroxy and 10-hydroxy warfarin levels were not statistically significant.
Table 4.
Levels of anticoagulation, clotting factors, and warfarin at both visits
| Visit 1 | Visit 2 | P Valuea | |
|---|---|---|---|
| INR | 6.9 ± 2.4 | 2.7 ± 1.3 | <0.001 |
| PIVKA-II | 2861.7 ± 1717.1 | 1685.1 ± 1501.7 | <0.001 |
| Vitamin K–dependent clotting | |||
| Factor II: F2 | 14.9% ± 7.9% | 30.7% ± 17.4% | <0.001 |
| Factor VII: F7 | 12.4% ± 9.8% | 52.4% ± 29.7% | <0.001 |
| Factor IX: F9 | 25.7% ± 18.3% | 81.4% ± 52.9% | <0.001 |
| Factor X: F10 | 9.5% ± 5.4% | 22.0% ± 16.1% | <0.001 |
|
| |||
| Total warfarin (mg) | 1409.9 ± 1216.8 | 1221.5 ± 1002.7 | 0.07 |
| Warfarin metabolites | |||
| 4-OH (mg) | 2.9 ± 3.2 | 1.5 ± 2.1 | <0.001 |
| 6-OH (mg) | 6.0 ± 9.6 | 4.4 ± 6.3 | 0.02 |
| 7-OH (mg) | 93.0 ± 102.0 | 82.4 ± 93.4 | 0.2 |
| 8-OH (mg) | 19.3 ± 20.8 | 15.2 ± 15.7 | 0.02 |
| 10-OH (mg) | 87.1 ± 162.8 | 82.2 ± 131.7 | 0.4 |
INR: Iinternational normalized ratio; OH, hydroxy; PIVKA-II: protein induced by vitamin K absence or antagonist II
Note: Total concentrations in all 74 warfarin reversal cohort patients. Values are given as mean ± standard deviation.
p is significant at α =0.05 and denotes statistical difference in measurements across visits 1 and 2
Influence of Kidney Function on INR Reversal and PIVKA-II Levels
After adjustment for age, race, gender, BMI, vitamin K dose, and genotype (CYP2C9, VKORC1, CYP4F2 and GGCX), kidney function had a significant influence on the rate of INR reversal (p=0.04). The rate of INR decline was faster (0.11 U/h) among patients with eGFR ≥45 mL/min/1.73 m2 compared to the rate (0.05 U/h) among those with eGFR <45 ml/min/1.73 m2. Additionally gender (p=0.02), vitamin K dose (p=0.001), and CYP2C9 (p=0.08) influenced rate of INR reversal. After adjustment for age, race, gender, BMI, vitamin K dose, and genotype (CYP2C9, VKORC1, CYP4F2 and GGCX) kidney function had a significant influence on the rate of decrease in PIVKA-II levels (p=0.008).
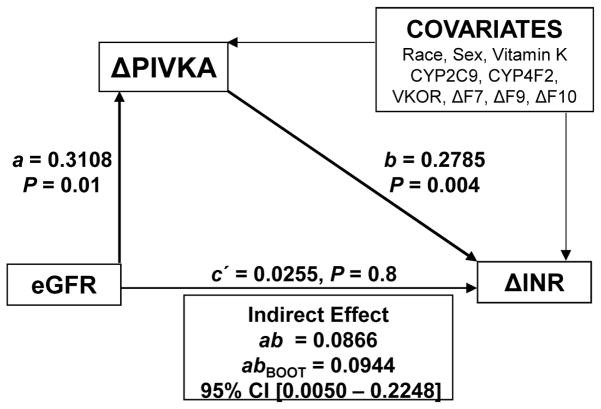
As kidney function has a significant influence on rate of decrease in INR and PIVKA-II levels and as the rate of decrease in PIVKA-II levels were significantly related to rate of change in INR (p = 0.004), we investigated the indirect effects of kidney function on rate of change in PIVKA-II and rate of change in INR using path analyses (Figure 2). Race; gender; vitamin K dose; variant CYP2C9, VKORC1, CYP4F2 and GGCX genotypes; and rate of change in F7, F9, and F10 accounted for 21.9% of the variance in rate of change in PIVKA-II, although this finding did not reach statistical significance (p = 0.07). However, addition of kidney function uniquely explained an additional 8.2% of the variance in rate of change in PIVKA-II (p=0.01). Overall these factors explained 30.1% of the variance in rate of change in PIVKA-II (p=0.01). Similarly race; gender; vitamin K dose; variant CYP2C9, VKORC1, CYP4F2 and GGCX genotypes; and rate of change in F7, F9, and F10 accounted for 59.5% of the variance in rate of change in INR (p<0.001). Although kidney function did not explain additional variance in rate of change in INR (p=0.3), addition of rate of change in PIVKA-II uniquely explained 6.8% (p= 0.004) of the variance in rate of change in INR. Overall the model explained 66.3% of the variation in rate of change in INR.
Figure 2.
Indirect Effect of eGFR on Change in INR via Change in PIVKA. The A coefficient represents the effect of GFR on rate of change in PIVKA-II and the b coefficient represents the association of rate of change in PIVKA-II.
Discussion
This prospective study demonstrates that supra-therapeutic INR (INR≥4) increases the risk of major hemorrhage among warfarin users with decreased kidney function (eGFR<45 mL/min/1.73 m2; CKD stages 3b, 4, and 5 and patients on dialysis), but not in those with eGFR≥45 mL/min/1.73 m2. Inclusion of patients across the spectrum of kidney function improves generalizability of these results.
There is extensive literature on the increased risk of hemorrhage among warfarin users during episodes of supra-therapeutic INR.1,19,21–26 We have previously shown that patients with eGFR<30 10 and those with eGFR<45 mL/min/1.73 m2 12 are at an increased risk of hemorrhage. To our knowledge, our work is the first to demonstrate that kidney function modifies the association between supra-therapeutic INR and the risk of hemorrhage. At INRs <4 the risk of hemorrhage is similar among warfarin users independent of their kidney function. Episodes of supra-therapeutic INR are more frequent in patients with eGFR<45 mL/min/1.73 m2 compared to patients with eGFR ≥45 mL/min/1.73 m2. Moreover, compared to patients with eGFR ≥60 mL/min/1.73 m2, those with eGFR<45 mL/min/1.73 m2 are at a 2.2-fold higher risk of hemorrhage and those with eGFR<30 mL/min/1.73 m2 are at a 5.8-fold higher risk. Among the warfarin pharmacogenetics cohort, 18% had eGFR <45 mL/min/1.73 m2 and accounted for 47 (34.3%) of major hemorrhages encountered, highlighting the importance of this finding.
Recognition of the hemorrhagic risk associated with supra-therapeutic INR has led to the development of guidelines to mitigate the risk.8,27 Reversal strategies are based on the patient’s INR level and the presence (or absence) of bleeding.8,27 In non-bleeding patients with elevated INRs, administration of vitamin K, which is the essential cofactor for synthesis of vitamin K–dependent proteins, is the first line treatment. In more urgent situations (actively bleeding patient or patient at imminent risk of bleeding), fresh frozen plasma and factor replacement (prothrombin complex concentrate or recombinant Factor VIIa) are administered. While factor replacement provides reliable warfarin reversal, the more commonly used strategy of temporarily withholding warfarin with or without administering vitamin K leads to unpredictable anticoagulation reversal, with significant variation in the rate and extent of INR reversal.2,28–30
To our knowledge, our work is the first to demonstrate that kidney function influences the rate of INR reversal among warfarin users with supra-therapeutic INR. Among patients with supra-therapeutic INR, those with poor kidney function (eGFR<30 mL/min/1.73 m2) experienced a slower rate of INR reversal. This indicates that these patients are at an increased risk of hemorrhage during episodes of supra-therapeutic INR and experience slower reversal of anticoagulation (holding doses with/ without administration of vitamin K), thereby prolonging the period of heightened risk.
Assessment of the indirect effects of decreased kidney function on reversal of anticoagulation (measured by INR decrease) and PIVKA-II (measured by decrease in PIVKA-II levels) enabled us to explore a potential mechanism through which decreased kidney function influences coagulation. In the presence of vitamin K, clotting factors II, VII, IX and X are carboxylated by GGCX. PIVKA-II, the un-carboxylated clotting factor II, represents a functional measure of vitamin K antagonism in patients on warfarin therapy.31–37 Therefore PIVKA-II is a functional measure of vitamin K status and the rate of decrease in PIVKA-II represents the rate of carboxylation of clotting factors.38,39 Our analysis shows that kidney function explains 9.4% of the variance in change in PIVKA-II. The slower decrease in PIVKA-II levels among patients with decreased kidney function implies that it is associated with a decreased rate of carboxylation of clotting factors.
The recognition of the influence of CKD on response to medications that are predominantly metabolized by the liver is growing.7,40,41 Animal studies in CKD have demonstrated significant down-regulation (40%-85%) of hepatic cytochrome P450 metabolism.42,43 Our findings lead us to hypothesize that the mechanism by which decreased kidney function influences anticoagulation is through slower rate of carboxylation of clotting factors as indicated by slower rate of INR and PIVKA-II reversal in patients presenting with overanticoagulation. This is supported by the influence of decreased kidney function on the carboxylation of matrix Gla-protein, another vitamin K-dependent protein.44–46 The slower rate of INR decline, together with other factors (e.g. uremia, platelet dysfunction) known to be associated with kidney disease, could explain the higher risk of hemorrhagic complications.
Our study had several strengths including the large sample size in the warfarin pharmacogenetics cohort with prospective data collection that enabled assessment of the influence of the eGFR-INR interaction on risk of hemorrhage.10,12,14 Our focus was on major hemorrhage, because these events are associated with morbidity, mortality and health care costs. Furthermore, clinical (comorbidity, medications) and genetic factors, overall anticoagulation control, and anticoagulation intensity (INR) at the time of hemorrhage were taken into account in our analysis. However we recognize its limitations. First, urine albumin was not uniformly ascertained and therefore could not be included in classifying CKD stages. Second, the small (n=74) warfarin reversal cohort only allowed for assessment of INR reversal in two broad categories (eGFR 45 vs. ≥45 mL/min/1.73 m2 ). Third, among patients in the warfarin reversal cohort, data on recent antibiotic use was not complete and therefore not included in the analysis.
Although the warfarin reversal cohort allowed us to evaluate the influence of kidney function on INR reversal and enabled us to propose a potential mechanism, our findings should be considered exploratory and hypothesis generating. Moreover, development in assay methodologies that can facilitate the assessment of changes in non-carboxylated forms of other vitamin K dependent clotting factors (factors VII, IX, X) would allow us to further vet this hypothesis. Finally, further research in larger cohorts is needed to confirm these findings and better understand the influence of kidney function on the coagulation processes.
The institution of oral anticoagulation therapy in patients with decreased kidney function is particularly challenging as these patients are underrepresented in clinical trials. The decision to initiate therapy should weigh risk of thromboembolism and risk of hemorrhage judiciously. Recently, investigators demonstrated that among hemodialysis patients with incident atrial fibrillation, warfarin use was associated with a decreased risk of all-cause mortality and a composite outcome of gastrointestinal bleeding, any stroke, and death, indicating net benefit of warfarin use in this indication.47,48 As the population ages and the prevalence of CKD increases, research that addresses the use and management of oral anticoagulation in this high-risk/ high-benefit population is greatly needed.
In summary, patients with poor kidney function have more frequent episodes of supra-therapeutic INR, are at a differentially higher risk of hemorrhage during episodes of supra-therapeutic INR, and experience slower reversal of anticoagulation (with vitamin K treatment) prolonging the period of heightened risk. Given the increased hemorrhagic risk, guidelines for reversing the effects of warfarin should provide specific guidance for patients with decreased kidney function.
Supplementary Material
Acknowledgments
This study has contributed samples to the National Institute of Neurological Disorders and Stroke (NINDS) Human Genetics Resource Center DNA and Cell Line Repository (http://ccr.coriell.org/ninds). NINDS Repository sample numbers corresponding to the samples used are ND04466, ND04556, ND04604, ND04605, ND04626, ND04869, ND04907, ND04934, ND04951, ND05036, ND05108, ND05175, ND05176, ND05239, ND05605, ND05606, ND05701, ND05702, ND05735, ND06147, ND06207, ND06385, ND06424, ND06480, ND06706, ND06814, ND06871, ND06983, ND07057, ND07234, ND07304, ND07494, ND07602, ND07711, ND07712, ND08065, ND08596, ND08864, ND08932, ND09079, ND09172, ND09760, ND09761, ND09809.
Support: This work was supported in part by grants from the National Heart Lung and Blood Institute (RO1HL092173, RO1HL092173-S2), National Institutes of Health Clinical and Translational Science Award program (grant UL1 TR000165), and the US Department of Agriculture ARS Cooperative Agreement (58-1950-7-707). Stago Diagnostica provided test reagents/kits and consumables for analysis of clotting factors and PIVKA-II. The study sponsors had no role in the study design; collection, analysis, and interpretation of data; writing the manuscript; and the decision to submit the manuscript for publication.
Footnotes
Financial Disclosure: The authors declare that they have no other relevant financial interests.
Contributions: Research idea and study design: N.A.L.; data acquisition: N.A.L, T.D.N, S.L.B, AC, M.B.M, M.R.C; data analysis/interpretation: N.A.L, T.D.N, S.L.B, AC, M.B.M, M.R.C; M.A.; statistical analysis: N.A.L, T.M.B. Each author contributed important intellectual content during manuscript drafting or revision and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved. NAL takes responsibility that this study has been reported honestly, accurately, and transparently; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned have been explained.
Item S1: Supplementary methods.
Note: The supplementary material accompanying this article (doi:_______) is available at www.ajkd.org
Descriptive Text for Online Delivery of Supplementary Material
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wittkowsky AK, Devine EB. Frequency and causes of overanticoagulation and underanticoagulation in patients treated with warfarin. Pharmacotherapy. 2004 Oct;24(10):1311–1316. doi: 10.1592/phco.24.14.1311.43144. [DOI] [PubMed] [Google Scholar]
- 2.Hylek EM, Chang YC, Skates SJ, Hughes RA, Singer DE. Prospective study of the outcomes of ambulatory patients with excessive warfarin anticoagulation. Arch Intern Med. 2000 Jun 12;160(11):1612–1617. doi: 10.1001/archinte.160.11.1612. [DOI] [PubMed] [Google Scholar]
- 3.Hylek EM, Go AS, Chang Y, et al. Effect of intensity of oral anticoagulation on stroke severity and mortality in atrial fibrillation. N Engl J Med. 2003 Sep 11;349(11):1019–1026. doi: 10.1056/NEJMoa022913. [DOI] [PubMed] [Google Scholar]
- 4.Budnitz DS, Lovegrove MC, Shehab N, Richards CL. Emergency hospitalizations for adverse drug events in older Americans. N Engl J Med. 2011 Nov 24;365(21):2002–2012. doi: 10.1056/NEJMsa1103053. [DOI] [PubMed] [Google Scholar]
- 5.Nolin TD, Naud J, Leblond FA, Pichette V. Emerging evidence of the impact of kidney disease on drug metabolism and transport. Clinical pharmacology and therapeutics. 2008 Jun;83(6):898–903. doi: 10.1038/clpt.2008.59. [DOI] [PubMed] [Google Scholar]
- 6.Momper JD, Venkataramanan R, Nolin TD. Nonrenal drug clearance in CKD: Searching for the path less traveled. Adv Chronic Kidney Dis. 2010 Sep;17(5):384–391. doi: 10.1053/j.ackd.2010.05.009. [DOI] [PubMed] [Google Scholar]
- 7.Huang SM, Temple R, Xiao S, Zhang L, Lesko LJ. When to conduct a renal impairment study during drug development: US Food and Drug Administration perspective. Clin Pharmacol Ther. 2009 Nov;86(5):475–479. doi: 10.1038/clpt.2009.190. [DOI] [PubMed] [Google Scholar]
- 8.Ageno W, Gallus AS, Wittkowsky A, et al. Oral anticoagulant therapy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012 Feb;141(2 Suppl):e44S–88S. doi: 10.1378/chest.11-2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Warfarin Package Insert. U.S. Food and Drug Administration; 2010. http://packageinserts.bms.com/pi/pi_coumadin.pdf. [Google Scholar]
- 10.Limdi NA, Beasley TM, Baird MF, et al. Kidney Function Influences Warfarin Responsiveness and Hemorrhagic Complications. J Am Soc Nephrol. 2009 Feb 18;20:912–921. doi: 10.1681/ASN.2008070802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Limdi NA, Limdi MA, Cavallari L, et al. Warfarin Dosing in Patients With Impaired Kidney Function. Am J Kidney Dis. 2010 Aug 13;56(5):823–831. doi: 10.1053/j.ajkd.2010.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Limdi MA, Crowley MR, Beasley TM, Limdi NA, Allon M. Influence of kidney function on risk of hemorrhage among patients taking warfarin: a cohort study. Am J Kidney Dis. 2013 Feb;61(2):354–357. doi: 10.1053/j.ajkd.2012.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Limdi NA, Beasley TM, Crowley MR, et al. VKORC1 polymorphisms, haplotypes and haplotype groups on warfarin dose among African-Americans and European-Americans. Pharmacogenomics. 2008 Oct;9(10):1445–1458. doi: 10.2217/14622416.9.10.1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Limdi NA, McGwin G, Goldstein JA, et al. Influence of CYP2C9 and VKORC1 1173C/T genotype on the risk of hemorrhagic complications in African-American and European-American patients on warfarin. Clin Pharmacol Ther. 2008 Feb;83(2):312–321. doi: 10.1038/sj.clpt.6100290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ageno W, Gallus AS, Wittkowsky A, Crowther M, Hylek EM, Palareti G. Oral Anticoagulant Therapy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012 Feb;141(2 Suppl):e44S–88S. doi: 10.1378/chest.11-2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009 May 5;150(9):604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Levey AS, Stevens LA. Estimating GFR using the CKD Epidemiology Collaboration (CKD-EPI) creatinine equation: more accurate GFR estimates, lower CKD prevalence estimates, and better risk predictions. Am J Kidney Dis. 2010 Apr;55(4):622–627. doi: 10.1053/j.ajkd.2010.02.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hylek EM, Skates SJ, Sheehan MA, Singer DE. An analysis of the lowest effective intensity of prophylactic anticoagulation for patients with nonrheumatic atrial fibrillation. N Engl J Med. 1996 Aug 22;335(8):540–546. doi: 10.1056/NEJM199608223350802. [DOI] [PubMed] [Google Scholar]
- 19.Fihn SD, McDonell M, Martin D, et al. Risk factors for complications of chronic anticoagulation. A multicenter study. Warfarin Optimized Outpatient Follow-up Study Group. Ann Intern Med. 1993 Apr 1;118(7):511–520. doi: 10.7326/0003-4819-118-7-199304010-00005. [DOI] [PubMed] [Google Scholar]
- 20.Therneau TM, Grambsch PM. Multiple Events per Subject (pages169–229) In: Therneau TM, Grambsch PM, editors. Modeling Survival Data: Extending the Cox Model (Statistics for Biology and Health) 1. New York, New York: Springer-Verlag; 2001. [Google Scholar]
- 21.Fihn SD, Callahan CM, Martin DC, McDonell MB, Henikoff JG, White RH. The risk for and severity of bleeding complications in elderly patients treated with warfarin. The National Consortium of Anticoagulation Clinics. Ann Intern Med. 1996 Jun 1;124(11):970–979. doi: 10.7326/0003-4819-124-11-199606010-00004. [DOI] [PubMed] [Google Scholar]
- 22.Hylek EM, Regan S, Go AS, Hughes RA, Singer DE, Skates SJ. Clinical predictors of prolonged delay in return of the international normalized ratio to within the therapeutic range after excessive anticoagulation with warfarin. Ann Intern Med. 2001 Sep 18;135(6):393–400. doi: 10.7326/0003-4819-135-6-200109180-00008. [DOI] [PubMed] [Google Scholar]
- 23.Lindh JD, Holm L, Dahl ML, Alfredsson L, Rane A. Incidence and predictors of severe bleeding during warfarin treatment. J Thromb Thrombolysis. 2008 Apr;25(2):151–159. doi: 10.1007/s11239-007-0048-2. [DOI] [PubMed] [Google Scholar]
- 24.Palareti G, Cosmi B. Bleeding with anticoagulation therapy - who is at risk, and how best to identify such patients. Thromb Haemost. 2009 Aug;102(2):268–278. doi: 10.1160/TH08-11-0730. [DOI] [PubMed] [Google Scholar]
- 25.Shalansky S, Lynd L, Richardson K, Ingaszewski A, Kerr C. Risk of warfarin-related bleeding events and supratherapeutic international normalized ratios associated with complementary and alternative medicine: a longitudinal analysis. Pharmacotherapy. 2007 Sep;27(9):1237–1247. doi: 10.1592/phco.27.9.1237. [DOI] [PubMed] [Google Scholar]
- 26.DiMarco JP, Flaker G, Waldo AL, et al. Factors affecting bleeding risk during anticoagulant therapy in patients with atrial fibrillation: observations from the Atrial Fibrillation Follow-up Investigation of Rhythm Management (AFFIRM) study. American heart journal. 2005 Apr;149(4):650–656. doi: 10.1016/j.ahj.2004.11.015. [DOI] [PubMed] [Google Scholar]
- 27.Holbrook A, Schulman S, Witt DM, et al. Evidence-based management of anticoagulant therapy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012 Feb;141(2 Suppl):e152S–184S. doi: 10.1378/chest.11-2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Crowther MA, Ageno W, Garcia D, et al. Oral vitamin K versus placebo to correct excessive anticoagulation in patients receiving warfarin: a randomized trial. Ann Intern Med. 2009 Mar 3;150(5):293–300. doi: 10.7326/0003-4819-150-5-200903030-00005. [DOI] [PubMed] [Google Scholar]
- 29.Duong T-M, Plowman BK, Morreale AP, Janetzky K. Retrospective and Prospective Analyses of the Treatment of Overanticoagulated Patients. Pharmacotherapy: The Journal of Human Pharmacology and Drug Therapy. 1998 Nov-Dec;18(6):1264–1270. [PubMed] [Google Scholar]
- 30.Glover JJ, Morrill GB. Conservative treatment of overanticoagulated patients. Chest. 1995 Oct;108(4):987–990. doi: 10.1378/chest.108.4.987. [DOI] [PubMed] [Google Scholar]
- 31.Wallin R, Suttie JW. Vitamin K-dependent carboxylation and vitamin K epoxidation. Evidence that the warfarin-sensitive microsomal NAD(P)H dehydrogenase reduces vitamin K1 in these reactions. Biochem J. 1981 Mar 15;194(3):983–988. doi: 10.1042/bj1940983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wallin R, Martin LF. Vitamin K-dependent carboxylation and vitamin K metabolism in liver. Effects of warfarin. J Clin Invest. 1985 Nov;76(5):1879–1884. doi: 10.1172/JCI112182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wallin R, Hutson SM. Warfarin and the vitamin K-dependent gamma-carboxylation system. Trends Mol Med. 2004 Jul;10(7):299–302. doi: 10.1016/j.molmed.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 34.Crosier MD, Peter I, Booth SL, Bennett G, Dawson-Hughes B, Ordovas JM. Association of sequence variations in vitamin K epoxide reductase and gamma-glutamyl carboxylase genes with biochemical measures of vitamin K status. Journal of nutritional science and vitaminology. 2009 Apr;55(2):112–119. doi: 10.3177/jnsv.55.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rishavy MA, Berkner KL. Vitamin K oxygenation, glutamate carboxylation, and processivity: defining the three critical facets of catalysis by the vitamin K-dependent carboxylase. Advances in nutrition (Bethesda, Md) 2012 Mar;3(2):135–148. doi: 10.3945/an.111.001719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shearer MJ, Fu X, Booth SL. Vitamin k nutrition, metabolism, and requirements: current concepts and future research. Advances in nutrition (Bethesda, Md) 2012;3(2):182–195. doi: 10.3945/an.111.001800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tsu LV, Dienes JE, Dager WE. Vitamin K dosing to reverse warfarin based on INR, route of administration, and home warfarin dose in the acute/critical care setting. The Annals of pharmacotherapy. 2012 Dec;46(12):1617–1626. doi: 10.1345/aph.1R497. [DOI] [PubMed] [Google Scholar]
- 38.Umeki S, Umeki Y. Levels of acarboxy prothrombin (PIVKA-II) and coagulation factors in warfarin-treated patients. Med Lab Sci. 1990 Apr;47(2):103–107. [PubMed] [Google Scholar]
- 39.Abdelhafez OM, Amin KM, Batran RZ, Maher TJ, Nada SA, Sethumadhavan S. Synthesis, anticoagulant and PIVKA-II induced by new 4-hydroxycoumarin derivatives. Bioorg Med Chem. 2010 Apr 8; doi: 10.1016/j.bmc.2010.04.009. [DOI] [PubMed] [Google Scholar]
- 40.Pichette V, Leblond FA. Drug metabolism in chronic renal failure. Current drug metabolism. 2003 Apr;4(2):91–103. doi: 10.2174/1389200033489532. [DOI] [PubMed] [Google Scholar]
- 41.Yeung CK, Shen DD, Thummel KE, Himmelfarb J. Effects of chronic kidney disease and uremia on hepatic drug metabolism and transport. Kidney Int. 2014;85(3):522–528. doi: 10.1038/ki.2013.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dreisbach AW, Lertora JJ. The effect of chronic renal failure on hepatic drug metabolism and drug disposition. Seminars in dialysis. 2003 Jan-Feb;16(1):45–50. doi: 10.1046/j.1525-139x.2003.03011.x. [DOI] [PubMed] [Google Scholar]
- 43.Leblond F, Guevin C, Demers C, Pellerin I, Gascon-Barre M, Pichette V. Downregulation of hepatic cytochrome P450 in chronic renal failure. J Am Soc Nephrol. 2001 Feb;12(2):326–332. doi: 10.1681/ASN.V122326. [DOI] [PubMed] [Google Scholar]
- 44.Boxma PY, van den Berg E, Geleijnse JM, et al. Vitamin k intake and plasma desphospho-uncarboxylated matrix Gla-protein levels in kidney transplant recipients. PloS one. 2012;7(10):e47991. doi: 10.1371/journal.pone.0047991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schlieper G, Westenfeld R, Kruger T, et al. Circulating nonphosphorylated carboxylated matrix gla protein predicts survival in ESRD. J Am Soc Nephrol. 2011 Feb;22(2):387–395. doi: 10.1681/ASN.2010040339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Voong K, Harrington D, Goldsmith D. Vitamin K status in chronic kidney disease: a report of a study and a mini-review. International urology and nephrology. 2013 Oct;45(5):1339–1344. doi: 10.1007/s11255-012-0367-x. [DOI] [PubMed] [Google Scholar]
- 47.Shen JI, Turakhia MP, Winkelmayer WC. Anticoagulation for atrial fibrillation in patients on dialysis: are the benefits worth the risks? Curr Opin Nephrol Hypertens. 2012 Nov;21(6):600–606. doi: 10.1097/MNH.0b013e32835856fd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shen JI, Montez-Rath ME, Chang TI, Winkelmayer WC. Warfarin Initiation after Newly-Diagnosed Atrial Fibrillation Associates with Better Outcomes in U.S. Patients on Hemodialysis (FR-OR138) J Am Soc Nephrol. 2013:24. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.