Abstract
Purpose
Inhibition of the vascular endothelial growth factor receptor (VEGFR) with tyrosine kinase inhibitors (TKIs) is associated with cutaneous adverse effects that increase patient morbidity. Our objective was to examine the skin toxicity profile of anti-VEGFR TKIs and determine the changing incidence in clinical trials.
Methods
PubMed was queried for Phase II or III trials of anti-VEGFR TKIs between 2000 and 2013 involving ≥50 patients. Adverse events were abstracted, with results presented in both fixed and random effects models. Odds ratios (OR) and 95% CIs were estimated for studies with at least two arms.
Results
Across 82 included studies, all grades rash (OR, 2.68; 95% CI, 2.45–2.94), hand foot skin reaction (HFSR) (OR, 2.70; 95% CI, 2.43–3.00) and pruritus (OR, 1.25; 95% CI, 1.12–1.39) were associated with anti-VEGFR TKIs. Vandetanib had the highest incidence of rash (41%), while sorafenib was most commonly associated with HFSR (37%) and pruritus (14%). The incidence of HFSR from 2000 to 2013 showed an upward trend (r2=0.042, p=0.10) and in sunitinib therapy increased significantly (r2=0.237, p=0.04).
Conclusion
The incidence of HFSR, rash and pruritus varies considerably by drug. Our data suggest a continued need to address skin toxicities and improve reporting strategies.
Keywords: Palmoplantar erythodysesthesia, Hand-Foot Syndrome, Hand-Foot Skin Reaction, Toxic Erythema of Chemotherapy, Tyrosine Kinase Inhibitor, Vascular Endothelial Growth Factor
INTRODUCTION
Tyrosine kinase inhibitors (TKIs) targeting the vascular endothelial growth factor receptor (VEGFR) are approved for the treatment of several aggressive malignancies[29, 31, 34, 36]. However, treatment with anti-VEGFR TKIs is limited by poor tolerability, including skin toxicity, that has resulted in rates of discontinuation in some cases exceeding that of conventional “cytotoxic” chemotherapy[30].
Dermatologic side effects include rash, alopecia, depigmentation, pruritus, xerosis, acneiform rashes and mucositis[11, 16, 19]. However, the most troublesome cutaneous side effect for most patients is often a hand-foot skin reaction (HFSR), characterized by painful, edematous, erythematous and keratotic lesions on acral surfaces, particularly weight-bearing sites, one to six weeks after therapy is initiated[11, 28]. Acral dysesthesia and paresthesia commonly precede the lesions[28].
HFSR occurring with anti-VEGFR TKIs can be distinguished clinically from hand-foot syndrome (HFS) associated with “cytotoxic” chemotherapeutic agents[15]. While HFSR produces localized, hyperkeratotic plaques on acral sites[18, 40], HFS is marked by symmetric, desquamative erythema and edema not typically extending beyond volar and plantar surfaces[18, 19]. The mechanism of the HFSR is poorly understood[21].
We undertook a meta-analysis of studies of TKIs with VEGFR inhibitory activity to determine the prevalence of skin toxicity amongst TKIs in clinical trials in the past thirteen years, and to examine individual skin toxicities and their relationship to each drug’s in vitro multikinase inhibition profiles.
MATERIAL AND METHODS
Data Sources
A PubMed search was performed for articles published between January 2000 and March 2013 using generic drug names and original designations (e.g. “BAY734506”) as keywords. When missing data were encountered, FDA package inserts were used as well as the adverse events listing in the “Results” tab of clinicaltrials.gov. Where adverse event data were reported below a particular threshold percentage, data were entered into the database as threshold % - 1% (e.g. if authors reported HFSR occurred in <10% of subjects, a value of 9% was entered for that adverse event).
Study Selection
The following inclusion criteria were applied to all identified clinical studies: 1) published in English; 2) Phase II or III trial; 3) ≥50 patients in the safety analysis; 4) ≥50 patients in the dose arm or schedule for that particular arm to be included. A minimum of 50 patients per study or treatment arm was used to limit the number of small trials. If one study arm met entry criteria but another did not, the arm with < 50 patients was omitted.
Data Extraction
Trials were reviewed independently by two study authors (P.M. and J.O.). The following data elements were abstracted: treatment, population under study, dose, administration method and schedule, year of publication, median date of patient enrollment, region of study, trial phase, trial design, number of patients on study and number of patients evaluated in the safety analysis. Adverse events data included HFSR, pruritus, rash, diarrhea, fatigue, number of patients who discontinued the trial and number who underwent dose reductions.
Statistical Analysis
Meta-analyses were performed on randomized studies that compared anti-VEGFR TKI therapy with a non-anti-VEGFR TKI therapy. For studies with more than two arms, each unique experimental/control arm combination was treated as a separate entry. The summary measure used for the pooling of studies in fixed effects (weighted with inverse variance) meta-analyses was an odds ratio (OR) and the DerSimonian-Laird method was used to estimate the between-study variance. A level of 0.95 was used to calculate confidence intervals for individual studies and pooled estimates. A value of 0.05 was added to all cell frequencies if at least one study had a zero cell count. When possible, drugs were organized according to the date of FDA approval.
Subgroup analyses were performed by specific TKI type and by anti-VEGFR TKIs use as monotherapy or in combination with a conventional chemotherapeutic agent. For Forest Plot analyses, all included trials used either placebo or non-VEGFR TKI as a control group.
Toxicity over time was assessed using linear regression of the proportions of adverse events against median study enrollment dates. Adverse events distributions among all studies, including single arm studies, were compared between anti-VEGFR TKIs by way of Tukey multiple comparisons of means (95% family-wise confidence level). All statistical analysis and graphical generation was done using R version 2.15.3 (2013-03-01).
Data Synthesis and Assessment of Study Quality and Bias
Jadad’s criteria was applied to assess for quality in randomized trials [14]. For non-randomized trials, the Newcastle-Ottawa Scale (NOS) was applied [38]. Cochrane Collaboration’s tool for assessing risk of bias was applied across multiple domains for each study. Statistical heterogeneity was determined by chi-squared test. Tests for funnel plot asymmetry were only performed if the number of studies was ten or larger[37]. The score method was used to test funnel plot asymmetry [13].
RESULTS
Anti-VEGFR therapy is associated with skin and systemic toxicity
Eighty-two studies encompassing eight anti-VEGFR TKIs and 13,857 patients met inclusion criteria (Fig 1; Supplemental Table 1). 33 (40%) randomized trials met inclusion criteria for Forest Plot meta-analyses. In all analyses, similar results were observed using either a fixed effect or a random effect model and fixed effect model data are reported in the results. Estimated effects of anti-VEGFR TKI intervention on rash, HFSR (Fig 2), pruritus and diarrhea are shown (Fig 3, Supplemental Fig 1). TKIs targeting the VEGFR were significantly associated with overall (all toxicity grades) rash (OR, 2.68; 95% CI, 2.45–2.94), HFSR (OR, 2.70; 95% CI, 2.43–3.00) and pruritus (OR, 1.25; 95% CI, 1.12–1.39). Similarly, diarrhea (OR, 2.71; 95% CI, 2.51–2.91) and fatigue (OR, 1.26; 95% CI, 1.17–1.35) were more likely to occur with a TKI targeting the VEGFR than placebo. The overall OR for discontinuation of an anti-VEGFR TKI treatment was 1.63 (95% CI, 1.48–1.79) and the overall OR for dose reduction was 3.56 (95% CI, 3.08–3.90).
Fig. 1. Flow Diagram of Included Studies.
Flow diagram demonstrating the selection of trials in this review of the literature and meta-analysis.
Fig. 2. Hand-Foot Skin Reaction.

Note desquamative hyperkeratosis and erythema over pressure-bearing areas of the foot in a patient during treatment with sorafenib.
Fig. 3. Meta-analysis of Adverse Events.
Forest Plot of all randomized trials demonstrating odds ratio of all grade rash, hand-foot skin reaction (HFSR), pruritus and diarrhea by drug versus placebo.
Comparison of cutaneous and systemic toxicities of anti-VEGFR TKIs
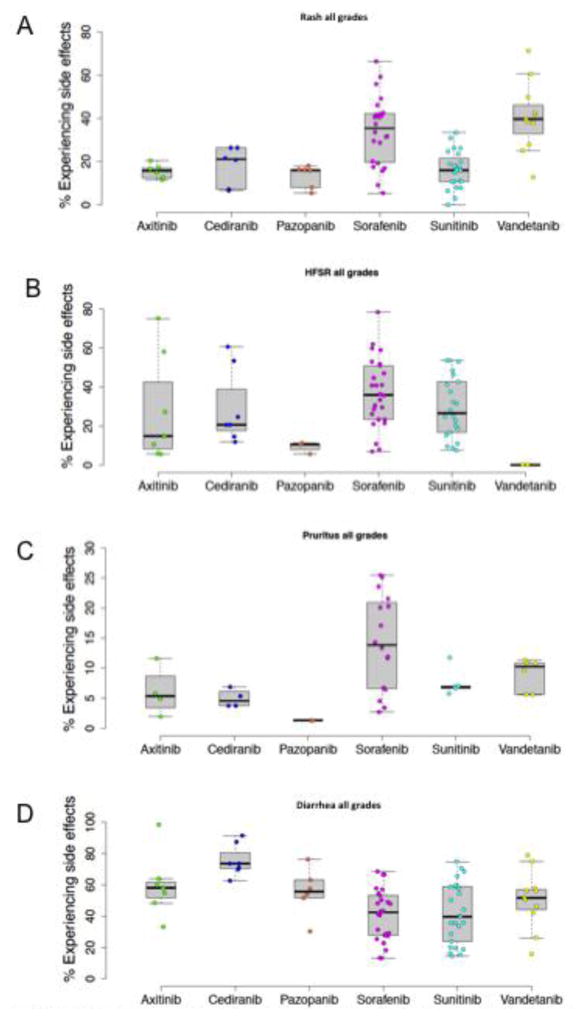
Six anti-VEGFR TKIs had sufficient data available from 78 trials for comparisons of toxicities (cabozantinib and regorafenib were not included due to a small number of trials) (Fig 4). The mean percentage of patients experiencing rash ranged between 13% with pazopanib and 41% with vandetanib. Overall, rash was significantly more common with sorafenib than with axitinib (p=0.01), pazopanib (p<.01) cediranib (p=0.05) and sunitinib (p<.0001). Vandetanib was associated with a higher risk of rash than sunitinib (p<.0001), pazopanib (p<.001), cediranib (p<.01), and axitinib (p=.001). The mean incidence of HFSR ranged from 0% for vandetanib to 37% for sorafenib. However, there was no statistical difference between TKI groups in the multiple comparisons test. Sorafenib was the most frequent cause of pruritus (14%). No statistically significant differences were detected with respect to pruritus, although there was a trend for sorafenib to cause pruritus more often than either cediranib (p=0.07) or pazopanib (p=0.053). Finally, patients taking cediranib were significantly more likely to experience diarrhea than patients taking sorafenib (p<0.001) or sunitinib (p<0.001). Boxplots for six anti-VEGFR TKIs separated by grades 1/2 and 3/4 toxicities with respect to rash, HFSR, pruritus and diarrhea are shown in Supplemental Fig 2.
Fig. 4. A–D. Box Plots of Adverse Events.
Box plots demonstrating percent of patients who experienced rash (A), HFSR (B), pruritus (C), and diarrhea (D), with data derived from both randomized and nonrandomized clinical trials (n= 78).
Reported HFSR over time
Time interval scatter plots were generated by plotting the percentage of patients with adverse effects versus the median date of patient enrollment from December 2002 – June 2010 (Fig 5). Scatter plots were generated for 1) all anti-VEGFR TKIs, 2) sunitinib and 3) sorafenib. Sunitinib and sorafenib were chosen for individual review due to their long tenure in clinical use. Between 2002 and 2010, the overall incidence of HFSR for all anti-VEGFR TKIs showed a tendency to increase over time, but did not reach statistical significance (r2=0.042, p=0.10). Sunitinib showed a significant increase in HFSR over time (r2=0.237, p=0.04) while the incidence in patients receiving sorafenib remained constant (all toxicity grades of HFSR r2=0.003, p=0.8). In contrast, the cumulative incidence of all toxicity grades of diarrhea over the same time period was unchanged over time (r2=0.001, p=0.8) and the overall incidence of sunitinib and sorafenib-associated diarrhea were similarly stable (r2=0.007, p=0.7 and r2=0.002, p=0.8). Scatter plots separating grades 1/2 and 3/4 toxicities over the same time are included in Supplemental Fig 3.
Fig. 5. Scatter Plots of Adverse Events By Time Interval.
Time interval scatter plots demonstrating percent of patients reporting all grade HFSR (top) and diarrhea (bottom) by drug class, sunitinib and sorafenib.
Cutaneous toxicity in combination therapy
The 33 randomized trials were dichotomized by the use of TKIs as monotherapy or in combination therapy with a non-TKI, and were analyzed by tests for subgroup differences (Fig 6). Anti-VEGFR TKIs in combination with conventional chemotherapy was associated with a decreased overall risk of rash (OR, 2.68; 95% CI 2.45–2.94) compared to monotherapy (OR, 4.03; 95% CI, 3.47–4.68). The risk of HFSR when the anti-VEGFR TKI was a component of a combination regimen involving a conventional chemotherapeutic agent (OR, 2.70; 95% CI 2.43–3.00) was not different from TKI use as monotherapy (OR, 2.65; 95% CI, 2.28–3.08). Rates of pruritus were similar in combination regimens (OR, 1.49; 95% CI 1.25–1.78) and monotherapy (OR, 1.12; 95% CI, 0.98–1.29). All grades of diarrhea were more common in monotherapy compared to combination therapy (OR, 3.60; 95% CI 3.17–4.08; OR, 2.32; 95% CI, 2.12–2.55).
Fig. 6. A–D. Subgroup Analysis of Monotherapy v. Combination Therapy.
Forest Plot subgroup analysis of randomized trials demonstrating odds ratio of all grade rash (A), HFSR (B), pruritus (C) and diarrhea (D) by drug versus placebo, according to whether drug was given as monotherapy or in combination with conventional cytotoxic chemotherapy.
Data Synthesis and Assessment of Study Quality and Bias
The 47 randomized controlled trials included in the analysis had an average Jadad score of 3.5 indicating a satisfactory quality level. No studies had a quality score below 3 and thus none were excluded from consideration. Non-randomized trials were assessed using NOS criteria and had an average score of 7.5. Studies had a low risk of bias according to the Cochrane Collaboration’s tool. Tests for statistical heterogeneity revealed significant heterogeneity in the meta-analyses, with I2 values ranging from 60–90% for all grade toxicities. However, when evaluated by individual drug, I2 values decreased in most cases to 0%. Examination of funnel plots and estimated degree of funnel plot asymmetry did not indicate evidence of reporting or publication bias. Only the data for diarrhea were found to have some evidence of asymmetry in the funnel plots.
DISCUSSION
Although TKIs directed against the VEGFR are “targeted therapeutics”, most inhibit a range of kinase receptors with varied potency and selectivity. The latter include platelet-derived growth factor (PDGF), fibroblast growth factor receptor, epidermal growth factor receptor (EGFR), c-kit, Flt-3 and insulin-like growth factor receptor [5]. These agents act by targeting the intracellular ATP-binding domain of the tyrosine kinase, preventing phosphorylation and downstream signaling [23, 35]. Rash, HFSR and pruritus have emerged as a “class effect,” impairing drug tolerability and contributing to dose reduction and treatment discontinuation [30]. However, the distinct multikinase inhibition profile of each agents may produce differences in the rate and severity of cutaneous side effects.
Of the commonly reported skin toxicities, HFSR is the most troublesome for patients and the most difficult to manage. We show here that the overall (all grade) incidence of HFSR, may be increasing over time. One possible explanation for this observation is that physician experience and treatment success may be leading to more aggressive dosing. However, the overall incidence of diarrhea, a common systemic side effect, has remained unchanged over the same time period, suggesting that heightened clinical and detection of HFSR may also be responsible.
As multiple TKIs have demonstrated similar efficacy against some cancers [8, 25], an awareness of specific cutaneous toxicity profiles of each drug may aid clinicians in the selection or adjustment of patients’ therapeutic regimens based on quality of life concerns [24]. Sorafenib, for example, was associated with HFSR in 37% of patients, compared to only 9% of patients receiving Pazopanib. In studies meeting our inclusion criteria, vandetanib was not a significant cause of HFSR.
The etiology of HFSR in association with anti-VEGFR TKI therapy is unclear. Bevacizumab, a monoclonal antibody against VEGF, is not associated with HFSR [2, 27]. Lacouture et al. proposed that VEGFR inhibition may be necessary but is not sufficient to cause HFSR, and that PDGFR, which is expressed in vascular stroma, must also be inhibited [1, 12]. Support for this concept includes two observations: (1) sorafenib and sunitinib, the two anti-VEGFR TKIs most strongly associated with HFSR, inhibit both VEGFR and PDGFR [5, 6], whereas vandetanib, which does not inhibit PDGFR, only rarely results in HFSR [17, 32]; and (2) imatinib mesylate, a potent PDGFR inhibitor (but not VEGFR inhibitor) does not cause HFSR [4]. It has been proposed that a synergistic effect of inhibition of VEGFR and PDGFR leads to compromised capillary endothelium and poor reparative response to ordinary trauma at high friction areas, such as the hands and feet [6, 7, 11]. Therefore, counseling patients at risk of developing HFSR to take extra precautions to avoid trauma on acral surfaces (e.g. soft shoes, gloves) is prudent.
New TKIs with increased specificity for VEGFR have been developed with the hope of reducing or eliminating off-target side effects [11, 33, 39]. If dermatologic adverse events due to anti-VEGFR agents are related to an off-target multikinase inhibitory profile, one would then expect fewer side effects with more highly selective drugs. However, our data demonstrate that HFSR and other dermatologic adverse events continue to be a significant problem with these newer agents. For example, axitinib, which was approved by the FDA in 2012 for the treatment of metastatic renal cell carcinoma, is 100x more potent in some assays than sunitinib and is far more selective [17, 22]. Nevertheless, the two drugs cause HFSR at comparable rates. The failure of enhanced selectivity in reducing skin toxicity suggests the pathophysiology of HFSR and other cutaneous toxicities of TKIs may lie in “on-target inhibition,” particularly of the VEGF and PDGF receptors. This is consistent with reports that the development of HFSR significantly correlates with tumor response [26]. The finding that TKI treatment in combination with other chemotherapeutic agents did not increase the dermatologic side effects of TKIs also supports an “on target” mechanism rather than nonspecific drug toxicity.
Finally, our study suggests a high degree of variability in the reporting of dermatologic adverse events in oncology trials. For example, the OR of HFSR in patients ranged from 5.55 to 28.47 in randomized trials utilizing identical doses of sorafenib monotherapy [9, 10]. Several factors may be affecting the reproducibility of dermatologic adverse event reporting, including varied patterns of presentation and inherent subjectivity in dermatologic event grading. Further collaboration between dermatologists and oncologists is warranted to standardize diagnostic terminology in order to better capture the adverse event diversity inherent to this class of agents.
We cannot exclude the possibility that some of the results were confounded by heterogeneity in patient populations across the 82 studies we reviewed. This is of special concern with respect to ethnicity [20] and neoplasm type [7] – two factors that have been shown to independently influence the development of cutaneous toxicity in TKI therapy. In addition, our analysis is susceptible to challenges in inter-observer clinical judgment, which we have highlighted in this discussion.
The management of cutaneous adverse events in cancer patients has emerged as a key issue in TKI, which unlike conventional chemotherapy, are administered on a long-term basis [3]. Here we have documented variability within the anti-VEGFR TKI class with respect to the incidence and severity of HFSR, rash, and pruritus as well as non-dermatologic toxicities including diarrhea. This information may be of use to dermatologists as well as oncologists seeking to develop new approaches to prevent and treat these toxicities that in many cases limit the dose and duration of the therapy. Better supportive dermatologic treatments can improve the quality of life of cancer patients receiving anti-VEGFR TKIs, and may allow for longer and more sustained treatment and improved survival.
Supplementary Material
Supplemental Fig 1A–G. Additional Meta-Analyses Organized by Drug. Forest Plot of all randomized trials organized by drug and displaying trial author name, demonstrating odds ratios of all grade rash (A), HFSR (B), pruritus (C), diarrhea (D), fatigue (E), adverse events leading to discontinuation (F), adverse events leading to dose reduction (G) of TKI versus placebo.
Supplemental Fig 2A–H. Additional Box Plots Separated by Adverse Events Grade. Box plots demonstrating percent of patients who experienced rash grades 1/2 (A), rash grades 3/4 (B), HFSR grades 1/2 (C), HFSR grades 3/4 (D), pruritus grades 1/2 (E), pruritus grades 3/4 (F), diarrhea grades 1/2 (G), diarrhea grades 3/4 (H).
Supplemental Fig 3. Additional Time Interval Scatter Plots Separated by Adverse Events Grade. Additional time interval scatter plots for rates of adverse events in patients taking sunitinib and sorafenib, organized by low grade (1/2) and high grade (3/4) HFSR and diarrhea.
Supplemental Fig 4A–D. Additional Subgroup Analysis of Monotherapy v. Combination Therapy By Drug. Forest plots of randomized trials demonstrating odds ratio for experiencing all grade rash (A), all grade HFSR (B), all grade pruritus (C), or all grade diarrhea (D), organized by drug and according to whether drug was given as monotherapy or in combination with conventional cytotoxic chemotherapy.
Supplemental Table 1. Summary Table of Included Studies. Summary table of 82 trials meeting inclusion criteria including treatment, author name, year of publication, dose of drug, number of patients and if randomization or use of placebo were present.
Acknowledgments
Funding/Support/Role of the Sponsors: This research was made possible in part by the Intramural Research Program of the NIH, National Cancer Institute.
We would like to thank Drs. Mark Udey and Heidi Kong of the NCI for assistance in editing this manuscript and Dr. Tito Fojo for his general advice regarding this project.
Footnotes
Financial disclosure: None reported.
CONFLICTS OF INTEREST
The authors have no conflicts of interest to report.
Author attribution: All authors had full access to all of the data in the study and take responsibility for the integrity of the data. Study concept and design: Massey, Cowen. Acquisition of data: Massey, Okman. Analysis and interpretation of data: all authors. Drafting of the manuscript: Massey, Okman, Cowen. Critical revision of the manuscript for important intellectual content: all authors. Statistical analysis: Wilkerson. Obtained funding: N/A. Administrative, technical, or material support: N/A. Study supervision: Cowen.
References
- 1.Andrae J, Gallini R, Betsholtz C. Role of platelet-derived growth factors in physiology and medicine. Genes Dev. 2008;22:1276–1312. doi: 10.1101/gad.1653708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Azad NS, Aragon-Ching JB, Dahut WL, Gutierrez M, Figg WD, Jain L, Steinberg SM, Turner ML, Kohn EC, Kong HH. Hand-foot skin reaction increases with cumulative sorafenib dose and with combination anti-vascular endothelial growth factor therapy. Clin Cancer Res. 2009;15:1411–1416. doi: 10.1158/1078-0432.CCR-08-1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Balagula Y, Rosen ST, Lacouture ME. The emergence of supportive oncodermatology: the study of dermatologic adverse events to cancer therapies. J Am Acad Dermatol. 2011;65:624–635. doi: 10.1016/j.jaad.2010.06.051. [DOI] [PubMed] [Google Scholar]
- 4.Breccia M, Carmosino I, Russo E, Morano SG, Latagliata R, Alimena G. Early and tardive skin adverse events in chronic myeloid leukaemia patients treated with imatinib. Eur J Haematol. 2005;74:121–123. doi: 10.1111/j.1600-0609.2004.00351.x. [DOI] [PubMed] [Google Scholar]
- 5.Chow LQ, Eckhardt SG. Sunitinib: from rational design to clinical efficacy. J Clin Oncol. 2007;25:884–896. doi: 10.1200/JCO.2006.06.3602. [DOI] [PubMed] [Google Scholar]
- 6.Chu D, Lacouture ME, Fillos T, Wu S. Risk of hand-foot skin reaction with sorafenib: a systematic review and meta-analysis. Acta Oncol. 2008;47:176–186. doi: 10.1080/02841860701765675. [DOI] [PubMed] [Google Scholar]
- 7.Chu D, Lacouture ME, Weiner E, Wu S. Risk of hand-foot skin reaction with the multitargeted kinase inhibitor sunitinib in patients with renal cell and non-renal cell carcinoma: a meta-analysis. Clin Genitourin Cancer. 2009;7:11–19. doi: 10.3816/CGC.2009.n.002. [DOI] [PubMed] [Google Scholar]
- 8.Escudier B, Eisen T, Stadler WM, Szczylik C, Oudard S, Siebels M, Negrier S, Chevreau C, Solska E, Desai AA, Rolland F, Demkow T, Hutson TE, Gore M, Freeman S, Schwartz B, Shan M, Simantov R, Bukowski RM, Group TS. Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med. 2007;356:125–134. doi: 10.1056/NEJMoa060655. [DOI] [PubMed] [Google Scholar]
- 9.Escudier B, Eisen T, Stadler WM, Szczylik C, Oudard S, Staehler M, Negrier S, Chevreau C, Desai AA, Rolland F, Demkow T, Hutson TE, Gore M, Anderson S, Hofilena G, Shan M, Pena C, Lathia C, Bukowski RM. Sorafenib for treatment of renal cell carcinoma: Final efficacy and safety results of the phase III treatment approaches in renal cancer global evaluation trial. J Clin Oncol. 2009;27:3312–3318. doi: 10.1200/JCO.2008.19.5511. [DOI] [PubMed] [Google Scholar]
- 10.Escudier B, Szczylik C, Hutson TE, Demkow T, Staehler M, Rolland F, Negrier S, Laferriere N, Scheuring UJ, Cella D, Shah S, Bukowski RM. Randomized phase II trial of first-line treatment with sorafenib versus interferon Alfa-2a in patients with metastatic renal cell carcinoma. J Clin Oncol. 2009;27:1280–1289. doi: 10.1200/JCO.2008.19.3342. [DOI] [PubMed] [Google Scholar]
- 11.Fischer A, Wu S, Ho AL, Lacouture ME. The risk of hand-foot skin reaction to axitinib, a novel VEGF inhibitor: a systematic review of literature and meta-analysis. Invest New Drugs. 2013;31:787–797. doi: 10.1007/s10637-013-9927-x. [DOI] [PubMed] [Google Scholar]
- 12.Gomez P, Lacouture ME. Clinical presentation and management of hand-foot skin reaction associated with sorafenib in combination with cytotoxic chemotherapy: experience in breast cancer. Oncologist. 2011;16:1508–1519. doi: 10.1634/theoncologist.2011-0115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harbord RM, Egger M, Sterne JA. A modified test for small-study effects in meta-analyses of controlled trials with binary endpoints. Stat Med. 2006;25:3443–3457. doi: 10.1002/sim.2380. [DOI] [PubMed] [Google Scholar]
- 14.Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, McQuay HJ. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17:1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 15.Janusch M, Fischer M, Marsch WCh, Holzhausen HJ, Kegel T, Helmbold P. The hand-foot syndrome--a frequent secondary manifestation in antineoplastic chemotherapy. Eur J Dermatol. 2006;16:494–499. [PubMed] [Google Scholar]
- 16.Kamba T, McDonald DM. Mechanisms of adverse effects of anti-VEGF therapy for cancer. Br J Cancer. 2007;96:1788–1795. doi: 10.1038/sj.bjc.6603813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Karaman MW, Herrgard S, Treiber DK, Gallant P, Atteridge CE, Campbell BT, Chan KW, Ciceri P, Davis MI, Edeen PT, Faraoni R, Floyd M, Hunt JP, Lockhart DJ, Milanov ZV, Morrison MJ, Pallares G, Patel HK, Pritchard S, Wodicka LM, Zarrinkar PP. A quantitative analysis of kinase inhibitor selectivity. Nat Biotechnol. 2008;26:127–132. doi: 10.1038/nbt1358. [DOI] [PubMed] [Google Scholar]
- 18.Lacouture ME, Reilly LM, Gerami P, Guitart J. Hand foot skin reaction in cancer patients treated with the multikinase inhibitors sorafenib and sunitinib. Ann Oncol. 2008;19:1955–1961. doi: 10.1093/annonc/mdn389. [DOI] [PubMed] [Google Scholar]
- 19.Lacouture ME, Wu S, Robert C, Atkins MB, Kong HH, Guitart J, Garbe C, Hauschild A, Puzanov I, Alexandrescu DT, Anderson RT, Wood L, Dutcher JP. Evolving strategies for the management of hand-foot skin reaction associated with the multitargeted kinase inhibitors sorafenib and sunitinib. Oncologist. 2008;13:1001–1011. doi: 10.1634/theoncologist.2008-0131. [DOI] [PubMed] [Google Scholar]
- 20.Lee JH, Chung YH, Kim JA, Shim JH, Lee D, Lee HC, Shin ES, Yoon JH, Kim BI, Bae SH, Koh KC, Park NH. Genetic predisposition of hand-foot skin reaction after sorafenib therapy in patients with hepatocellular carcinoma. Cancer. 2013;119:136–142. doi: 10.1002/cncr.27705. [DOI] [PubMed] [Google Scholar]
- 21.Lipworth AD, Robert C, Zhu AX. Hand-foot syndrome (hand-foot skin reaction, palmar-plantar erythrodysesthesia): focus on sorafenib and sunitinib. Oncology. 2009;77:257–271. doi: 10.1159/000258880. [DOI] [PubMed] [Google Scholar]
- 22.McTigue M, Murray BW, Chen JH, Deng YL, Solowiej J, Kania RS. Molecular conformations, interactions, and properties associated with drug efficiency and clinical performance among VEGFR TK inhibitors. Proc Natl Acad Sci U S A. 2012;109:18281–18289. doi: 10.1073/pnas.1207759109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morabito A, De Maio E, Di Maio M, Normanno N, Perrone F. Tyrosine kinase inhibitors of vascular endothelial growth factor receptors in clinical trials: current status and future directions. Oncologist. 2006;11:753–764. doi: 10.1634/theoncologist.11-7-753. [DOI] [PubMed] [Google Scholar]
- 24.Motzer RJ, Hutson TE, Cella D, Reeves J, Hawkins R, Guo J, Nathan P, Staehler M, de Souza P, Merchan JR, Boleti E, Fife K, Jin J, Jones R, Uemura H, De Giorgi U, Harmenberg U, Wang J, Sternberg CN, Deen K, McCann L, Hackshaw MD, Crescenzo R, Pandite LN, Choueiri TK. Pazopanib versus sunitinib in metastatic renal-cell carcinoma. N Engl J Med. 2013;369:722–731. doi: 10.1056/NEJMoa1303989. [DOI] [PubMed] [Google Scholar]
- 25.Motzer RJ, Hutson TE, Tomczak P, Michaelson MD, Bukowski RM, Rixe O, Oudard S, Negrier S, Szczylik C, Kim ST, Chen I, Bycott PW, Baum CM, Figlin RA. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med. 2007;356:115–124. doi: 10.1056/NEJMoa065044. [DOI] [PubMed] [Google Scholar]
- 26.Nakano K, Komatsu K, Kubo T, Natsui S, Nukui A, Kurokawa S, Kobayashi M, Morita T. Hand-foot skin reaction is associated with the clinical outcome in patients with metastatic renal cell carcinoma treated with sorafenib. Jpn J Clin Oncol. 2013;43:1023–1029. doi: 10.1093/jjco/hyt110. [DOI] [PubMed] [Google Scholar]
- 27.Perren TJ, Swart AM, Pfisterer J, Ledermann JA, Pujade-Lauraine E, Kristensen G, Carey MS, Beale P, Cervantes A, Kurzeder C, du Bois A, Sehouli J, Kimmig R, Stähle A, Collinson F, Essapen S, Gourley C, Lortholary A, Selle F, Mirza MR, Leminen A, Plante M, Stark D, Qian W, Parmar MK, Oza AM Investigators I. A phase 3 trial of bevacizumab in ovarian cancer. N Engl J Med. 2011;365:2484–2496. doi: 10.1056/NEJMoa1103799. [DOI] [PubMed] [Google Scholar]
- 28.Porta C, Paglino C, Imarisio I, Bonomi L. Uncovering Pandora’s vase: the growing problem of new toxicities from novel anticancer agents. The case of sorafenib and sunitinib. Clin Exp Med. 2007;7:127–134. doi: 10.1007/s10238-007-0145-8. [DOI] [PubMed] [Google Scholar]
- 29.Posadas EM, Limvorasak S, Sharma S, Figlin RA. Targeting angiogenesis in renal cell carcinoma. Expert Opin Pharmacother. 2013 doi: 10.1517/14656566.2013.832202. [DOI] [PubMed] [Google Scholar]
- 30.Prasad V, Massey P, Fojo T. J Clin Oncol. Oral Anticancer Drugs: How Limited Dosing Options and Dose Reductions May Affect Outcomes in Comparative Trials and Efficacy in Patients. Book Oral Anticancer Drugs: How Limited Dosing Options and Dose Reductions May Affect Outcomes in Comparative Trials and Efficacy in Patients. In press., City. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ranieri G, Mammì M, Donato Di Paola E, Russo E, Gallelli L, Citraro R, Gadaleta CD, Marech I, Ammendola M, De Sarro G. Pazopanib a tyrosine kinase inhibitor with strong anti-angiogenetic activity: A new treatment for metastatic soft tissue sarcoma. Crit Rev Oncol Hematol. 2013 doi: 10.1016/j.critrevonc.2013.08.012. [DOI] [PubMed] [Google Scholar]
- 32.Rosen AC, Wu S, Damse A, Sherman E, Lacouture ME. Risk of rash in cancer patients treated with vandetanib: systematic review and meta-analysis. J Clin Endocrinol Metab. 2012;97:1125–1133. doi: 10.1210/jc.2011-2677. [DOI] [PubMed] [Google Scholar]
- 33.Sahade M, Caparelli F, Hoff PM. Cediranib: a VEGF receptor tyrosine kinase inhibitor. Future Oncol. 2012;8:775–781. doi: 10.2217/fon.12.73. [DOI] [PubMed] [Google Scholar]
- 34.Shin JW, Chung YH. Molecular targeted therapy for hepatocellular carcinoma: Current and future. World J Gastroenterol. 2013;19:6144–6155. doi: 10.3748/wjg.v19.i37.6144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shukla S, Chen ZS, Ambudkar SV. Tyrosine kinase inhibitors as modulators of ABC transporter-mediated drug resistance. Drug Resist Updat. 2012;15:70–80. doi: 10.1016/j.drup.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Smaglo BG, Hwang J. Continuum of care with anti-angiogenic therapies in metastatic colorectal cancer. J Gastrointest Oncol. 2013;4:299–307. doi: 10.3978/j.issn.2078-6891.2013.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sterne JA, Sutton AJ, Ioannidis JP, Terrin N, Jones DR, Lau J, Carpenter J, Rücker G, Harbord RM, Schmid CH, Tetzlaff J, Deeks JJ, Peters J, Macaskill P, Schwarzer G, Duval S, Altman DG, Moher D, Higgins JP. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ. 2011;343:d4002. doi: 10.1136/bmj.d4002. [DOI] [PubMed] [Google Scholar]
- 38.Wells G, Shea B, O’Connell D, Peterson J, Welch V, Losos M, Tugwell P. Book The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Ottawa Hospital Research Institute; City: The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. [Google Scholar]
- 39.Wilhelm SM, Dumas J, Adnane L, Lynch M, Carter CA, Schütz G, Thierauch KH, Zopf D. Regorafenib (BAY 73-4506): a new oral multikinase inhibitor of angiogenic, stromal and oncogenic receptor tyrosine kinases with potent preclinical antitumor activity. Int J Cancer. 2011;129:245–255. doi: 10.1002/ijc.25864. [DOI] [PubMed] [Google Scholar]
- 40.Yang CH, Lin WC, Chuang CK, Chang YC, Pang ST, Lin YC, Kuo TT, Hsieh JJ, Chang JW. Hand-foot skin reaction in patients treated with sorafenib: a clinicopathological study of cutaneous manifestations due to multitargeted kinase inhibitor therapy. Br J Dermatol. 2008;158:592–596. doi: 10.1111/j.1365-2133.2007.08357.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Fig 1A–G. Additional Meta-Analyses Organized by Drug. Forest Plot of all randomized trials organized by drug and displaying trial author name, demonstrating odds ratios of all grade rash (A), HFSR (B), pruritus (C), diarrhea (D), fatigue (E), adverse events leading to discontinuation (F), adverse events leading to dose reduction (G) of TKI versus placebo.
Supplemental Fig 2A–H. Additional Box Plots Separated by Adverse Events Grade. Box plots demonstrating percent of patients who experienced rash grades 1/2 (A), rash grades 3/4 (B), HFSR grades 1/2 (C), HFSR grades 3/4 (D), pruritus grades 1/2 (E), pruritus grades 3/4 (F), diarrhea grades 1/2 (G), diarrhea grades 3/4 (H).
Supplemental Fig 3. Additional Time Interval Scatter Plots Separated by Adverse Events Grade. Additional time interval scatter plots for rates of adverse events in patients taking sunitinib and sorafenib, organized by low grade (1/2) and high grade (3/4) HFSR and diarrhea.
Supplemental Fig 4A–D. Additional Subgroup Analysis of Monotherapy v. Combination Therapy By Drug. Forest plots of randomized trials demonstrating odds ratio for experiencing all grade rash (A), all grade HFSR (B), all grade pruritus (C), or all grade diarrhea (D), organized by drug and according to whether drug was given as monotherapy or in combination with conventional cytotoxic chemotherapy.
Supplemental Table 1. Summary Table of Included Studies. Summary table of 82 trials meeting inclusion criteria including treatment, author name, year of publication, dose of drug, number of patients and if randomization or use of placebo were present.