Abstract
Purpose
To determine the relative effectiveness, major complications, and refractive errors associated with intravitreal bevacizumab (IVB) versus panretinal photocoagulation (PRP) to treat Type 1 retinopathy of prematurity (ROP).
Subjects
Consecutive infants with Type 1 ROP who received either IVB or PRP between January 2008 and December 2012 and had at least six months of follow-up.
Design
Retrospective case series.
Methods
The data from infants treated with either IVB or PRP for Type 1 ROP between January 2008 and December 2012 were recorded from two medical centers in Atlanta, Georgia.
Main Outcome Measures
Recurrence rate, complication rate, refractive error.
Results
A total of 54 eyes (28 patients) with Type 1 ROP were evaluated: 22 eyes (11 patients) received IVB, and 32 eyes (17 patients) received PRP. Among the 22 eyes treated with IVB, 16 eyes had Zone I ROP and 6 eyes had posterior Zone II ROP. The number of Zone I and Zone II ROP eyes treated with PRP were 5 and 27 eyes, respectively. Mean gestational age, birth weight, postmenstrual age at the initial treatment, and follow-up period for the infants receiving IVB were 24.2 weeks, 668.1 grams, 35.1 weeks, and 21.7 weeks, respectively, and for the infants receiving PRP were 24.8, 701.4 grams, 36.1 weeks, and 34.5 weeks, respectively. ROP recurred in 3/22 (14%) IVB-treated eyes and in 1/32 (3%) PRP-treated eyes. None of IVB-treated eyes progressed to retinal detachment or developed macular ectopia. Only one eye went on to retinal detachment and five eyes developed macular ectopia in PRP-treated eyes. Mean spherical equivalent and postgestational age at the last refraction for IVB-treated eyes were −2.4 D and 22.4 months, respectively, and for PRP-treated eyes were −5.3 D and 37.1 months, respectively. Mean spherical equivalent for Zone I ROP eyes treated with IVB and PRP were −3.7 D and −10.1 D, respectively, and for Zone II ROP eyes were 0.6 D and −4.7 D, respectively.
Conclusions
Both IVB and PRP are effective treatment options for Type 1 ROP with low complication rates. Zone I ROP was associated with high minus refractive errors in eyes treated with either IVB or PRP.
Introduction
Retinopathy of prematurity (ROP) is a leading cause of blindness in children worldwide. It is a proliferative vascular disorder of the retina that exclusively affects premature infants. Although the pathogenesis of ROP is not completely understood, one of the causative factors leading to ROP is dysregulation of vascular endothelial growth factor (VEGF),1 leading to abnormal vasculogenesis and neovascularization.2–4
Panretinal photocoagulation (PRP) has been used for the last two decades to treat ROP. However, the side effect profile of PRP is substantial, including permanent destruction of a considerable portion of the retina, visual field loss, and high myopia.5–11 Moreover, despite treatment with PRP, some eyes progress to retinal detachment. In recent years, the off-label use of VEGF inhibitors like bevacizumab,12 which had been used effectively to treat other types of retinopathies like age-related macular degeneration and diabetic retinopathy,13–17 has been used to treat ROP.18–24
Although the BEAT-ROP study25 showed improved outcomes with IVB compared to PRP for Zone I ROP, confirmatory studies are lacking. While recent studies have assessed the effectiveness of IVB or PRP to treat ROP, only a single treatment modality was analyzed, and no direct comparison was made between IVB and PRP.23, 24, 26, 27 Moreover, many of these studies were conducted in developing countries in which patient profiles differ significantly from the patient profiles from the patients in the BEAT ROP study.
This study compares the clinical outcome of babies with Type 1 ROP treated with IVB versus PRP. The infants in this study had similar patient characteristics to the infants enrolled in the BEAT-ROP study,25 but had a longer follow-up period.
Methods
After approval from the Institutional Review Board at Emory University School of Medicine, we conducted a retrospective chart review of infants who underwent treatment for Type 1 ROP to assess and compare the use of IVB (Avastin; Genentech, Inc., South San Francisco, CA) versus PRP. Included in the study are consecutive infants with Type 1 ROP who received either IVB or PRP between January 2008 and December 2012 at Children’s Healthcare of Atlanta at Egleston Hospital and Emory Midtown Hospital in Atlanta, GA and had at least 6 months of follow-up. From January 2008 to January 2011, patients with either Zone I or Zone II Type 1 ROP were treated exclusively with PRP. After the publication of the BEAT-ROP study in 2011, we also offered IVB to patients with either Zone I or posterior Zone II Type 1 ROP as an alternative to PRP. However, starting in October 2011 after the publication of several studies raising awareness of potential, deleterious systemic effects from IVB, we offered IVB exclusively to Zone I ROP patients. Infants whose ROP met the criteria for Type 1 ROP, as defined by the Early Treatment for Retinopathy of Prematurity study as Zone I with any stage with plus disease, Zone I with stage 3 without plus disease, and Zone II with stage 2 or 3 with plus disease, were treated.28 After the treatment with either IVB or PRP, each infant was initially examined every 1 to 2 weeks until the ROP completely regressed, and in the case of IVB, until the retinal vessels extended into Zone III. Subsequently, each infant was examined less frequently in a gradual fashion, from every 4 weeks to every 3–6 months. For IVB, a lid speculum was first placed in the eye and then 2–3 tetracaine 0.5% drops were placed into the eye. The eye was sterilized with 10% povidone solution with a swab. An injection of bevacizumab (0.025 ml solution) 0.625 mg was performed through the pars plicata using a 30-gauge needle, aiming the needle directly towards the optic nerve in direction of visual axis. For PRP, the infant was intubated and sedated under the supervision of an attending neonatologist. An indirect laser at wavelength 810 nm was then used to apply photocoagulation to the entire avascular retina. The screening and treatment were administered by two experienced, attending physicians, one of whom specializes in vitreoretinal surgery and the other in pediatric ophthalmology.
The following characteristics were recorded for each patient in the study: estimated gestational age at birth, birth weight, sex, ROP zone, ROP stage, race of the mother, comorbidities, 1- and 5-minute Apgar scores, mean age at treatment, and follow-up period. The clinical status of the retina at the last dilated fundus exam was noted for each patient, and the status was classified as attached with no dragging or macular ectopia, attached with macular ectopia, attached with dragging, detached stage 4, detached stage 5, and others. The main outcome measures included ROP recurrences requiring retreatment and/or progressing to retinal detachment, major complications associated with each treatment group, and refractive errors. We defined recurrence as any of the following: recurrent plus disease, recurrent neovascularization, o r progression of traction in spite of treatment.. The time from the initial treatment to the recurrence, the type of treatment administered following the recurrence, and the clinical outcome were recorded for each recurrence. Major complications were defined as corneal opacity requiring corneal transplant, lens opacity requiring cataract surgery, pre-retinal or intravitreal hemorrhage requiring vitrectomy, and “ROP crunch” (rapid progression to tractional retinal detachment after IVB). Intraocular pressure (IOP) was not routinely measured for each patient, but when there were visible signs of increased IOP such as corneal clouding and mucoid discharge, IOP was calculated based on the average of three separate tonopen measurements. The refractive error data were comprised of spherical power, cylindrical power, spherical equivalent, and age at examination. Cycloplegic refractions were performed after instilling one drop of a mixture of 1% cyclopentolate, 1% tropicamide, and 2.5% neosynephrine in each eye and then waiting 30–40 minutes for cycloplegia to be achieved.
For statistical analyses, t-test for independent means, Mann-Whitney rank sum test, chi-square test, and Fisher’s exact test were used. A p value < 0.05 was considered statistically significant. Numerical data are expressed as mean ± SD unless otherwise stated.
Results
Out of 39 infants who were treated for Type 1 ROP from January 2008 to December 2012, 11 infants had less than 6 months of follow-up and were excluded from this study. A total of 54 eyes from 28 patients were included in this study (Table 1). The composition of the maternal race for the IVB group and PRP was similar. The IVB-group was comprised of 45% black, 27% white, 0% Hispanic, 18% Asian, and 9% other, while the PRP group was comprised of 41% black, 35% white, 23% Hispanic, 0% Asian. Twenty-two eyes from 11 patients were treated with IVB, and 16/22 eyes (8 patients) had Zone I ROP, while 6/22 eyes (3 patients) had Zone II ROP. Twenty out of 22 eyes treated with IVB had stage 3 ROP and 2/22 had stage 2 ROP. All 22 eyes treated with IVB had plus disease. Thirty-two eyes from 17 patients were treated with PRP, and 5/32 eyes (3 patients) had Zone I ROP, while 27/32 eyes (14 patients) had Zone II ROP. All 32 eyes treated with PRP had stage 3 ROP. Among the 32 eyes treated with PRP, 31/32 had plus disease and 1/32 had Zone I Stage 3 pre-plus ROP. Regression of the disease was seen within 48 hours in the IVB-treated eyes and 1–2 weeks in the PRP-treated eyes. The mean time to reach Zone III for the IVB treated-eyes was 5.5 ± 1.8 months (range 2.2–7.4). At the last dilated fundus exam, 22/22 eyes (100%) treated with IVB had attached retina with no macular ectopia, while among 32 eyes treated with PRP, 26/32 eyes (81%) had attached retina with no macular ectopia, 5/32 eyes (16%) had attached retina with macular ectopia, and 1/32 eyes (3%) progressed to stage 5 retinal detachment. Only one eye in two patients out of the 17 ROP patients treated with PRP was included in the study. In one patient, one eye had Zone III stage 2 ROP without plus disease and did not require treatment. In another patient, one eye had Zone II pre-plus ROP, which was treated with PRP based on the patient’s family preference, but did not meet the criteria for Type1 ROP, and the data from that eye was omitted from the study.
Table 1.
Characteristics of retinopathy of prematurity patients receiving intravitreal bevacizumab (IVB) or panretinal photocoagulation (PRP).*
| IVB | PRP | P value | |
|---|---|---|---|
| No of eyes (patients) | 22 (11) | 32 (17) | |
| - Zone 1 | 16 (8) | 5 (3) | |
| - Zone 2 | 6 (3) | 27 (14) | |
| Mean birth age (weeks) | 24.2 ± 1.0 (23–26) | 24.8 ± 1.2 (23–28) | |
| - Zone 1 | 24.3 ± 1.0 (23–26) | 24.4 ± 0.0 (24–24) | 0.70 |
| - Zone 2 | 24.0 ± 1.0 (23–25) | 24.9 ± 1.3 (23–28) | 0.26 |
| Mean birth weight (g) | 668.1 ± 127.3 (473–850) | 701.4 ± 118.8 (525–970) | |
| - Zone 1 | 667.6 ± 117.4 (515–850) | 697.7 ± 89.6 (629–799) | 0.70 |
| - Zone 2 | 669.3 ± 181.2 (473–830) | 702.2 ± 127.0 (525–970) | 0.71 |
| Male sex (%) | 55% | 76% | 0.24 |
| - Zone 1 | 63% | 100% | |
| - Zone 2 | 33% | 71% | |
| Mother's race (%) | 0.13 | ||
| - Black | 45% | 41% | |
| - White | 27% | 35% | |
| - Hispanic | 0% | 23% | |
| - Asian | 18% | 0% | |
| - Other | 9% | 0% | |
| Comorbidities (%) | 0.21 | ||
| Intraventricular hemorrhage | 64% | 47% | |
| - Grade I | 27% | 0% | |
| - Grade II | 18% | 12% | |
| - Grade III | 9% | 18% | |
| - Grade IV | 1% | 18% | |
| Necrotizing enterocolitis requiring surgery | 73% | 59% | |
| Sepsis with positive cultures | 45% | 41% | |
| Patent ductus arteriosus corrected with ligation | 18% | 18% | |
| Mean Apgar score - 1 min | 3.1 ± 2.5 (1–8) | 3.8 ± 2.1 (1–7) | |
| - Zone 1 | 3.5 ± 2.9 (1–8) | 5.0 ± 2.0 (3–7) | 0.43 |
| - Zone 2 | 2.0 ± 1.0 (1–3) | 3.6 ± 2.1 (1–7) | 0.23 |
| Mean Apgar score - 5 min | 5.8 ± 2.0 (2–8) | 6.3 ± 2.4 (1–9) | |
| - Zone 1 | 6.3 ± 1.8 (4–8) | 7.3 ± 1.5 (6–9) | 0.37 |
| - Zone 2 | 4.7 ± 2.5 (2–7) | 6.1 ± 2.6 (1–9) | 0.31 |
| Mean age at treatment (PMA) | 35.1 ± 2.2 (31.7–38.0) | 36.1 ± 2.3 (32.7–39.9) | |
| - Zone 1 | 34.9 ± 2.2 (31.7–38.0) | 33.6 ± 0.8 (32.7–34.1) | 0.76 |
| - Zone 2 | 35.5 ± 2.7 (32.4–37.6) | 37.4 ± 2.0 (33.4–39.9) | 0.58 |
| Follow-up period (months) | 21.7 ± 8.8 (9.8–33.7) | 34.5 ± 20.4 (6.8–72.8) | |
| - Zone 1 | 19.1 ± 8.1 (9.8–33.7) | 38.6 ± 20.7 (14.9–52.8) | 0.04 |
| - Zone 2 | 28.8 ± 7.5 (20.2–33.7) | 33.7 ± 21.0 (6.8–72.8) | 0.52 |
Data represent mean ± standard deviation (range) unless otherwise noted.
A total of 4 eyes, all of which had Zone I ROP, had recurrence (Table 2). ROP recurred in 3/16 (19%) Zone I ROP eyes initially treated with IVB and in 1/5 (20%) Zone I ROP eyes initially treated with PRP. There was no significant difference in the recurrence rate between the two groups (p = 1.0). In all three recurrence cases in the IVB group, ROP regressed. Two cases were retreated with IVB while for the third case, PRP was utilized as the second treatment. The average time between the initial IVB and additional treatment was 9.0 ± 5.7 weeks (range, 2.4–12.3), and the mean age at which the recurrence occurred was PMA 45.0 ± 6.3 weeks (range, 37.7–48.6). In the PRP group, one infant had a recurrence 2.6 weeks after the initial treatment at a PMA of 35.3 weeks old. This patient had progression to traction detachment due to fibrosis and blood after laser. There were no skip areas and no additional laser was performed. Laser was applied only to the avascular retina and not posterior to the ridge. The eye developed fibrosis and traction in association with blood in the first few weeks after laser, and the eye underwent vitrectomy, but the eye progressed to stage 5 despite this intervention.
Table 2.
Retinopathy of prematurity recurrence rates in intravitreal bevacizumab (IVB) and panretinal photocoagulation groups (PRP)
| No of Eyes with Recurrence (%) |
Type | Outcome | |
|---|---|---|---|
| IVB | 3/22 (14%) | ||
| - Zone 1 | 3/16 (19%) | Plus (n=2) NVI* (n=1) | regressed after laser photocoagulation (n=2) regressed after second IVB (n=1) |
| - Zone 2 | 0/6 (0%) | ||
| PRP | 1/32 (3%) | ||
| - Zone 1 | 1/5 (20%) | RD** | Stage 5 retinal detachment |
| - Zone 2 | 0/27 (0%) |
NVI = neovascularization of the iris
RD = retinal detachment
Pre-retinal or vitreous hemorrhage occurred in 4/22 eyes (18%) treated with IVB, but none of them involved the visual axis or required vitrectomy. No corneal or lenticular opacities, vascular sheathing, or ROP crunch were identified, and no systemic effects related to IVB were reported. In eyes treated with PRP, pre-retinal or vitreous hemorrhage not involving the visual axis or requiring vitrectomy occurred in 3/32 eyes (9%) One patient developed signs of increased IOP including corneal clouding and mucoid discharge in one eye several weeks after PRP, which prompted the evaluation for and discovery of ocular hypertension. No eyes developed corneal or lenticular opacities.
Refractive error data were available for 49/54 eyes (93%) from 26/28 patients (93%, Table 4). Twenty eyes in 10 patients were treated with IVB, and 14/20 eyes (70%) had Zone I ROP, while 6/20 eyes (30%) had posterior Zone II ROP. Twenty-nine eyes in 16 patients were treated with PRP, and 4/29 eyes (14%) had Zone I ROP and 25/29 eyes (86%) had Zone II ROP. The mean postgestational age at which the latest refractive data was available was 22.4 ± 8.1 months (range, 11.8–36.6) for the infants treated with IVB and 37.1 ± 19.8 months (range, 9.6–76.2) for the infants treated with PRP. The average spherical power, cylindrical power, and spherical equivalent were −3.0 ± 3.7 diopters (D; range, −9.5 to 2.5), 1.0 ± 0.8 D (range, 0 to 2.5), and −2.4 ± 3.5 D (range, −8.9 to 2.5), respectively, for the infants in the IVB group and −6.1 ± 5.6 D (range, −17.5 to 1.8), 1.6 ± 1.5 D (range, 0 to 5), and −5.3 ± 5.4 D (range, −16.5 to 1.8), respectively, for the infants in the PRP group. The mean spherical power, mean cylindrical power, and mean spherical equivalent were not significantly different between Zone I ROP eyes treated with IVB versus PRP (p = 0.09, 0.13, and 0.41, respectively). However, in babies with Zone II ROP, the mean spherical power and mean spherical equivalent were significantly greater in the infants treated with PRP than IVB (p = 0.004 and 0.002, respectively). The mean cylindrical power was not significantly different between the two groups for Zone II ROP (p = 0.19). For ROP eyes treated with IVB and PRP, mean spherical equivalent was significantly more severe in Zone I than in Zone II (p = 0.007 and 0.03, respectively).
Table 4.
Comparison of the present study with other published studies reporting the results of treating Type 1 retinopathy of prematurity.
| Study | Location | Treatment type |
# of eyes |
# of eyes in zone 1 (%) |
Race | Mean PMA* at birth (wk) |
Mean PMA at treatment (wk) |
Mean birth weight (g) |
Follow- up (mo) |
Recurrence rate No (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| Mintz-Hittner 2011 | USA | |||||||||
| IVB | 75 | 33 (44%) | Mixed | 24.4 | 35.1 | 652.1 | ~ 5 | 4/70 (6%) | ||
| Laser | 75 | 34 (44%) | Mixed | 24.4 | 34.8 | 669.3 | ~ 5 | 19/73 (26%) | ||
| Jalali 2011 | India | Laser | 161 | 161 (100%) | Asian | 29.6 | 34.6 | 1228 | 10.3 | 13/169 (7.7%) |
| Sanghi 2013 | India | Laser | 109 | 39 (36%) | Asian | 30.2 | 35.3 | 1392.8 | ≥ 6 | 19/109 (17.4%) |
| Wu 2013 | Taiwan | IVB | 162 | 17 (10%) | Asian | 26.3 | 36.6 | 930.1 | 13.7 | 14/162 (9%) |
| Present study | USA | |||||||||
| IVB | 22 | 16 (73%) | Mixed | 24.2 | 35.1 | 668.1 | 21.7 | 3/22 (14%) | ||
| Laser | 32 | 5 (16%) | Mixed | 24.8 | 36.2 | 701.4 | 32.5 | 1/32 (3%) |
PMA = post-menstrual age
Discussion
We found that eyes with Type 1 ROP had similarly low recurrence and complication rates after treatment with either IVB or PRP. Moreover, we observed high myopic refractive errors in eyes with Zone I ROP in both the IVB- and PRP- treatment groups.
Both IVB and PRP are effective treatment options for ROP. Studies assessing the effectiveness of IVB in treating Zone I and II ROP have shown similarly low recurrence rates (6–10%).23–25 Likewise, recurrence rates of Zone I and Zone II ROP after treatment with PRP coincide with our results.26, 27 One study reported treating161 eyes with Zone I ROP with PRP, and after a mean follow-up period of 10.3 months, ROP only recurred in 13/169 eyes (7.7%).26 Moreover, another study reported that ROP only recurred in 19/109 eyes (17.4%) with Zone I or II ROP treated with PRP after six months or longer of follow-up.27 However, the BEAT-ROP study showed significantly higher recurrence rate after PRP versus IVB (22% versus 4% overall; 35% versus 3.2% for Zone I ROP). These recurrence rates are much higher than what we report in the present study even though the patient characteristics of our study and the BEAT-ROP study are similar.25 While a few studies have reported a high recurrence rate (21%–78%) in eyes with Zone I ROP eyes treated with PRP,29–31, these studies treated eyes with threshold ROP, which is associated with a higher recurrence rate than eyes with Type 1 ROP.28
While our study was not powered to evaluate safety, we did not detect significant complications with either IVB or PRP. In our study, similar rates of pre-retinal or vitreous hemorrhages were noted after treatment with IVB and PRP, and none of these hemorrhages involved the visual axis or required a vitrectomy. These results are in sharp contrast to those reported in the BEAT-ROP study that showed significantly higher complication rates in the PRP-treated group compared to the IVB-treated group. The reasons for the discrepancy between the results from our study and BEAT-ROP study are not entirely clear. It is possible that the ethnic composition could play a role as a majority of BEAT-ROP patient population was Hispanic, while our study was mainly comprised of non-Hispanics. Nonetheless, the use of intravitreal bevacizumab (IVB) to treat ROP is not without shortcomings or potentially harmful consequences. For example, optimal dose of IVB for treating ROP has yet to be established.32, 33 Moreover, intravitreal IVB has been shown to significantly suppress systemic VEGF levels for at least two weeks in infants with ROP,34 which may result in deleterious systemic effects. In addition, the long-term ocular effects of IVB in infants are not known.
Our study included three infants with posterior Zone II ROP who were treated with IVB. After the publication of the results of the BEAT-ROP study but prior to October 2011, we offered IVB to patients with either Zone I or posterior Zone II Type 1 ROP as an alternative to conventional PRP. Occasionally, IVB was chosen over PRP in a patient whose systemic status was considered too fragile to withstand laser treatment. All had been appropriately counseled as to the known and unknown risks of IVB. However, after the release of several publications that raised awareness of decreased systemic VEGF levels in patients receiving IVB,34–36 we only offered IVB to infants with Zone I ROP.
Both IVB-treated and PRP-treated ROP eyes were associated with high refractive errors. Our results from eyes treated with PRP are consistent with those published in other studies, that showed mean myopic refractive errors ranging from −2.3 to −6.7 D.5–8, 11, 37–40 Although the number of studies reporting refractive error data for IVB-treated eyes is limited, two studies showed a mean myopic refractive error of −1.8 D and −1.1 D at ages 5 and 2.5 years, respectively.11, 41 Another study reported minimal refractive errors (mean spherical equivalent of −0.1 D) in IVB-treated eyes at a mean age of 1.5 years.23 Interestingly, in Zone II ROP eyes treated with IVB in our study, the mean spherical equivalent refractive error was 0.6 D, which was significantly different from the mean refractive error in Zone II ROP eyes treated with PRP (−4.7 D), although the mean refractive error between Zone I ROP eyes treated with IVB versus PRP was not significantly different. This result suggests that Zone I disease may be an independent risk factor for high refractive errors even for IVB-treated eyes.
Although the mean age for the last refraction for Zone II ROP eyes was similar in both groups, the mean age for the last refraction was significantly higher in Zone I ROP eyes treated with PRP than those treated with IVB. The discrepancy is primarily due to the fact that we did not start treating eyes with Zone I ROP with IVB until the publication of the results of the BEAT-ROP study.25 Therefore, more recent cases of Zone I ROP were treated with IVB, while older cases of Zone I ROP were treated with PRP.
This study has a number of limitations. First, it was a retrospective study and as a result, the follow-up period was variable, and appropriate controls could not be implemented into the study design. Second, the sample size was small limiting the power of the findings, which might have underestimated the difference in myopia in PRP versus IVB treated eye, which was recently reported to be significantly worse in PRP treated eyes of the infants in the BEAT-ROP study.11 Third, the generalizability of our results may be limited given that the distribution of Zone I and Zone II disease differed between the PRP and IVB groups. Lastly, at the time of this study, we did not routinely perform fluorescein angiography. Delayed development of the retinal vasculature is a potential problem in ROP eyes treated with IVB. Unlike in PRP-treated eyes, late recurrences could occur in IVB-treated eyes after several months of regression. In our IVB-treated group, it took as long as 7.4 months before the retina vascularized into Zone III. Because our follow-up threshold was 6 months, we could have missed recurrence in some patients if there were much delayed vascularizations. Fluoroscein angiography should therefore be routinely performed on these patients to assess delayed vascularization of the peripheral retina. On the other hand, the strengths of this study included the fact that it was a consecutive series with relatively few patients lost to follow-up, the mean follow-up was longer than 2 years, and all patients were treated at only two hospitals that both used the same treatment protocols. Nevertheless, future studies that are prospective in design and large sample size are needed to confirm our results.
In conclusion, no difference outcome was observed between Type 1 ROP eyes treated with IVB versus PRP in this study. Both IVB and PRP appear to be effective methods to treat Type 1 ROP with low complication and recurrence rates. Only 1 patient developed a retinal detachment after treatment.
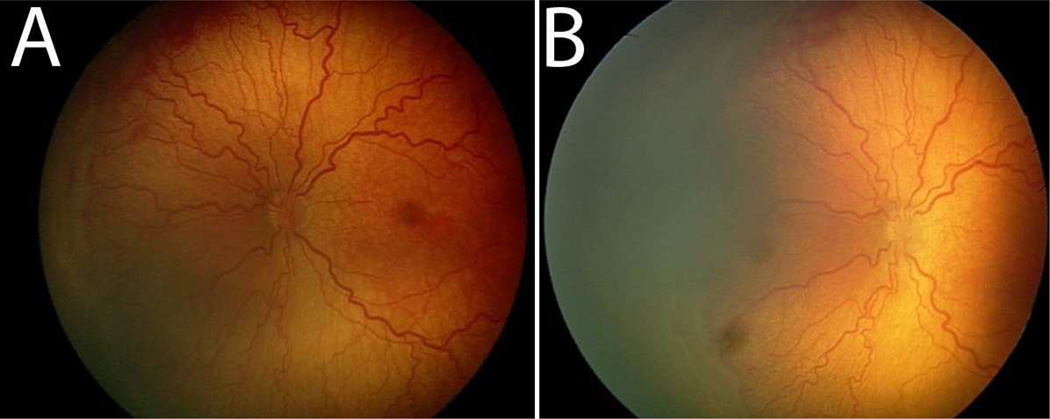
Figure 1.
Pre- and post-treatment images of the retina after intravitreal bevacizumab. Prior to treatment, plus disease with prominent tortuosity and congestion of the retinal vessels are observed in the left eye of a patient with Type 1 ROP (A). Two weeks post-treatment, the retinal vesels appear significantly less tortuous and congested (B).
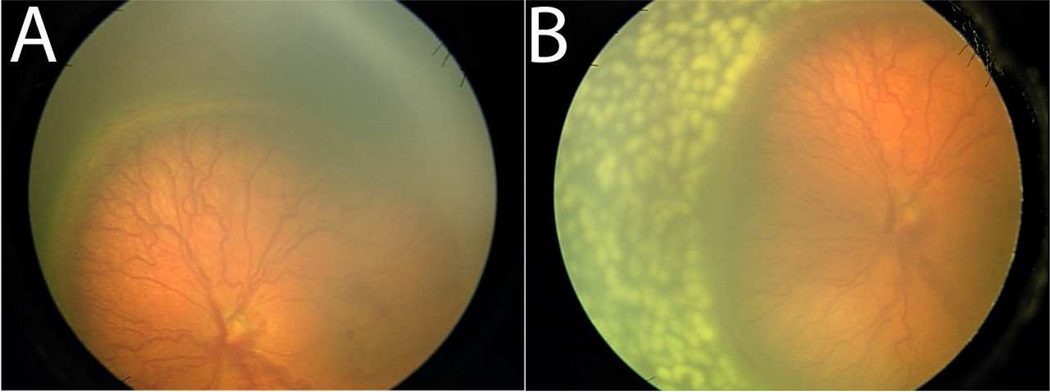
Figure 2.
Pre- and post-treatment images of the retina after pan-retinal photocoagulation. Plus disease with prominent tortuosity and congestion of the retinal vessels are observed in the left eye of a patient with Type 1 ROP prior to treatment (A). The retinal vesels appear significantly less tortuous and congested two weeks post-PRP (B).
Table 3.
Refractive errors in retinopathy of prematurity infants after treatment with intravitreal bevacizumab (IVB) or panretinal photocoagulation (PRP).*
| IVB | PRP | P value |
|
|---|---|---|---|
| Mean Spherical power | |||
| - Zone 1 | −4.3 ± 3.4 (−9.5 to 0) | −11.2 ± 11.0 (−17.5 to 1.5) | 0.09 |
| - Zone 2 | 0.3 ± 2.0 (−2.0 to 2.5) | −5.5 ± 4.6 (−16.3 to 0) | 0.004 |
| Mean Cylindrical power | |||
| - Zone 1 | 1.2 ± 0.8 (0 to 2.5) | 2.1 ± 1.1 (1.0 to 3.25) | 0.13 |
| - Zone 2 | 0.6 ± 0.8 (0 to +1.75) | 1.6 ± 1.6 (0 to 5.0) | 0.19 |
| Mean Spherical equivalent | |||
| - Zone 1 | −3.7 ± 3.3 (−8.9 to 0.3) | −10.1 ± 10.5 (−16.5 to 2.0) | 0.41 |
| - Zone 2 | 0.6 ± 1.7 (−1.1 to 2.5) | −4.7 ± 4.6 (−16 to 0) | 0.002 |
| Mean age at last refraction (months) | |||
| - Zone 1 | 18.4 ± 5.1 (11.8 to 25.1) | 52.8 ± 2.4 (50.7 to 54.8) | 0.004 |
| - Zone 2 | 31.5 ± 6.3 (23.4 to 36.6) | 34.6 ± 20.2 (9.6 to 76.2) | 1.0 |
Data represent mean ± standard deviation (range).
Both intravitreal bevacizumab (IVB) and panretinal photocoagulation (PRP) treated Type 1 retinopathy of prematurity (ROP) effectively with low complication rates. Zone 1 ROP was associated with high myopia after treatment with either IVB or PRP.
Acknowledgments
Financial Support: This work was supported in part by an NEI Core Grant for Vision Research (P30 EY 006360) and an unrestricted departmental grant from Research to Prevent Blindness (RPB)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The material has been previously presented at the American Academy of Ophthalmology Annual Meeting, November, 2013.
No conflicting relationship exists for any author.
References
- 1.Alon T, Hemo I, Itin A, et al. Vascular endothelial growth factor acts as a survival factor for newly formed retinal vessels and has implications for retinopathy of prematurity. Nat Med. 1995;1(10):1024–1028. doi: 10.1038/nm1095-1024. [DOI] [PubMed] [Google Scholar]
- 2.Kim KJ, Li B, Winer J, et al. Inhibition of vascular endothelial growth factor induced angiogenesis suppresses tumour growth in vivo. Nature. 1993;362(6423):841–844. doi: 10.1038/362841a0. [DOI] [PubMed] [Google Scholar]
- 3.Pierce EA, Avery RL, Foley ED, et al. Vascular endothelial growth factor/vascular permeability factor expression in a mouse model of retinal neovascularization. Proc Natl Acad Sci U S A. 1995;92(3):905–909. doi: 10.1073/pnas.92.3.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hartnett ME, Penn JS. Mechanisms and management of retinopathy of prematurity. N Engl J Med. 2012;367(26):2515–2526. doi: 10.1056/NEJMra1208129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.White JE, Repka MX. Randomized comparison of diode laser photocoagulation versus cryotherapy for threshold retinopathy of prematurity: 3-year outcome. J Pediatr Ophthalmol Strabismus. 1997;34(2):83–87. doi: 10.3928/0191-3913-19970301-06. quiz 121-2. [DOI] [PubMed] [Google Scholar]
- 6.Shalev B, Farr AK, Repka MX. Randomized comparison of diode laser photocoagulation versus cryotherapy for threshold retinopathy of prematurity: seven year outcome. Am J Ophthalmol. 2001;132(1):76–80. doi: 10.1016/s0002-9394(01)00956-4. [DOI] [PubMed] [Google Scholar]
- 7.Fallaha N, Lynn MJ, Aaberg TM, Jr, Lambert SR. Clinical outcome of confluent laser photoablation for retinopathy of prematurity. J aapos. 2002;6(2):81–85. doi: 10.1067/mpa.2002.121452. [DOI] [PubMed] [Google Scholar]
- 8.Yang CS, Wang AG, Sung CS, et al. Long-term visual outcomes of laser-treated threshold retinopathy of prematurity: a study of refractive status at 7 years. Eye (Lond) 2010;24(1):14–20. doi: 10.1038/eye.2009.63. [DOI] [PubMed] [Google Scholar]
- 9.Sanghi G, Dogra MR, Das P, et al. Aggressive posterior retinopathy of prematurity in Asian Indian babies: spectrum of disease and outcome after laser treatment. Retina. 2009;29(9):1335–1339. doi: 10.1097/IAE.0b013e3181a68f3a. [DOI] [PubMed] [Google Scholar]
- 10.Gonzalez VH, Giuliari GP, Banda RM, et al. Confluent laser photocoagulation for the treatment of retinopathy of prematurity. J Pediatr Ophthalmol Strabismus. 2010;47(2):81–85. doi: 10.3928/01913913-20100308-05. quiz 6–7. [DOI] [PubMed] [Google Scholar]
- 11.Geloneck MM, Chuang AZ, Clark WL, et al. Refractive Outcomes Following Bevacizumab Monotherapy Compared With Conventional Laser Treatment: A Randomized Clinical Trial. JAMA Ophthalmol. 2014 doi: 10.1001/jamaophthalmol.2014.2772. [DOI] [PubMed] [Google Scholar]
- 12.Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350(23):2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 13.Bashshur ZF, Haddad ZA, Schakal AR, et al. Intravitreal bevacizumab for treatment of neovascular age-related macular degeneration: the second year of a prospective study. Am J Ophthalmol. 2009;148(1):59.e1–65.e1. doi: 10.1016/j.ajo.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 14.Spaide RF, Fisher YL. Intravitreal bevacizumab (Avastin) treatment of proliferative diabetic retinopathy complicated by vitreous hemorrhage. Retina. 2006;26(3):275–278. doi: 10.1097/00006982-200603000-00004. [DOI] [PubMed] [Google Scholar]
- 15.Spaide RF, Laud K, Fine HF, et al. Intravitreal bevacizumab treatment of choroidal neovascularization secondary to age-related macular degeneration. Retina. 2006;26(4):383–390. doi: 10.1097/01.iae.0000238561.99283.0e. [DOI] [PubMed] [Google Scholar]
- 16.Avery RL, Pearlman J, Pieramici DJ, et al. Intravitreal bevacizumab (Avastin) in the treatment of proliferative diabetic retinopathy. Ophthalmology. 2006;113(10):1695, e1–e15. doi: 10.1016/j.ophtha.2006.05.064. [DOI] [PubMed] [Google Scholar]
- 17.Mason JO, 3rd, Nixon PA, White MF. Intravitreal injection of bevacizumab (Avastin) as adjunctive treatment of proliferative diabetic retinopathy. Am J Ophthalmol. 2006;142(4):685–688. doi: 10.1016/j.ajo.2006.04.058. [DOI] [PubMed] [Google Scholar]
- 18.Mintz-Hittner HA, Kuffel RR., Jr Intravitreal injection of bevacizumab (avastin) for treatment of stage 3 retinopathy of prematurity in zone I or posterior zone II. Retina. 2008;28(6):831–838. doi: 10.1097/IAE.0b013e318177f934. [DOI] [PubMed] [Google Scholar]
- 19.Quiroz-Mercado H, Martinez-Castellanos MA, Hernandez-Rojas ML, et al. Antiangiogenic therapy with intravitreal bevacizumab for retinopathy of prematurity. Retina. 2008;28(3 Suppl):S19–S25. doi: 10.1097/IAE.0b013e318159ec6b. [DOI] [PubMed] [Google Scholar]
- 20.Kusaka S, Shima C, Wada K, et al. Efficacy of intravitreal injection of bevacizumab for severe retinopathy of prematurity: a pilot study. Br J Ophthalmol. 2008;92(11):1450–1455. doi: 10.1136/bjo.2008.140657. [DOI] [PubMed] [Google Scholar]
- 21.Travassos A, Teixeira S, Ferreira P, et al. Intravitreal bevacizumab in aggressive posterior retinopathy of prematurity. Ophthalmic Surg Lasers Imaging. 2007;38(3):233–237. doi: 10.3928/15428877-20070501-09. [DOI] [PubMed] [Google Scholar]
- 22.Harder BC, von Baltz S, Jonas JB, Schlichtenbrede FC. Intravitreal bevacizumab for retinopathy of prematurity. J Ocul Pharmacol Ther. 2011;27(6):623–627. doi: 10.1089/jop.2011.0060. [DOI] [PubMed] [Google Scholar]
- 23.Wu WC, Kuo HK, Yeh PT, et al. An updated study of the use of bevacizumab in the treatment of patients with prethreshold retinopathy of prematurity in taiwan. Am J Ophthalmol. 2013;155(1):150 e1–158 e1. doi: 10.1016/j.ajo.2012.06.010. [DOI] [PubMed] [Google Scholar]
- 24.Wu WC, Yeh PT, Chen SN, et al. Effects and complications of bevacizumab use in patients with retinopathy of prematurity: a multicenter study in taiwan. Ophthalmology. 2011;118(1):176–183. doi: 10.1016/j.ophtha.2010.04.018. [DOI] [PubMed] [Google Scholar]
- 25.Mintz-Hittner HA, Kennedy KA, Chuang AZ. Efficacy of intravitreal bevacizumab for stage 3+ retinopathy of prematurity. N Engl J Med. 2011;364(7):603–615. doi: 10.1056/NEJMoa1007374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jalali S, Kesarwani S, Hussain A. Outcomes of a protocol-based management for zone 1 retinopathy of prematurity: the Indian Twin Cities ROP Screening Program report number 2. Am J Ophthalmol. 2011;151(4):719 e2–724 e2. doi: 10.1016/j.ajo.2010.10.007. [DOI] [PubMed] [Google Scholar]
- 27.Sanghi G, Dogra MR, Katoch D, Gupta A. Aggressive posterior retinopathy of prematurity: risk factors for retinal detachment despite confluent laser photocoagulation. Am J Ophthalmol. 2013;155(1):159 e2–164 e2. doi: 10.1016/j.ajo.2012.07.012. [DOI] [PubMed] [Google Scholar]
- 28.Early Treatment For Retinopathy Of Prematurity Cooperative G. Revised indications for the treatment of retinopathy of prematurity: results of the early treatment for retinopathy of prematurity randomized trial. Arch Ophthalmol. 2003;121(12):1684–1694. doi: 10.1001/archopht.121.12.1684. [DOI] [PubMed] [Google Scholar]
- 29.Kychenthal A, Dorta P, Katz X. Zone I retinopathy of prematurity: clinical characteristics and treatment outcomes. Retina. 2006;26(7 Suppl):S11–S15. doi: 10.1097/01.iae.0000244285.79004.e6. [DOI] [PubMed] [Google Scholar]
- 30.Foroozan R, Connolly BP, Tasman WS. Outcomes after laser therapy for threshold retinopathy of prematurity. Ophthalmology. 2001;108(9):1644–1646. doi: 10.1016/s0161-6420(01)00695-9. [DOI] [PubMed] [Google Scholar]
- 31.O'Keefe M, Lanigan B, Long VW. Outcome of zone 1 retinopathy of prematurity. Acta Ophthalmol Scand. 2003;81(6):614–616. doi: 10.1111/j.1395-3907.2003.00171.x. [DOI] [PubMed] [Google Scholar]
- 32.Fleck BW. Management of retinopathy of prematurity. Arch Dis Child Fetal Neonatal Ed. 2013;98(5):F454–F456. doi: 10.1136/archdischild-2013-303933. [DOI] [PubMed] [Google Scholar]
- 33.Harder BC, von Baltz S, Jonas JB, Schlichtenbrede FC. Intravitreal low-dosage bevacizumab for retinopathy of prematurity. Acta Ophthalmol. 2014;92(6):577–581. doi: 10.1111/aos.12266. [DOI] [PubMed] [Google Scholar]
- 34.Sato T, Wada K, Arahori H, et al. Serum concentrations of bevacizumab (avastin) and vascular endothelial growth factor in infants with retinopathy of prematurity. Am J Ophthalmol. 2012;153(2):327 e1–333 e1. doi: 10.1016/j.ajo.2011.07.005. [DOI] [PubMed] [Google Scholar]
- 35.Chakravarthy U, Harding SP, Rogers CA, et al. Ranibizumab versus bevacizumab to treat neovascular age-related macular degeneration: one-year findings from the IVAN randomized trial. Ophthalmology. 2012;119(7):1399–1411. doi: 10.1016/j.ophtha.2012.04.015. [DOI] [PubMed] [Google Scholar]
- 36.Hoerster R, Muether P, Dahlke C, et al. Serum concentrations of vascular endothelial growth factor in an infant treated with ranibizumab for retinopathy of prematurity. Acta Ophthalmol. 2013;91(1):e74–e75. doi: 10.1111/j.1755-3768.2012.02469.x. [DOI] [PubMed] [Google Scholar]
- 37.Sahni J, Subhedar NV, Clark D. Treated threshold stage 3 versus spontaneously regressed subthreshold stage 3 retinopathy of prematurity: a study of motility, refractive, and anatomical outcomes at 6 months and 36 months. Br J Ophthalmol. 2005;89(2):154–159. doi: 10.1136/bjo.2004.045815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pearce IA, Pennie FC, Gannon LM, et al. Three year visual outcome for treated stage 3 retinopathy of prematurity: cryotherapy versus laser. Br J Ophthalmol. 1998;82(11):1254–1259. doi: 10.1136/bjo.82.11.1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Connolly BP, McNamara JA, Sharma S, et al. A comparison of laser photocoagulation with trans-scleral cryotherapy in the treatment of threshold retinopathy of prematurity. Ophthalmology. 1998;105(9):1628–1631. doi: 10.1016/S0161-6420(98)99029-7. [DOI] [PubMed] [Google Scholar]
- 40.McLoone EM, O'Keefe M, McLoone SF, Lanigan BM. Long-term refractive and biometric outcomes following diode laser therapy for retinopathy of prematurity. J AAPOS. 2006;10(5):454–459. doi: 10.1016/j.jaapos.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 41.Martinez-Castellanos MA, Schwartz S, Hernandez-Rojas ML, et al. Long-term effect of antiangiogenic therapy for retinopathy of prematurity up to 5 years of follow-up. Retina. 2013;33(2):329–338. doi: 10.1097/IAE.0b013e318275394a. [DOI] [PubMed] [Google Scholar]